Neuroprotective Properties of Picroside II in a Rat Model of Focal Cerebral Ischemia
Abstract
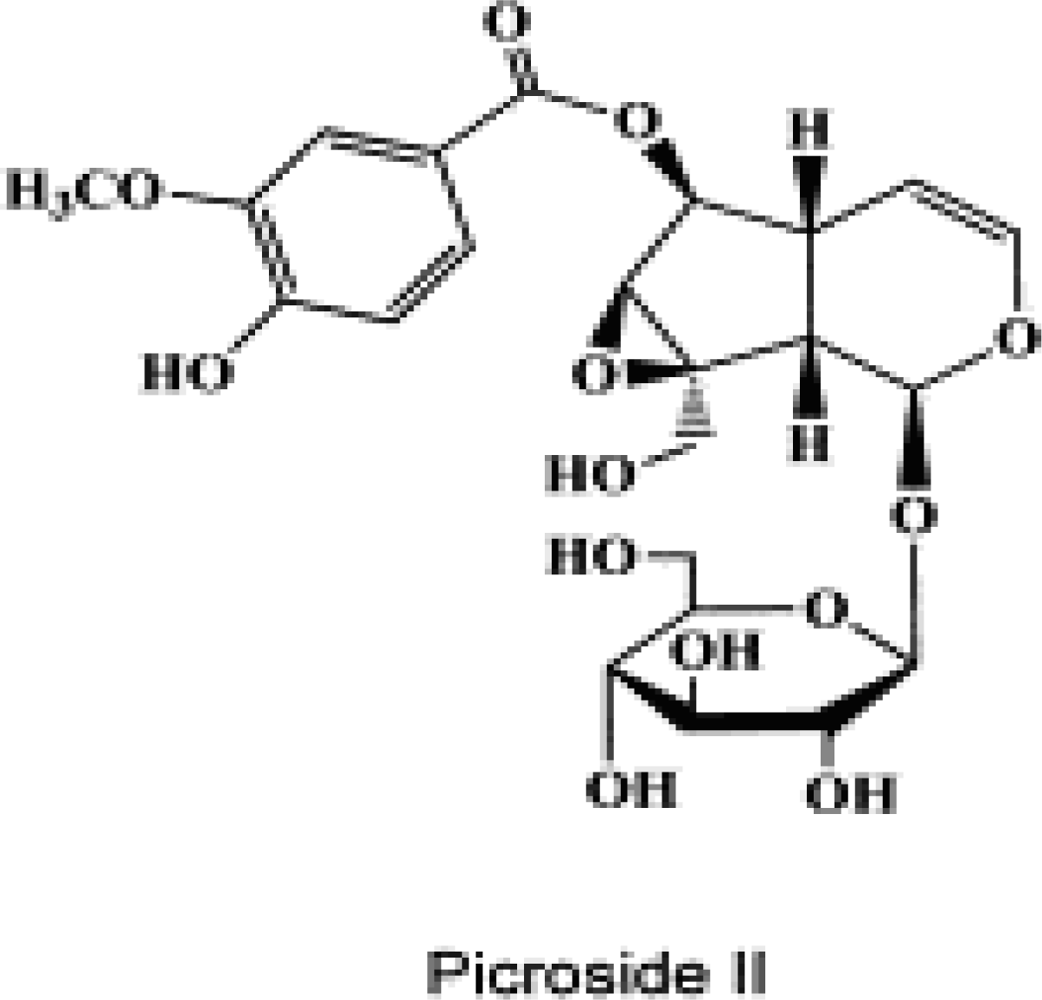
:1. Introduction
2. Results and Discussion
2.1. Neurobehavioral Deficit Score
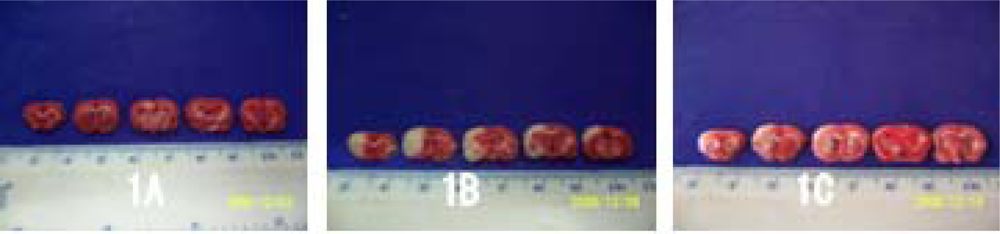
2.2. Volume of Cerebral Infarction
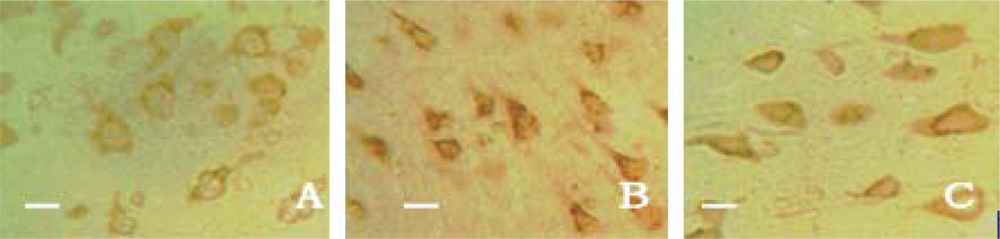
2.3. Neuronal Apoptosis
2.4. Caspase-3 Expression
2.5. PARP Expression
2.6. The Concentration of Caspase-3 and PARP in Brain Tissue
2.7. Discussion
3. Experimental Section
3.1. Animal Model of MCAO/R
3.2. Intervention Study
3.3. Neurobehavioral Dysfunction Score
3.4. TTC Staining
3.5. Neuronal Apoptosis Assay
3.6. Immunohistochemical Staining
3.7. Enzyme Linked Immunosorbent Assay (ELISA)
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Cho, BB; Toledo-Pereyra, LH. Caspase-independent programmed cell death following ischemic stroke. J. Invest. Surg 2008, 21, 141–147. [Google Scholar]
- Broughton, BR; Reutens, DC; Sobey, CG. Apoptotic mechanisms after cerebral ischemia. Stroke 2009, 40, e331–e339. [Google Scholar]
- Moroni, F. Poly (ADP-ribose) polymerase-1 (PARP-1) and postischemic brain damage. Curr. Opin. Pharmacol 2008, 8, 96–103. [Google Scholar]
- Li, JX; Li, P; Tezuka, Y; Namba, T; Kadota, S. Three phenylethanoid glycosides and an iridoid glycoside from Picrorhiza scrophulariiflora. Phytochemistry 1998, 48, 537–542. [Google Scholar]
- Wang, DQ; He, ZD; Feng, BS; Yang, CR. Chemical constituents from Picrorhiza scrophulariiflora. Acta Botanica Yunnanica 1993, 15, 83–88. [Google Scholar]
- Zou, LC; Zhu, TF; Xiang, H; Yu, L; Yan, ZH; Gan, SC; Wang, DC; Zeng, S; Deng, XM. New secoiridoid glycosides from the roots of Picrorhiza Scrophulariiflora. Molecules 2008, 13, 2049–2057. [Google Scholar]
- Li, P; Matsunaga, K; Yamakuni, T; Ohizumi, Y. Potentiation of nerve growth factor-action by picrosides I and II, natural iridoids, in PC12D cells. Eur. J. Pharmacol 2000, 406, 203–208. [Google Scholar]
- Li, T; Liu, JW; Zhang, XD; Guo, MC; Ji, G. The neuroprotective effect of picroside II from hu-huang-lian against oxidative stress. Am. J. Chin. Med 2007, 35, 681–691. [Google Scholar]
- Cao, Y; Liu, JW; Yu, YJ; Zheng, PY; Zhang, XD; Li, T; Guo, MC. Synergistic protective effect of picroside II and NGF on P12 cells against oxidative stress induced by H2O2. Pharmacol. Rep 2007, 59, 573–579. [Google Scholar]
- Smit, HF; Kroes, BH; van den Berg, AJ; van der Wal, D; van den Worm, E; Beukelman, CJ; van Dijk, H; Labadie, RP. Immunomodulatory and anti-inflammatory activity of Picrorhiza scrophulariiflora. J. Ethnopharmacol 2000, 73, 101–109. [Google Scholar]
- He, LJ; Liang, M; Hou, FF; Guo, ZJ; Xie, D; Zhang, X. Ethanol extraction of Picrorhiza scrophulariiflora prevents renal injury in experimental diabetes via anti-inflammation action. J. Endocrinol 2009, 200, 347–355. [Google Scholar]
- Gao, H; Zhou, YW. Inhibitory effect of picroside II on hepatocyte apoptosis. Acta Pharmacol Sin 2005, 26, 729–736. (in Chinese). [Google Scholar]
- Gao, H; Zhou, YW. Anti-lipid peroxidation and protection of liver mitochondria against injuries by picroside II. World J. Gastroenterol 2005, 11, 3671–3674. [Google Scholar]
- Li, P; Matsunaga, K; Yamakuni, T; Ohizumi, Y. Potentiation of nerve growth factor-action by picrosides I and II, natural iridoids, in PC12D cells. Eur. J. Pharmacol 2000, 406, 203–208. [Google Scholar]
- Li, P; Matsunaga, K; Yamakuni, T; Ohizumi, Y. Picrosides Iand II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells. Life Sci 2002, 71, 1821–1835. [Google Scholar]
- Tao, YW; Liu, JW; Wei, DZ; Su, W; Zhou, WY. Protective effect of Picroside II on the damage of PC12 cells in vitro. Chin J Clin Pharmacol Ther 2003, 8, 27–30. (in Chinese). [Google Scholar]
- Yin, JJ; Zhang, W; Du, F. Effects of extraction of Huhuanglian on apoptosis and Bcl-2 gene in penumbra area in ischemia reperfusion rats. Shandong J Tradit Chin Med 2005, 24, 364–366. (in Chinese). [Google Scholar]
- Li, Z; Xu, XY; Shen, W; Guo, YL. The interferring effects of picroside II on the expressions of NF-κB and I-κB following cerebral ischemia reperfusion injury in rats. Chin Pharmacol Bull 2010, 26, 56–59. (in Chinese). [Google Scholar]
- Yang, X; Zhang, L; Jiang, S; Gong, P; Zeng, F. Effect of dauricine on apoptosis and expression of apoptogenic protein after transient focal cerebral ischemia-reperfusion injury in rats. Chin J Chin Materia Medica 2009, 34, 78–83. (in Chinese). [Google Scholar]
- Xu, XH; Chen, Y; Zheng, XX. Effects of puerarin on neuronal apoptosis induced by cerebral ischemia in rats. Chin Pharm J 2006, 41, 1628–1631. (in Chinese). [Google Scholar]
- Li, H; Deng, CQ; Chen, BY; Chen, RF; Zhang, SP; Liang, Y. Effects of panax notoginseng saponins on expression of caspase after focal cerebral ischemia reperfusion in rats. Chin Pharmacol Bull 2006, 22, 189–193. (in Chinese).. [Google Scholar]
- Tang, YH; Li, H; Chen, BY. Effect of active fraction of buyang huanwu decoction on caspase expression in rats after focal cerebral ischemic reperfusion. Chin. J. Internet. Med 2006, 26, 533–537. [Google Scholar]
- Liu, D; Gharavi, R; Pitta, M. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromol. Med 2009, 11, 28–42. [Google Scholar]
- Chaitanya, GV; Babu, PP. Differential PARP cleavage: An indication of heterogeneous forms of cell death and involvement of multiple proteases in the infarct of focal cerebral ischemia in rat. Cell Mol. Neurobiol 2009, 29, 563–573. [Google Scholar]
- Li, Z; Xu, XY; Li, Q; Zhang, MZ; Shen, W. Protective mechanisms of picroside II on aquaporin-4 expression in a rat model of cerebral ischemia/reperfusion injury. Neural Regen Res 2010, 5, 411–417. [Google Scholar]
- Guidance Suggestion of Caring Laboratory Animals; The Ministry of Science and Technology of the People’s Republic of China: Beijing, China, 30 September 2006. (in Chinese).
- Longa, EZ; Weinstein, PR; Carlson, S; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar]
- Xiao, YJ; Jiang, ZZ; Yao, JC; Huang, X; Huang, JF; Wang, T; Zhang, LY. Investigation of picrosideII’s impacts on the P450 activities using a cockail method. Chin J Nat Med 2008, 6, 292–297. (in Chinese).. [Google Scholar]
- Bederson, JB; Pitts, LH; Tsuji, M; Nishimura, MC; Davis, RL; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar]


| Groups | n | Neurological function scores | Infarction volume |
|---|---|---|---|
| Sham-operation group | 5 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Control group | 5 | 2.16 ± 0.28Δ | 77.32 ± 3.06Δ |
| Treatment group | 5 | 1.27 ± 0.26* | 68.73 ± 3.46* |
| Groups | n | Cortex | Striatum | Hippocampus |
|---|---|---|---|---|
| Sham-operation group | 5 | 4.53 ± 1.13 | 3.79 ± 1.36 | 3.67 ± 1.15 |
| Control group | 5 | 67.62 ± 8.25Δ | 53.30 ± 6.26Δ | 41.30 ± 4.24 |
| Treatment group | 5 | 15.64 ± 4.28* | 14.12 ± 4.46* | 12.13 ± 2.46* |
| Groups | n | Cortex | Striatum | Hippocampus |
|---|---|---|---|---|
| Sham-operation group | 5 | 5.25 ± 1.70 | 4.25 ± 1.95 | 3.75 ± 1.69 |
| Control group | 5 | 33.4 ± 4.07Δ | 32.80 ± 3.77Δ | 28.00 ± 3.58 Δ |
| Treatment group | 5 | 15.82 ± 3.30* | 14.12 ± 2.83* | 13.22 ± 2.29* |
| Groups | n | Cortex | Striatum | Hippocampus |
|---|---|---|---|---|
| Sham-operation group | 5 | 4.34 ± 1.24 | 3.72 ± 1.18 | 3.28 ± 1.16 |
| Control group | 5 | 34.25 ± 4.21Δ | 31.75 ± 3.70Δ | 27.50 ± 3.29 Δ |
| Treatment group | 5 | 16.00 ± 2.16* | 15.00 ± 2.58* | 12.20 ± 1.92* |
| Sham-operation group | n | Caspase-3 | PARP |
|---|---|---|---|
| Control group | 5 | 12.35 ± 2.21 | 12.24 ± 2.23 |
| Treatment group | 5 | 54.23 ± 7.22Δ | 48.12 ± 5.17Δ |
| Sham-operation group | 5 | 30.45 ± 4.44* | 28.36 ± 3.62* |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Q.; Li, Z.; Xu, X.-y.; Guo, Y.-l.; Du, F. Neuroprotective Properties of Picroside II in a Rat Model of Focal Cerebral Ischemia. Int. J. Mol. Sci. 2010, 11, 4580-4590. https://doi.org/10.3390/ijms11114580
Li Q, Li Z, Xu X-y, Guo Y-l, Du F. Neuroprotective Properties of Picroside II in a Rat Model of Focal Cerebral Ischemia. International Journal of Molecular Sciences. 2010; 11(11):4580-4590. https://doi.org/10.3390/ijms11114580
Chicago/Turabian StyleLi, Qin, Zhen Li, Xin-ying Xu, Yun-liang Guo, and Fang Du. 2010. "Neuroprotective Properties of Picroside II in a Rat Model of Focal Cerebral Ischemia" International Journal of Molecular Sciences 11, no. 11: 4580-4590. https://doi.org/10.3390/ijms11114580




