Heats of Mixing Using an Isothermal Titration Calorimeter: Associated Thermal Effects
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Calorimetric Model
3.2. Effect due to the Difference between the Temperature of the Injected Liquid and the Temperature of the Mixture during the Mixing Process
3.3. Effect due to the Increase of Liquid Volume in the Mixing Cell
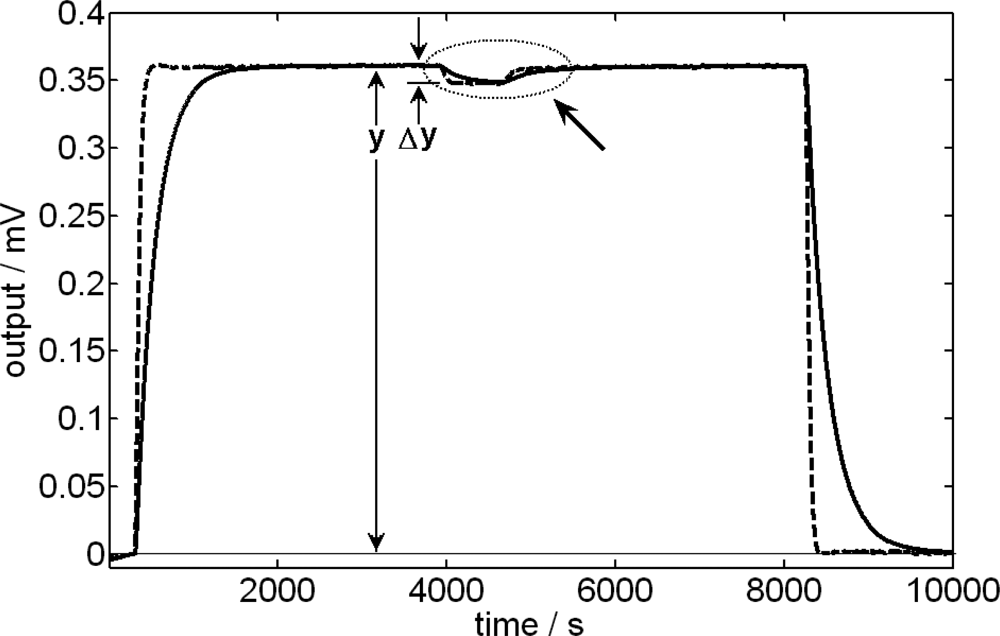
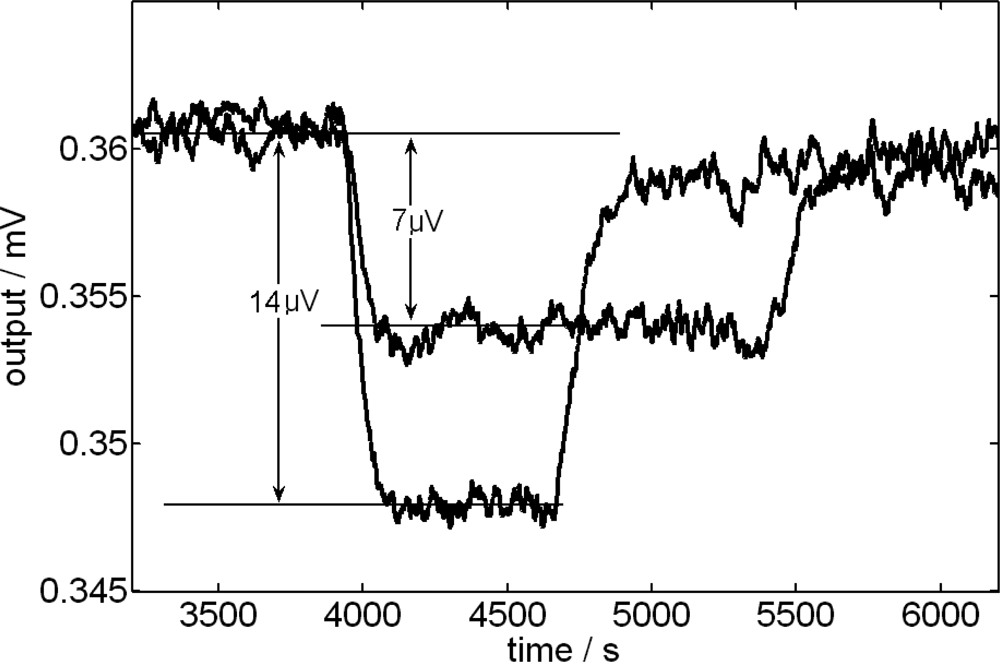
3.4. Effect due to the Stirring Velocity
4. Conclusions
References and Notes
- Harsted, BS; Thomsen, ES. Excess enthalpies from flow microcalorimetry 1. Experimental method and excess enthalpies for carbon tetrachloride + cyclohexane, + benzene, and + octamethylcyclotetrasiloxane, and of n-hexane + cyclohexane. J. Chem. Thermodyn 1974, 6, 549–555. [Google Scholar]
- Tanaka, R; D’Arcy, PJ; Benson, GC. Application of a flow microcalorimeter to determine the excess enthalpies of binary mixtures of non-electrolytes. Thermochim. Acta 1975, 11, 163–175. [Google Scholar]
- Socorro, F; Rodríguez de Rivera, M. Modellization of the mixture dissipation in a flow microcalorimeter. J. Therm. Anal. Calorim 2007, 88, 741–744. [Google Scholar]
- Socorro, F; de la Nuez, I; Rodríguez de Rivera, M. Calibration of isothermal heat conduction calorimeters: case of flow calorimeters. Measurement 2003, 33, 241–250. [Google Scholar]
- Ott, JB; Wormald, CJ. Excess enthalpy by flow calorimetry. In Solution Calorimetry; Marsh, KN, O’Hare, PAG, Eds.; Blackwell Scientific Publications: London, UK, 1994. [Google Scholar]
- Grolier, JE; Wormald, CJ; Fontaine, JC; Sosnkowska-Kehiaian, K; Kehiaian, HV. Heats of mixing and solution: Binary liquid systems of nonelectrolytes; Springer-Verlag: Berlin, Germany, 2004; Volume IX, pp. 1–564, ISBN: 978-3-540-60347-4, Introduction. [Google Scholar]
- Mathonat, C; Hynek, V; Majer, V; Grolier, J-PE. Measurements of excess enthalpies at high temperature and pressure using a new type of mixing unit. J. Solution Chem 1994, 23, 1161–1181. [Google Scholar]
- Tachoire, H; Torra, V. Thermokinetics by heat-conduction calorimetry, Thermochim. Acta 1987, 110, 171–181. [Google Scholar]
- Socorro, F; Rodríguez de Rivera, M; Dubès, JP; Tachoire, H; Torra, V. Computer-controlled experimental set-up and signal processing in calorimetric studies of liquid mixtures. Meas. Sci. Technol 1990, 1, 1285–1290. [Google Scholar]
- Grolier, J-PE; Dan, F. The use of advanced calorimetric techniques in polymer synthesis and characterization. Thermochim. Acta 2006, 450, 47–55. [Google Scholar]
- Rodríguez de Rivera, M; Socorro, F. Signal processing and uncertainty in an isothermal titration calorimeter. J. Therm. Anal. Calorim 2007, 88, 745–750. [Google Scholar]
- Sabbah, R; An, Xu-wu; Chickos, JS; Planas Leitão, ML; Roux, MV; Torres, LA. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar]
- Stokes, RH; Marsh, KN; Tomlins, RP. An isothermal displacement for endothermic enthalpies of mixing. J. Chem. Thermodyn 1969, 1, 211–221. [Google Scholar]
- Ott, JB; Stouffer, CE; Cornett, GV; Woodfield, BF; Guanquan, Ch; Christensen, JJ. Excess enthalpies for (ethanol + water) at 398.15, 423.15, 448.15, and 473.15 K and at pressures of 5 and 15 MPa. Recommendations for choosing (ethanol + water) as an HmE reference mixture. J. Chem. Thermodyn 1987, 19, 337–448. [Google Scholar]
- Rodríguez de Rivera, M; Socorro, F; Dubes, JP; Tachoire, H; Torra, V. Microcalorimetrie: Identification et déconvolution automatique à l’aide de modéles physiques. Thermochim. Acta 1989, 150, 11–25. [Google Scholar]
- Socorro, F; Rodríguez de Rivera, M; Jesús, Ch. A thermal model of a flow calorimeter. J. Therm. Anal. Calorim 2001, 64, 357–366. [Google Scholar]
- Kirchner, R; Rodríguez de Rivera, M; Seidel, JM; Torra, V. Identification of micro-scale calorimetric devices. Part VI. An approach by RC-representative model to improvements in TAM Microcalorimeters. J. Therm. Anal. Calorim 2005, 82, 179–184. [Google Scholar]
- Socorro, F; Rodríguez de Rivera, M. Modellization of the spatial localization effect of the mixture dissipation on the sensitivity in a flow microcalorimeter. J. Therm. Anal. Calorim 2006, 84, 285–289. [Google Scholar]
- Marco, F; Rodríguez de Rivera, M; Ortin, J; Serra, T; Torra, V. Signal processing in time-varying calorimeters for study of continous liquid mixtures. Thermochim. Acta 1986, 107, 149–162. [Google Scholar]
- Rodríguez de Rivera, M; Socorro, F. Baseline changes in an isothermal titration microcalorimeter. J. Therm. Anal. Calorim 2005, 80, 769–773. [Google Scholar]
- Rodríguez de Rivera, M; Socorro, F; Matos, JS. Study of the stirring effect in a TAM2277-205 isothermal titration calorimeter. J. Therm. Anal. Calorim 2008, 92, 79–82. [Google Scholar]
- Marco, F; Rodríguez de Rivera, M; Ortin, J; Serra, T; Torra, V. A frequential analysis of the numerical algorithms used for inverse filtering in calorimetry. Thermochim. Acta 1986, 102, 173–178. [Google Scholar]



© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rodríguez de Rivera, M.; Socorro, F.; Matos, J.S. Heats of Mixing Using an Isothermal Titration Calorimeter: Associated Thermal Effects. Int. J. Mol. Sci. 2009, 10, 2911-2920. https://doi.org/10.3390/ijms10072911
Rodríguez de Rivera M, Socorro F, Matos JS. Heats of Mixing Using an Isothermal Titration Calorimeter: Associated Thermal Effects. International Journal of Molecular Sciences. 2009; 10(7):2911-2920. https://doi.org/10.3390/ijms10072911
Chicago/Turabian StyleRodríguez de Rivera, Manuel, Fabiola Socorro, and José S. Matos. 2009. "Heats of Mixing Using an Isothermal Titration Calorimeter: Associated Thermal Effects" International Journal of Molecular Sciences 10, no. 7: 2911-2920. https://doi.org/10.3390/ijms10072911



