Green Chemistry in Protected Horticulture: The Use of Peroxyacetic Acid as a Sustainable Strategy
Abstract
:1. Sustainable Protected Horticulture and Its Challenges
2. Principles of Green Chemistry
- To prevent the generation of waste; prevention is better than treating waste after its formation.
- To design synthesis methods to generate new products from start to finish.
- To design chemical syntheses that are less toxic to human health and the environment.
- To (re)design chemical products to preserve their efficacy but reduce toxicity.
- To use safe solvents, separating agents and catalysts and not using these when possible.
- To recognise the energy requirements for their environmental and economical impacts. The synthesis methods should take place at room temperature and pressure.
- To use renewable materials.
- To avoid chemical derivates. To use safe solvents and reaction conditions.
- To use catalytic reagents (as selective as possible) in greater proportions than the stoichiometric reagents.
- To design biodegradable products.
- To analyse the chemical processes in real time to avoid contamination.
- To minimise the risk of accident spills or other releases of chemicals.
3. Strategies of Green Chemistry in Protected Sustainable Horticulture: The Use of Peroxyacetic Mixtures
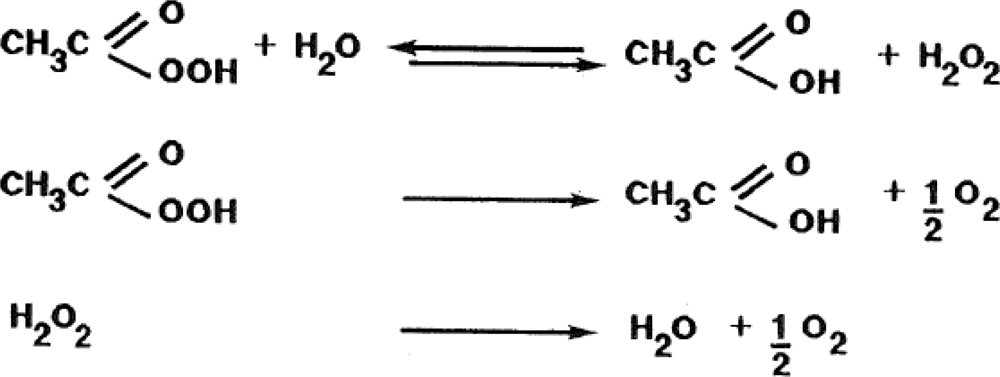
3.1. Use of Peroxyacetic Acid Mixtures for the Maintenance of Irrigation Facilities: Clogging, Environmental and Biosafety Control
3.1.1. Effects on Clogging
3.1.2. Effects on Environmental
3.1.3. Effects on Biosafety
3.2. Strategies for the Use of Peroxyacetic Acid Mixtures to Increase the Radical Oxygenation of Vegetables Growing in Soilless Cultures
3.3. Strategies for Using Peroxyacetic Acid Mixtures to Increase the Postharvest Life of Vegetables
3.4. Other Uses of Peroxyacetic Mixtures in the Food Industry
4. Conclusions
Acknowledgments
References
- Armenta, S; Garrigues, S; de la Guardia, M. Green analytical chemistry. Trends Anal. Chem 2008, 27, 497–511. [Google Scholar]
- García-Serna, J; Pérez-Barrigón, L; Cocero, MJ. New trends for design towards sustainability in chemical engineering: Green engineering. Chem. Eng. J 2007, 133, 7–30. [Google Scholar]
- Warriner, K; Huber, A; Namvar, A; Fan, W; Dunfield, K. Recent advances in the microbial safety of fresh fruits and vegetables. Adv Food Nutr Res 2009, 57, 155–208. [Google Scholar]
- Vicente, AR; Manganaris, GA; Sozzi, GO; Crisosto, CH. Chapter 5. Nutritional Quality of Fruits and Vegetables. In Postharvest Handling A Systems Approach, 2nd ed; Elsevier Inc: New York, NY, USA, 2009; pp. 57–106. [Google Scholar]
- Kirchoff, MN. Promoting sustainability through green chemistry. Resour. Conserv. Recycl 2005, 44, 237–243. [Google Scholar]
- Manley, JB; Anastas, P; Cue, BW, Jr. Frontiers in Green Chemistry: Meeting the grand challenges for sustainability in R&D and manufacturing. J. Clean. Product 2008, 16, 743–750. [Google Scholar]
- Mestres, R. Green Chemistry: Views and Strategies. Environ. Sci. Pollut. Res 2005, 12, 128–132. [Google Scholar]
- Poliakoff, M; Fitzpatrick, JM; Farren, TR; Anastas, P. Green chemistry: Science and politics of change. Science 2002, 297, 807–808. [Google Scholar]
- Merck. Safety data sheet. Perchloric acid. Ficha de datos de seguridad. Ácido Perclórico. Available at: http://es.vwr.com/es_ES/content/servicios/consejos_practicos/ac_perclorico_merck.pdf (accessed on 24 January 2010).
- Panreac. Peracetic acid solution 15% w/w RE. Technical Bulletin. Ácido Peracético solución 15% p/p RE. Boletín Técnico. Available at: http://www.panreac.com/new/esp/fds/ESP/X173495.htm (accessed on 18 January 2010).
- Álvaro, JE; Moreno, S; Dianez, F; Santos, M; Carrasco, G; Urrestarazu, M. Effects of peracetic acid disinfectant on the postharvest of some fresh vegetables. J. Food Eng 2009, 95, 11–15. [Google Scholar]
- Dell'Erba, A; Falsanisi, D; Liberti, L; Notarnicola, M; Santoro, D. Disinfection by-products formation during wastewater disinfection with peracetic acid. Desalination 2007, 215, 177–186. [Google Scholar]
- Kornegay, BH; Andrews, JF. Kinetics of fixed film biological reactor. J. Wat. Pollut. Control. Fed 1968, 40, 460–468. [Google Scholar]
- Bungay, HR; Whalen, WJ; Sanders, WM. Microprobe technique for determining diffusivities and respiration rates in microbial slime systems. Biotechnol. Bioeng 1969, 11, 765–772. [Google Scholar]
- Urrestarazu, M; Salas, MC; Mazuela, PC; Morales, E. Bioseguridad a través del agua de riego en la horticultura protegida. Vida Rural 2006, 239, 56–58. [Google Scholar]
- Urrestarazu, M; Mazuela, PC. Effect of slow-release oxygen supply by fertigation on horticultural crops under soilless culture. Sci. Horticulturae 2005, 106, 484–490. [Google Scholar]
- Urrestarazu, M; Mazuela, P; Ventura, F; Guillén, C. Beneficios de la aplicación de oxígeno en cultivos sin suelo. Agrícola Vergel: Fruticultura, horticultura, floricultura. Vida Rural 2006, 292, 195–200. [Google Scholar]
- Eguía, E; Trueba, A; Girón, A; Río-Calonge, B; Otero, F; Bielva, C. Optimisation of biocide dose as a function of residual biocide in a heat exchanger pilot plant effluent. Biofouling 2007, 23, 231–247. [Google Scholar]
- Verlicchi, P; Galletti, L; Masotti, L. A promising practice to reclaim treated wastewater for reuse: Chemical disinfection followed by natural systems. Desalination 2009, 247, 490–508. [Google Scholar]
- Carrasco, G; Gajardo, JM; Álvaro, JE; Urrestarazu, M. Rocket production (Eruca sativa Mill.) in floating system using peracetic acid using as oxygen source compared with substrate culture. J Plant Nutr 2010, 23. [Google Scholar]
- Vandekinderen, I; Devlieghere, F; Van Camp, J; Denon, Q; Sánchez-Alarcon, S; Ragaert, P; De Meulenaer, B. Impact of a decontamination step with peroxyacetic acid on the shelf-life, sensory quality and nutrient. Postharvest Biol. Technol 2009, 54, 141–152. [Google Scholar]
- Pedersen, L; Pedersen, PB; Nielsen, JL; Nielsen, PH. Peracetic acid degradation and effects on nitrification in recirculating aquaculture systems. Aquaculture 2009, 296, 246–254. [Google Scholar]
- Verween, A; Vincx, M; Degraer, S. Comparative toxicity of chlorine and peracetic acid in the biofouling control of Mytilopsis leucophaeata and Dreissena polymorpha embryos (Mollusca, Bivalvia). Int. Biodeterior. Biodegrad 2009, 63, 523–528. [Google Scholar]
- Bar-Yosef, B. Advances in fertigation. Adv. Agron 1999, 65, 1–77. [Google Scholar]
- Medina, JA. Riego Por Goteo; Teoría y Práctica; Ediciones Mundi-Prensa: Madrid, España, 2000. [Google Scholar]
- Urrestarazu, M. Tratado de Cultivos Sin Suelo; Grupo Mundi-Prensa: Madrid, España, 2004. [Google Scholar]
- Arvanitoyannis, IA; Varzakas, TH. Vegetable Waste Management: Treatment Methods and Potential Uses of Treated Waste; Elsevier Ltd: New York, NY, USA, 2008; Chapter 11, pp. 703–761. [Google Scholar]
- Lazarova, V; Janex, ML; Fiksdal, L; Oberg, C; Barcina, I; Pommepuy, M. Advanced wastewater disinfection technologies: Short and long term efficiency. Water Sci. Technol 1998, 38, 109–117. [Google Scholar]
- Anastas, PT; Warner, J. Green Chemistry Theory and Practice; Oxford Univ. Press: Oxford, UK, 2002. [Google Scholar]
- Nakayama, FS; Bucks, DA. Water quality in drip/trickle irrigation: A review. Irrigat. Sci 1991, 12, 187–192. [Google Scholar]
- Nakayama, FS; Bucks, DA. Emitter clogging effects on trickle irrigation uniformity. Trans. ASAE 1981, 24, 77–80. [Google Scholar]
- Segura, ML; Martín, E; Contreras, JI. Reutilización de aguas residuales urbanas para la horticultura. Horticultura 2006, 196, 16–19. [Google Scholar]
- Gobierno de España. Boletín Oficial del Estado BOE 1999; Orden 18907, Nº 23. 1999. [Google Scholar]
- Gill, DL. Pathogenic Pythium from irrigation ponds. Plant Disease Rep 1970, 54, 1077–1079. [Google Scholar]
- Shokes, FM; McCarter, SM. Occurrence, dissemination and survival of plant pathogens in surface irrigation ponds in southern Georgia. Phytopathology 1979, 69, 510–516. [Google Scholar]
- Maréchal, JP; Hellio, C. Challenges for the development of new non-toxic antifouling solutions. Int J Mol Sci 2009, 10, 4623–2637. [Google Scholar]
- Jiménez, SMC; Domínguez, FA; Silverio, CJM. Reaction kinetics of humic acid with sodium hypochlorite. Water Res 1993, 27, 815–820. [Google Scholar]
- O’Toole, GA. Biofilm formation as microbial development. Ann. Rev. Microbiol 2000, 54, 49–79. [Google Scholar]
- Sapers, GM. Washing and sanitizing raw materials for minimally processed fruits and vegetables. In Microbial Safety of Minimally Processed Foods; Novaks, JS, Sapers, GM, Juneja, VK, Eds.; CRS Press: Boca Ratón, FL, USA, 2003; pp. 221–253. [Google Scholar]
- Reckhow, DA; Singer, PC; Malcom, RL. Chlorination of humic materials: Byproduct formation and chemical interpretations. Environ Sci Technol 1990, 24, 1655–1664. [Google Scholar]
- Chu, I; Villeneuve, DC; Secours, VE; Becking, GC; Valli, VE. Toxicity of trihalomethanes: The acute and sucacute toxicity of chloroform, bromodichloromethane, chlorodibromethene and bromoform in rats. J. Environ. Sci. Health 1982, 17, 205–224. [Google Scholar]
- Carpenter, LM; Beresford, SA. Cancer mortality and type of water source: Findings from a study in the UK. Int. J. Epidemiol 1986, 15, 312–320. [Google Scholar]
- Maxwell, NI; Burmaster, DE; Ozonoff, D. Trihalometanes and maximum contaminant levels: The significance of inhalation and dermal exposures to chloroform in hosefold water. Regulat. Toxicol. Pharmacol 1991, 14, 297–312. [Google Scholar]
- Pilotto, LS. Disinfection of drinking water, disinfection by-products and cancer: What about Australia. Austr J Public Health 1995, 19, 89–93. [Google Scholar]
- Alarcón, A. Los Cultivos de Hortalizas Extra Tempranas; Dpto. Producción Agraria-ETSIA. Universidad Politécnica de Cartagena: Cartagena, Spain, 2005. [Google Scholar]
- Carrasco, G; Izquierdo, J. Manual Técnico Almaciguera Flotante para la Producción de Almácigos Hortícolas; Universidad de Talca and FAO: Talca, Chile, 2005. [Google Scholar]
- Moreno, S; Álvaro, J; Urrestarazu, M. Bioseguridad en la producción hortícola y en el consumo de alimentos. Vida Rural 2007, 259, 40–46. [Google Scholar]
- Artés, F; Gómez, P; Aguayo, E; Escalona, V; Artéz-Hernández, F. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharv. Biol. Technol 2009, 51, 287–296. [Google Scholar]
| Clogging risk | Number of bacteria mL−1 |
|---|---|
| Low | <10,000 |
| Medium | 10,000–50,000 |
| High | >50,000 |
| Clogging factors | Clogging risk | ||
|---|---|---|---|
| Low | Medium | High | |
| pH | < 7.0 | 7.0–8.0 | >8.0 |
| Dissolved solids (mg L−1) | < 50 | 50–100 | >100 |
| Manganese (mg L−1) | < 0.1 | 0.1–1.5 | >1.5 |
| Total iron (mg L−1) | < 0.2 | 0.2–2.0 | >2.0 |
| Hydrogen sulphide (mg L−1) | < 0.2 | 0.2–2.0 | >2.0 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carrasco, G.; Urrestarazu, M. Green Chemistry in Protected Horticulture: The Use of Peroxyacetic Acid as a Sustainable Strategy. Int. J. Mol. Sci. 2010, 11, 1999-2009. https://doi.org/10.3390/ijms11051999
Carrasco G, Urrestarazu M. Green Chemistry in Protected Horticulture: The Use of Peroxyacetic Acid as a Sustainable Strategy. International Journal of Molecular Sciences. 2010; 11(5):1999-2009. https://doi.org/10.3390/ijms11051999
Chicago/Turabian StyleCarrasco, Gilda, and Miguel Urrestarazu. 2010. "Green Chemistry in Protected Horticulture: The Use of Peroxyacetic Acid as a Sustainable Strategy" International Journal of Molecular Sciences 11, no. 5: 1999-2009. https://doi.org/10.3390/ijms11051999




