Immunomodulatory Activity of Dietary Fiber: Arabinoxylan and Mixed-Linked Beta-Glucan Isolated from Barley Show Modest Activities in Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Barley Dietary Fiber Fractions
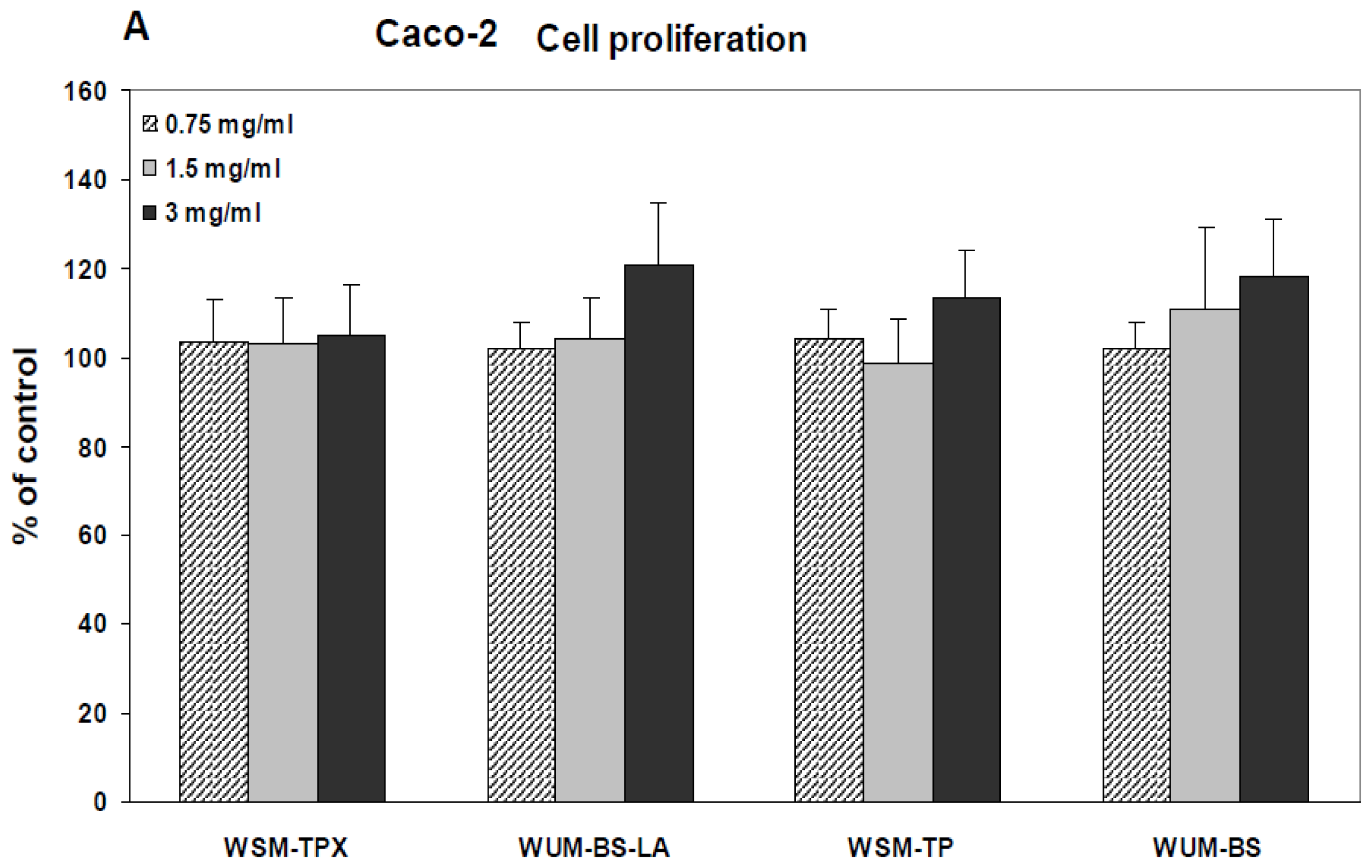
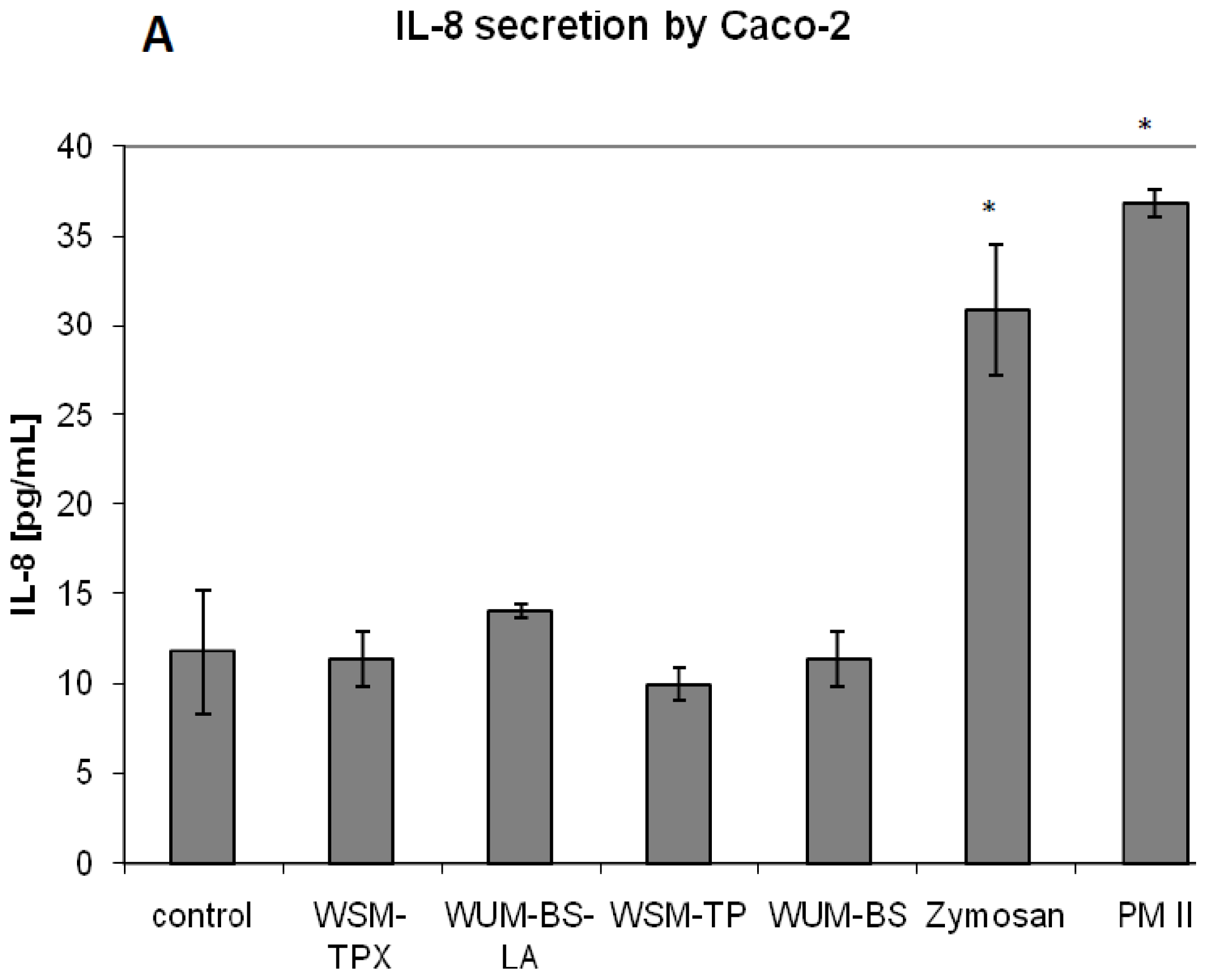
2.2. Effect on IL-8 Secretion and Cell Proliferation in Caco-2 and HT-29
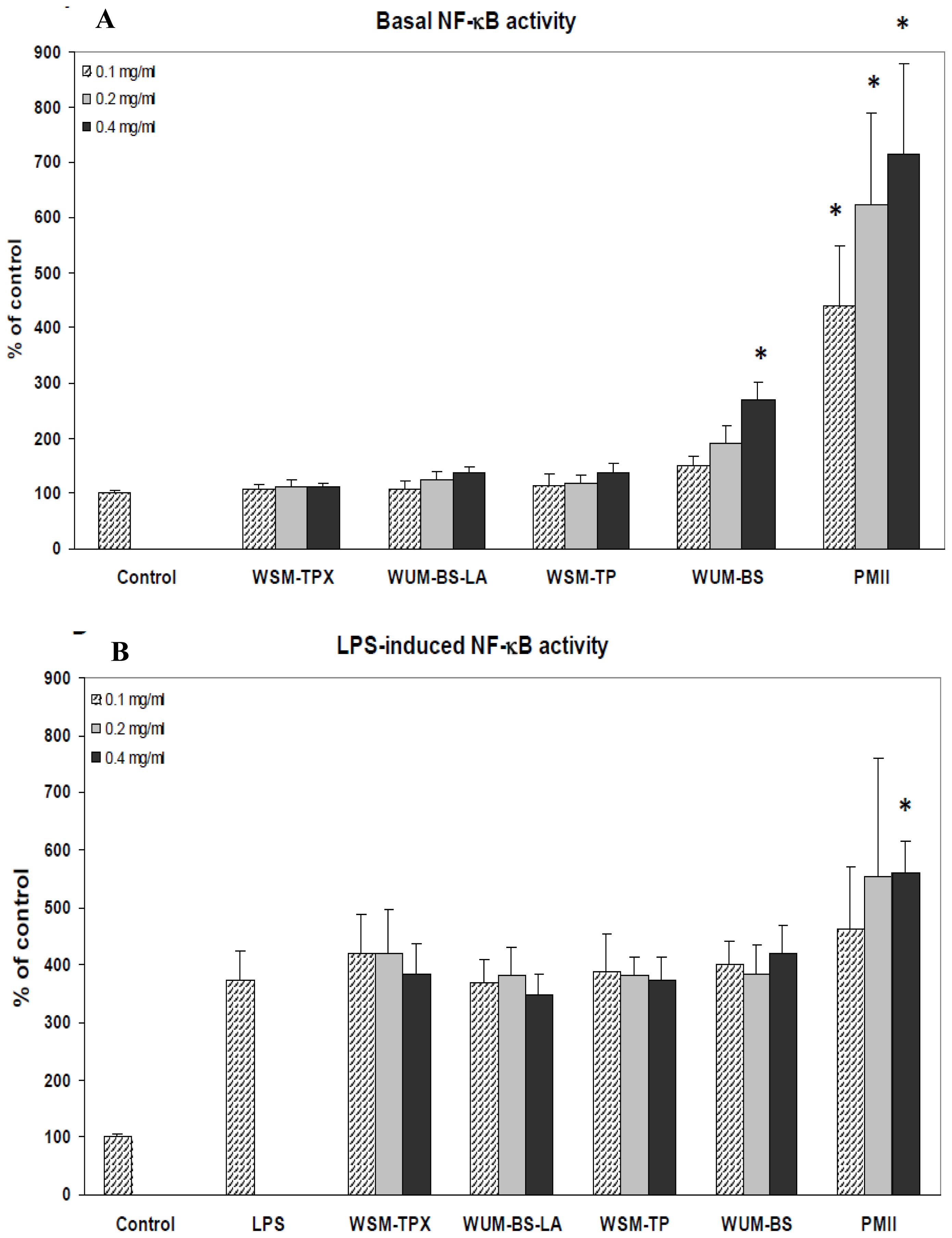
2.3. The Ability of the Fiber Fractions to Modulate NF-κB Activity in Monocytes
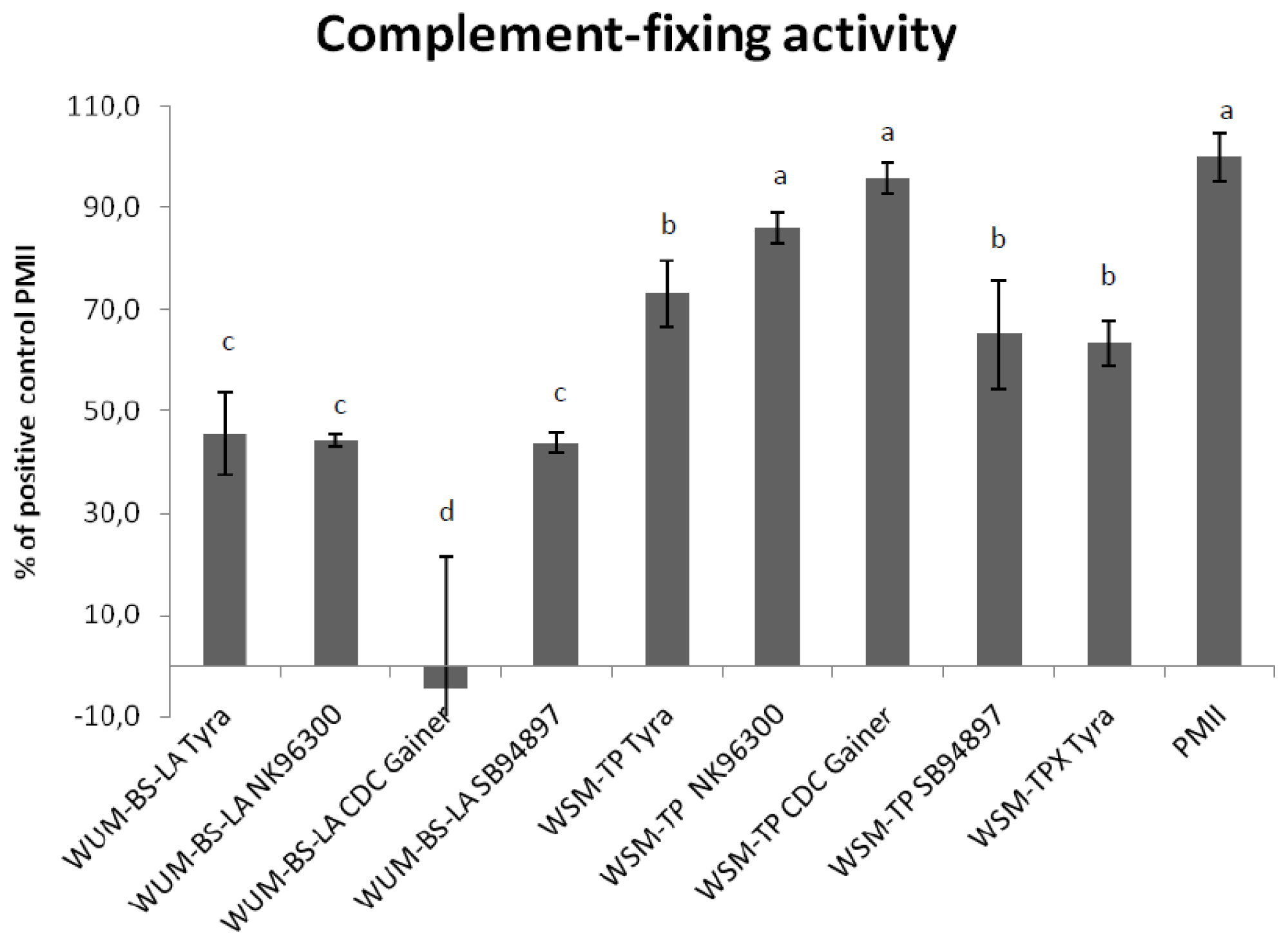
2.4. Complement Fixing Test
3. Experimental Section
3.1. Isolation of Fiber Fractions
3.2. Monosaccharide Composition
3.3. 1H-NMR
3.4. HPLC
3.5. Cell Cultures
3.6. Measurement of Cell Proliferation and IL-8 Secretion
3.7. NF-kB Activity Assay
3.8. Complement Fixing Test
3.9. Statistics
4. Conclusions
Acknowledgements
References
- Burkitt, DP. Some diseases characteristic of modern westernised civilisation. Br. Med. J 1973, 1, 274–278. [Google Scholar]
- Asano, TK; McLeod, RS. Dietary fibre for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2002, 1, CD003430. [Google Scholar]
- WHO;, FAO. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series; Volume 916, WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Marshall, JR. Nutrition and colon cancer prevention. Curr. Opin. Clin. Nutri. Metab Care 2009, 12, 539–343. [Google Scholar]
- Bray, F; Wibe, A; Dorum, LMR; Møller, B. Tykktarms-og endetarmskreft i Norge-epidemiologi. J. Nor. Med. Assoc 2007, 127, 2682–2687. [Google Scholar]
- Kirkegaard, H; Johnsen, NF; Christensen, J; Frederiksen, K; Overvad, K; Tjønneland, A. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ 2010, 341, c5504. [Google Scholar]
- Grivennikov, SI; Greten, FR; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar]
- Coussens, LM; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar]
- Erlinger, TP; Platz, EA; Rifai, N; Helzlsouer, KJ. C-Reactive Protein and the risk of incident colorectal cancer. JAMA 2004, 291, 585–590. [Google Scholar]
- King, DE; Egan, BM; Woolson, RF; Mainous, AG; A-Solaiman, Y; Jesri, A. Effect of a high-fiber diet vs. a fiber-supplemented diet on D-reactive protein level. Arch. Intern. Med 2007, 167, 502–506. [Google Scholar]
- Ajani, UA; Ford, ES; Mokdad, AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J. Nutri 2004, 134, 1181–1184. [Google Scholar]
- Ma, Y; Griffith, JA; Chasan-Taber, L; Olendzki, BC; Jackson, E; Stanek, EJ; Li, W; Pagoto, SL; Hafner, AR; Ockene, IS. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutri 2006, 83, 760–766. [Google Scholar]
- Ma, Y; Hebert, JR; Li, W; Bertone-Johnson, ER; Olendzki, B; Pagoto, SL; Tinker, L; Rosal, MC; Ockene, IS; Ockene, JK; Griffith, JA; Liu, S. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initative Observational Study. Nutrition 2008, 24, 941–949. [Google Scholar]
- Dunn, GP; Old, LJ; Schreiber, RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar]
- Hong, F; Yan, J; Baran, JT; Allendorf, DJ; Hansen, RD; Ostroff, GR; Xing, PX; Cheung, N-KV; Ross, GD. Mechanism by which orally administered beta-1,3-glucans enhance tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol 2004, 173, 797–806. [Google Scholar]
- Cheung, N-K; Modak, S; Vickers, A; Knuckles, B. Orally administered β-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol. Immunother 2002, 51, 557–564. [Google Scholar]
- Andoh, A; Fujiyama, Y. Anti-inflammatory roles of dietary fiber and short-chain fatty acids as regards inflammatory bowel diseases. Agro Food Ind Hi-Tech 2004, 1, 42–43. [Google Scholar]
- Lupton, JR. Microbial Degradation Products Influence Colon Cancer Risk: the Butyrate Controversy. J. Nutri 2004, 134, 479. [Google Scholar]
- Bordonaro, M; Lazarova, DL; Sartorellil, AC. Butyraate and Wnt signaling. Cell Cycle 2008, 7, 1178–1183. [Google Scholar]
- Volman, JJ; Rmakers, JD; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav 2008, 94, 276–284. [Google Scholar]
- Yamada, H; Kiyohara, H. Boons, G-J, Lee, YC, Suzuki, A, Taniguchi, N, Voragen, AGJ, Eds.; Cell Glycobiology and Development Health and Disease in Glycomedicine; Elsevier: Oxford, UK, 2007; pp. 664–693. [Google Scholar]
- Holtekjølen, AK; Uhlen, AK; Bråthen, E; Sahlstrøm, S; Knutsen, SH. Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem 2006, 94, 348–358. [Google Scholar]
- Knutsen, SH; Holtekjolen, AK. Preparation and analysis of dietary fibre constituents in whole grain from hulled and hull-less barley. Food Chem 2007, 102, 707–715. [Google Scholar]
- Westerlund, E; Andersson, R; Åman, P. Isolation and chemical characterization of water soluble mixed-linked beta-glucans and arabinoxylans in oat milling fractions. Carbohydr. Polym 1993, 20, 115–123. [Google Scholar]
- Misra, CK; Das, BK; Mukherjee, SC; Pattnaik, P. Effect of multiple injections of β-glucan on non-specific immune response and disease resistance in Labeo rohita fingerlings. Fish Shellfish Immunol 2006, 20, 305–319. [Google Scholar]
- Czop, JK; Austen, KF. Properties of glycans that activate the human alternative complement pathway and interact with the human monocyte β-glucan receptor. J. Immunol 1985, 135, 3388–3393. [Google Scholar]
- Akramiene, D; Grazeliene, G; Didziapetrine, J; Kevelaitis, E. Treatment of Lewis lung carcinoma by photodynamic therapy and glucan from barley. Medicina (Kaunas) 2009, 45, 480–485. [Google Scholar]
- Ross, GD; Cain, JA; Lachmann, PJ. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin and functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J. Immunol 1985, 134, 3307–3315. [Google Scholar]
- Xia, Y; Vetvicka, V; Yan, J; Hanikyrová, M; Mayadas, T; Ross, GD. The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generationg a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol 1999, 162, 2281–2290. [Google Scholar]
- Thornton, BP; Vetvicka, V; Pitman, M; Goldman, RC; Ross, GD. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor Type 3 (CD11b/CD18). J. Immunol 1996, 156, 1235–1246. [Google Scholar]
- Cheung, N-KV; Modak, S. Oral (1→3),(1→4)-β-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin. Cancer Res 2002, 8, 1217–1223. [Google Scholar]
- Tada, R; Adachi, Y; Ishibashi, K-I; Tsubaki, K; Ohno, N. Binding capacity of a barley β-glucan to the β-glucan recognition molecule Dectin-1. J. Agric. Food Chem 2008, 56, 1442–1450. [Google Scholar]
- Tada, R; Ikeda, F; Aoki, K; Yoshikawa, M; Kato, Y; Adachi, Y; Tanioka, A; Ishibashi, K; Tsubaki, K; Ohno, N. Barley-derived β-D-glucan induces immunostimulation via a dectin-1-mediated pathway. Immunol. Lett 2009, 123, 144–148. [Google Scholar]
- Karin, M; Yamamoto, Y; Wang, QM. The IKK NF-kappaB system: a treasure trove for drug development. Nat. Rev Drug Discovery 2004, 3, 17–26. [Google Scholar]
- Michaelsen, TE; Gilje, A; Samuelsen, AB; Høgåsen, K; Paulsen, BS. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantago major L. Scand. J. Immunol 2000, 52, 483–490. [Google Scholar]
- Di Carlo, FJ; Fiore, JV. On the composition of Zymosan. Science 1958, 127, 756–757. [Google Scholar]
- Soltanian, S; Stuyven, E; Cox, E; Sorgeloos, P; Bossier, P. Beta-glucans as immunostimulants in vertebrates and invertebrates. Crit. Rev. Microbiol 2009, 35, 109–138. [Google Scholar]
- Hetland, G; Samuelsen, AB; Lovik, M; Paulsen, BS; Aaberge, IS; Groeng, EC; Michaelsen, TE. Protective effect of Plantago major L. pectin polysaccharide against systemic Streptococcus pneumoniae infection in mice. Scand. J. Immunol 2000, 52, 348–355. [Google Scholar]
- Samuelsen, AB; Paulsen, BS; Wold, JK; Otsuka, H; Kiyohara, H; Yamada, H; Knutsen, SH. Characterization of a biologically active pectin from Plantago major L. Carbohydr Polym 1996, 30, 37–44. [Google Scholar]
- Li, Q; Verma, IM. NF-KB regulation in the immune system. Nat. Rev 2002, 2, 725–734. [Google Scholar]
- Carlsen, H; Moskaug, JØ; Fromm, SH; Blomhoff, R. In vivo imaging of NF-KB activity. J. Immunol 2002, 168, 1441–1446. [Google Scholar]
- Austenaa, LM; Carlsen, H; Hollung, K; Blomhoff, HK; Blomhoff, R. Retionoic acid dampens LPS-induced NF-κB activity: results from human monoblasts and in vivo imaging of NF-κB reporter mice. J. Nutri. Biochem 2009, 20, 726–734. [Google Scholar]
- Palma, AS; Feizi, T; Zhang, Y; Stoll, MS; Lawson, AM; Díaz-Rodríguez, E; Campanero-Rhodes, MA; Costa, J; Gordon, S; Brown, GD; Chai, W. Ligands for the β-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J. Biol. Chem 2006, 281, 5771–5779. [Google Scholar]
- Izydorczyk, MS; Dextre, JE. Barley β-glucans and arabinoglucans: Molecular structure, physiochemical properties, and uses in food products—a review. Food Res. Int 2008, 41, 850–868. [Google Scholar]
- Holtekjølen, AK; Uhlen, AK; Bråthen, E; Sahlstrøm, S; Knutsen, SH. Contents of starch and son-starch polysaccharides in barley varieties of different origin. Food Chem 2006, 94, 348–358. [Google Scholar]
- Janeway, CA; Travers, P. Immunbiology the Immune System in Health and Disease; Current Biology. Ltd: London, UK, 1996; pp. 31–50. [Google Scholar]
- Roitt, I; Brostoff, J; Male, D. Immunology; Mandarin Offset Ltd: Hong Kong, China, 1993. [Google Scholar]
- Samuelsen, AB. Polysaccharides in Plantago major L. Studies of structure and biological activity. Ph. D. Thesis, University of Oslo, Oslo, Norway, 1998. [Google Scholar]
- Moure, A; Gullon, P; Dominguez, H; Parajo, JC. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem 2006, 41, 1913–1923. [Google Scholar]
- Femia, AP; Salvadori, M; Broekaert, WF; Francois, IEJA; Delcour, JA; Courtin, CM; Caderni, G. Arabinoxylan-oligosaccharides (AXOS) reduce preneoplastic lesions in the colon of rats treated with 1,2-dimethylhydrazine (DMH). Eur. J. Nutri 2010, 49, 127–132. [Google Scholar]
- Knutsen, SH; Holtekjølen, AK. Preparation and analysis of dietary fibre constituents in whole grain from hulled and hull-less barley. Food Chem 2007, 102, 707–715. [Google Scholar]
- Chambers, RE; Clamp, JR. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. J. Biochem 1971, 125, 1009–1018. [Google Scholar]
- Samuelsen, AB; Paulsen, BS; Wold, JK; Otsuka, H; Yamada, H; Espevik, T. Isolation and partial characterization of biologically active polysaccharides from Plantago major L. Phytother. Res 1995, 9, 211–218. [Google Scholar]
- Vistica, DT; Skehan, P; Scudiero, D; Monks, A; Pittman, A; Boyd, MR. Tetrazolium-based Assays for Cellular Viability: A Critical Examination of Selected Parameters Affecting Formazan Production. Cancer Res 1991, 51, 2515–2520. [Google Scholar]


| Monosaccharide Composition (mol%) | Molecular Weight (kDa) | |||||
|---|---|---|---|---|---|---|
| Fraction | Glc | Man | Xyl | Ara | Mw | Mn |
| WSM-TP | 91.0 | 0.0 | 5.8 | 3.3 | 1090 | 599 |
| WSM-TPX | 96.5 | 0.0 | 1.9 | 1.6 | 886 | 501 |
| WUM-BS | 30.0 | 0.0 | 52.7 | 17.3 | 412 | 98 |
| WUM-BS-LA | 2.0 | 1.6 | 66.0 | 30.4 | 156 | 42 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Samuelsen, A.B.; Rieder, A.; Grimmer, S.; Michaelsen, T.E.; Knutsen, S.H. Immunomodulatory Activity of Dietary Fiber: Arabinoxylan and Mixed-Linked Beta-Glucan Isolated from Barley Show Modest Activities in Vitro. Int. J. Mol. Sci. 2011, 12, 570-587. https://doi.org/10.3390/ijms12010570
Samuelsen AB, Rieder A, Grimmer S, Michaelsen TE, Knutsen SH. Immunomodulatory Activity of Dietary Fiber: Arabinoxylan and Mixed-Linked Beta-Glucan Isolated from Barley Show Modest Activities in Vitro. International Journal of Molecular Sciences. 2011; 12(1):570-587. https://doi.org/10.3390/ijms12010570
Chicago/Turabian StyleSamuelsen, Anne Berit, Anne Rieder, Stine Grimmer, Terje E. Michaelsen, and Svein H. Knutsen. 2011. "Immunomodulatory Activity of Dietary Fiber: Arabinoxylan and Mixed-Linked Beta-Glucan Isolated from Barley Show Modest Activities in Vitro" International Journal of Molecular Sciences 12, no. 1: 570-587. https://doi.org/10.3390/ijms12010570




