Quantum Dots — Characterization, Preparation and Usage in Biological Systems
Abstract
:1. Introduction
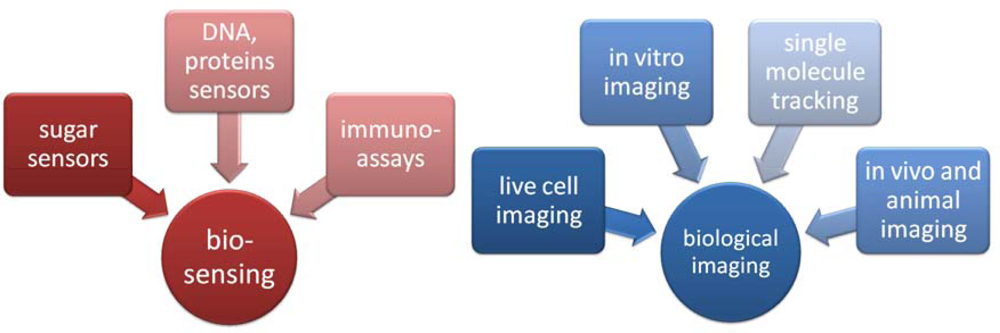
2. Quantum dots usage
2.1. The main criteria for using QDs in Medicine
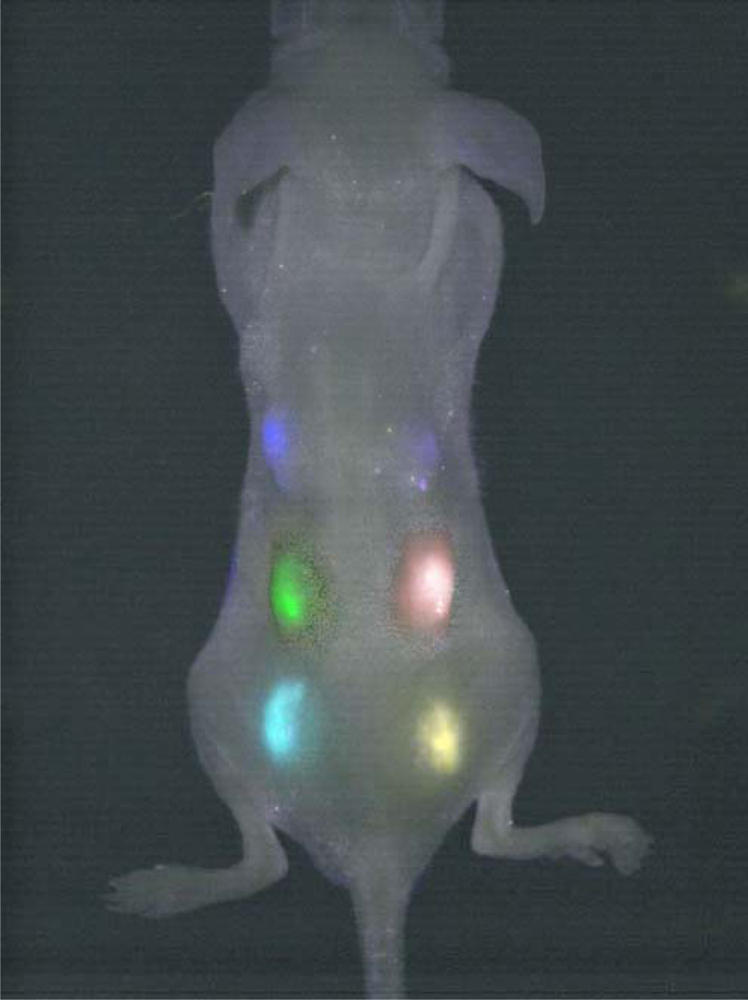
2.2. Some important applications of QDs
3. Quantum dot preparation and characterization
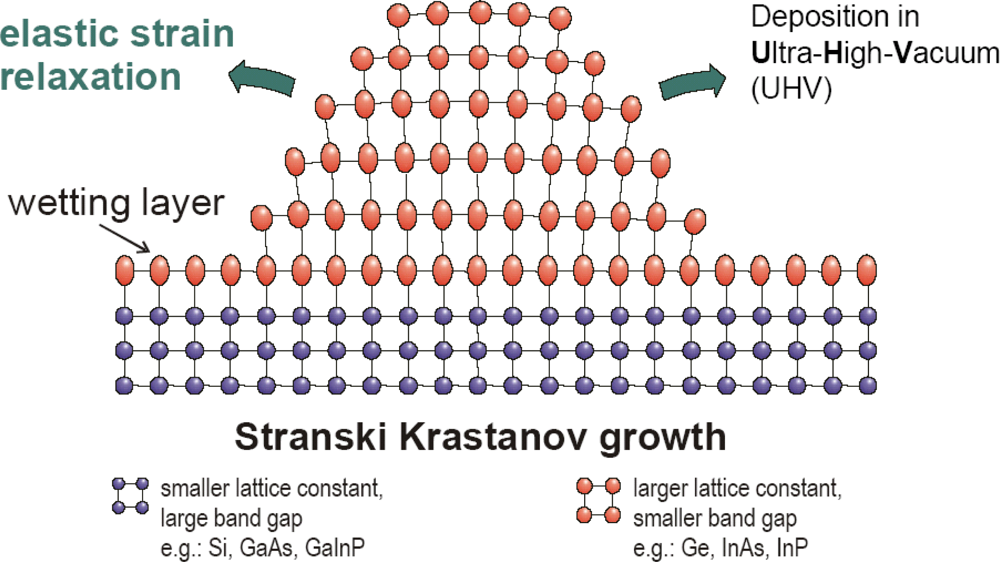
3.1. QD preparation
3.2. QD characterization
4. Conclusions
Acknowledgments
References and Notes
- Wang, W; Chen, C; Lin, KH; Fang, Y; Lieber, CM. Nanosensors. US 2007/0264623 A1, 2007.
- Kumar, CSSR. Nanomaterials for Medical Applications. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley, 2007.
- Kluson, P; Drobek, M; Bartkova, H; Budil, I. Welcome in the Nanoworld. Chem. Listy 2007, 101, 262–272. [Google Scholar]
- Ferancova, A; Labuda, J. DNA Biosensors based on nanostrucutred materials. In Nanostrucutred Materials in Electrochemistry; Eftekhari, A, Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 409–434. [Google Scholar]
- Kral, V; Sotola, J; Neuwirth, P; Kejik, Z; Zaruba, K; Martasek, P. Nanomedicine - Current status and perspectives: A big potential or just a catchword? Chem. Listy 2006, 100, 4–9. [Google Scholar]
- Ghanem, MA; Bartlett, PN; de Groot, P; Zhukov, A. A double templated electrodeposition method for the fabrication of arrays of metal nanodots. Electrochem. Commun 2004, 6, 447–453. [Google Scholar]
- Fujioka, K; Hiruoka, M; Sato, K; Manabe, N; Miyasaka, R; Hanada, S; Hoshino, A; Tilley, RD; Manome, Y; Hirakuri, K; Yamamoto, K. Luminescent passive-oxidized silicon quantum dots as biological staining labels and their cytotoxicity effects at high concentration. Nanotechnology 2008, 19, 7. [Google Scholar]
- Mićić, OI; Nozik, AJ. Colloidal quantum dots of III–V semiconductors. In Nanostructured Materials and Nanotechnology; Concise, Nalwa, HS, Eds.; Academic Press: San Diego, California, 2002; pp. 183–205. [Google Scholar]
- Matagne, P; Leburton, JP. Quantum Dots: Artificial Atoms and Molecules. In Quantum Dots and Nanowires; Nalwa, HS, Bandyopadhyay, S, Eds.; American Scientific Publishers: Stevenson Ranch, California, 2003; pp. 2–66. [Google Scholar]
- Ng, J; Missous, M. Improvements of stacked self-assembled InAs/GaAs quantum dot structures for 1.3 mu m applications. Microelectron. J 2006, 37, 1446–1450. [Google Scholar]
- Anantathanasarn, S; Notzel, R; van Veldhoven, PJ; van Otten, FWM; Barbarin, Y; Servanton, G; de Vries, T; Smalbrugge, E; Geluk, EJ; Eijkemans, TJ; Bente, E; Oei, YS; Smit, MK; Wolter, JH. Wavelength controlled InAs/InP quantum dots for telecom laser applications. Microelectron. J 2006, 37, 1461–1467. [Google Scholar]
- Gerion, D. Fluorescence imaging in biology using nanoprobes. In Nanosystem Characterization Tools in the Life Sciences, 1st; Kumar, CSSR, Ed.; Wiley-VCH: Weinheim, Germany, 2006; Volume 3, pp. 1–37. [Google Scholar]
- Walling, MA; Novak, JA; Shepard, JRE. Quantum dots for live cell and in vivo imaging. Int. J. Mol. Sci 2009, 10, 441–491. [Google Scholar]
- Byrne, SJ; Williams, Y; Davies, A; Corr, SA; Rakovich, A; Gun’ko, YK; Rakovich, YR; Donegan, JF; Volkov, Y. “Jelly dots”: Synthesis and cytotoxicity studies of CdTe quantum dot-gelatin nanocomposites. Small 2007, 3, 1152–1156. [Google Scholar]
- Qian, HF; Dong, CQ; Weng, JF; Ren, JC. Facile one-pot synthesis of luminescent, water-soluble, and biocompatible glutathione-coated CdTe nanocrystals. Small 2006, 2, 747–751. [Google Scholar]
- Iyer, G; Pinaud, F; Tsay, J; Weiss, S. Solubilization of quantum dots with a recombinant peptide from Escherichia coli. Small 2007, 3, 793–798. [Google Scholar]
- Chang, E; Thekkek, N; Yu, WW; Colvin, VL; Drezek, R. Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small 2006, 2, 1412–1417. [Google Scholar]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect 2006, 114, 165–172. [Google Scholar]
- Choi, AO; Brown, SE; Szyf, M; Maysinger, D. Quantum dot-induced epigenetic and genotoxic changes in human breast cancer cells. J. Mol. Med 2008, 86, 291–302. [Google Scholar]
- Gao, XH; Dave, SR. Quantum dots for cancer molecular imaging. In Bio-Applications of Nanoparticles; Springer-Verlag: Berlin: Berlin, 2007; Volume 620, pp. 57–73. [Google Scholar]
- Gao, XH; Yang, LL; Petros, JA; Marshal, FF; Simons, JW; Nie, SM. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol 2005, 16, 63–72. [Google Scholar]
- Li, HC; Zhou, QF; Liu, W; Yan, B; Zhao, Y; Jiang, GB. Progress in the toxicological researches for quantum dots. Sci. China Ser. B 2008, 51, 393–400. [Google Scholar]
- Geys, J; Nemmar, A; Verbeken, E; Smolders, E; Ratoi, M; Hoylaerts, MF; Nemery, B; Hoet, PHM. Acute toxicity and prothrombotic effects of quantum dots: Impact of surface charge. Environ. Health Perspect 2008, 116, 1607–1613. [Google Scholar]
- Yong, KT. Mn-doped near-infrared quantum dots as multimodal targeted probes for pancreatic cancer imaging. Nanotechnology 2009, 20, 10. [Google Scholar]
- Chen, HY; Wang, YQ; Xu, J; Ji, JZ; Zhang, J; Hu, YZ; Gu, YQ. Non-invasive near infrared fluorescence imaging of CdHgTe quantum dots in mouse model. J. Fluoresc 2008, 18, 801–811. [Google Scholar]
- Depalo, N; Mallardi, A; Comparelli, R; Striccoli, M; Agostiano, A; Curri, ML. Luminescent nanocrystals in phospholipid micelles for bioconjugation: An optical and structural investigation. J. Colloid Interface Sci 2008, 325, 558–566. [Google Scholar]
- Emerich, DF; Thanos, CG. Multifunctional peptide-based nanosystems for improving delivery and molecular imaging. Curr. Opin. Mol. Ther 2008, 10, 132–139. [Google Scholar]
- De La Fuente, JM; Berry, CC; Riehle, M; Cronin, L; Curtis, ASG. Quantum dots and their uses. US 2007/0249064 A1, 2007.
- Lin, CAJ; Liedl, T; Sperling, RA; Fernandez-Arguelles, MT; Costa-Fernandez, JM; Pereiro, R; Sanz-Medel, A; Chang, WH; Parak, WJ. Bioanalytics and biolabeling with semiconductor nanoparticles (quantum dots). J. Mater. Chem 2007, 17, 1343–1346. [Google Scholar]
- Liu, TC; Zhang, HL; Wang, JH; Wang, HQ; Zhang, ZH; Hua, XF; Cao, YC; Luo, QM; Zhao, YD. Study on molecular interactions between proteins on live cell membranes using quantum dot-based fluorescence resonance energy transfer. Anal. Bioanal. Chem 2008, 391, 2819–2824. [Google Scholar]
- Tan, WH; Wang, KM; He, XX; Zhao, XJ; Drake, T; Wang, L; Bagwe, RP. Bionanotechnology based on silica nanoparticles. Med. Res. Rev 2004, 24, 621–638. [Google Scholar]
- Hu, JT; Li, LS; Yang, WD; Manna, L; Wang, LW; Alivisatos, AP. Linearly polarized emission from colloidal semiconductor quantum rods. Science 2001, 292, 2060–2063. [Google Scholar]
- Dembski, S; Graf, C; Kruger, T; Gbureck, U; Ewald, A; Bock, A; Ruhl, E. Photoactivation of CdSe/ZnS quantum dots embedded in silica colloids. Small 2008, 4, 1516–1526. [Google Scholar]
- Zhang, Y; Mi, L; Wang, PN; Lu, SJ; Chen, JY; Guo, J; Yang, WL; Wang, CC. Photoluminescence decay dynamics of thiol-capped CdTe quantum dots in living cells under microexcitation. Small 2008, 4, 777–780. [Google Scholar]
- Zheng, JJ; Zheng, ZH; Gong, WW; Hu, XB; Gao, W. Abnormal temperature behavior of photoluminescence in CdSe/ZnSe self-assembled quantum dots. Solid State Commun 2008, 147, 429–432. [Google Scholar]
- Sapsford, KE; Pons, T; Medintz, IL; Mattoussi, H. Biosensing with luminescent semiconductor quantum dots. Sensors 2006, 6, 925–953. [Google Scholar]
- Huang, FH; Chen, GN. Preparation and application of L-cysteine-modified CdSe/CdS core/shell nanocrystals as a novel fluorescence probe for detection of nucleic acid. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr 2008, 70, 318–323. [Google Scholar]
- Tansil, NC; Gao, ZQ. Nanoparticles in biomolecular detection. Nano Today 2006, 1, 28–37. [Google Scholar]
- Chen, Z; Li, G; Zhang, L; Jiang, J; Li, Z; Peng, Z; Deng, L. A new method for the detection of ATP using a quantum-dot-tagged aptamer. Anal. Bioanal. Chem 2008, 392, 1185–1188. [Google Scholar]
- Chen, WB; Wang, X; Tu, XJ; Pei, DJ; Zhao, Y; Guo, XQ. Water-soluble off-on spin-labeled quantum-dots conjugate. Small 2008, 4, 759–764. [Google Scholar]
- Chen, QF; Yang, DZ; Xu, SK. Conjugations between cysteamine-stabilized CdTe quantum dots and single stranded DNA. Anal. Lett 2008, 41, 1964–1974. [Google Scholar]
- Shingyoji, M; Gerion, D; Pinkel, D; Gray, JW; Chen, FQ. Quantum dots-based reverse phase protein microarray. Talanta 2005, 67, 472–478. [Google Scholar]
- Liu, TC; Wang, JH; Wang, HQ; Zhang, HL; Zhang, ZH; Hua, XF; Cao, YC; Zhao, YD; Luo, QM. Bioconjugate recognition molecules to quantum dots as tumor probes. J. Biomed. Mater. Res. Part A 2007, 83A, 1209–1216. [Google Scholar]
- Shan, YM; Wang, LP; Shi, YH; Zhang, H; Li, HM; Liu, HZ; Yang, B; Li, TY; Fang, XX; Li, W. NHS-mediated QDs-peptide/protein conjugation and its application for cell labeling. Talanta 2008, 75, 1008–1014. [Google Scholar]
- Susumu, K; Uyeda, HT; Medintz, IL; Mattoussi, H. Design of biotin-functionalized luminescent quantum dots. J Biomed Biotechnol 2007, 7. [Google Scholar]
- Liedl, T; Dietz, H; Yurke, B; Simmel, F. Controlled trapping and release of quantum dots in a DNA-Switchable hydrogel. Small 2007, 3, 1688–1693. [Google Scholar]
- Goldman, ER; Medintz, IL; Mattoussi, H. Luminescent quantum dots in immunoassays. Anal. Bioanal. Chem 2006, 384, 560–563. [Google Scholar]
- Murcia, MJ; Naumann, CA. Biofunctionalization of fluorescent nanoparticles. In Biofunctionalization of Nanomaterials, 1st Ed; Kumar, CSSR, Ed.; Wiley-VCH: Weinheim, Germany, 2005; Volume 1, pp. 1–40. [Google Scholar]
- Protiere, M; Reiss, P. Highly luminescent Cd1-xZnxSe/ZnS core shell nanocrystals emitting in the blue-green spectral range. Small 2007, 3, 399–403. [Google Scholar]
- Bera, D; Qian, L; Holloway, PH. Time-evolution of photoluminescence properties of ZnO/MgO core/shell quantum dots. J. Phys. D-Appl. Phys 2008, 41, 4. [Google Scholar]
- Chen, Y; Munechika, K; Plante, IJL; Munro, AM; Skrabalak, SE; Xia, Y; Ginger, DS. Excitation enhancement of CdSe quantum dots by single metal nanoparticles. Appl. Phys. Lett 2008, 93, 3. [Google Scholar]
- Issac, A; Jin, SY; Lian, TQ. Intermittent electron transfer activity from single CdSe/ZnS quantum dots. J. Am. Chem. Soc 2008, 130, 11280–11281. [Google Scholar]
- Al-Jamal, WT; Al-Jamal, KT; Bomans, PH; Frederik, PM; Kostarelos, K. Functionalized-quantum-dot-liposome hybrids as multimodal nanoparticles for cancer. Small 2008, 4, 1406–1415. [Google Scholar]
- Pan, J; Wang, Y; Feng, SS. Formulation, characterization, and in vitro evaluation of quantum dots loaded in poly(lactide)-vitamin E TPGS nanoparticles for cellular and molecular imaging. Biotechnol. Bioeng 2008, 101, 622–633. [Google Scholar]
- Kerman, K; Endo, T; Tsukamoto, M; Chikae, M; Takamura, Y; Tamiya, E. Quantum dot-based immunosensor for the detection of prostate-specific antigen using fluorescence microscopy. Talanta 2007, 71, 1494–1499. [Google Scholar]
- Wang, J; Liu, GD; Wu, H; Lin, YH. Quantum-dot-based electrochemical immunoassay for high-throughput screening of the prostate-specific antigen. Small 2008, 4, 82–86. [Google Scholar]
- Dahan, M. From analog to digital: Exploring cell dynamics with single quantum dots. Histochem.Cell Biol 2006, 125, 451–456. [Google Scholar]
- Deerinck, TJ. The Application of fluorescent quantum dots to confocal, multiphoton, and electron microscopic imaging. Toxicol. Pathol 2008, 36, 112–116. [Google Scholar]
- Kim, K; Kim, D; Cho, EJ; Huh, YM. A quantitative analysis of the intracellular transport of quantum dot-peptide in live cells using total internal reflection and confocal microscopy. Prog. Biomed.Opt. Imag 2007, 8, 64490K.1–64490K.7. [Google Scholar]
- Michalet, X; Pinaud, FF; Bentolila, LA; Tsay, JM; Doose, S; Li, JJ; Sundaresan, G; Wu, AM; Gambhir, SS; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar]
- Jin, T; Fujii, F; Komai, Y; Seki, J; Seiyama, A; Yoshioka, Y. Preparation and characterization of highly fluorescent, glutathione-coated near infrared quantum dots for in vivo fluorescence imaging. Int. J. Mol. Sci 2008, 9, 2044–2061. [Google Scholar]
- Lin, S; Xie, XY; Patel, MR; Yang, YH; Li, ZJ; Cao, F; Gheysens, O; Zhang, Y; Gambhir, SS; Rao, JH; Wu, JC. Quantum dot imaging for embryonic stem cells. BMC Biotechnol 2007, 7, 10. [Google Scholar]
- Lin, SY; Chen, NT; Sum, SP; Lo, LW; Yang, CS. Ligand exchanged photoluminescent gold quantum dots functionalized with leading peptides for nuclear targeting and intracellular imaging. Chem Commun 2008, 4762–4764. [Google Scholar]
- Chen, FQ; Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett 2004, 4, 1827–1832. [Google Scholar]
- Lieleg, O; Lopez-Garcia, M; Semmrich, C; Auernheimer, J; Kessler, H; Bausch, AR. Specific integrin Labeling in living Celts using functionalized nanocrystals. Small 2007, 3, 1560–1565. [Google Scholar]
- Medintz, IL; Mattoussi, H; Clapp, AR. Potential clinical applications of quantum dots. Int. J. Nanomed 2008, 3, 151–167. [Google Scholar]
- Mahler, B; Spinicelli, P; Buil, S; Quelin, X; Hermier, JP; Dubertret, B. Towards non-blinking colloidal quantum dots. Nat. Mater 2008, 7, 659–664. [Google Scholar]
- Shi, YF; He, P; Zhu, XY. Photoluminescence-enhanced biocompatible quantum dots by phospholipid functionalization. Mater. Res. Bull 2008, 43, 2626–2635. [Google Scholar]
- Bodas, D; Khan-Malek, C. Direct patterning of quantum dots on structured PDMS surface. Sens. Actuator B-Chem 2007, 128, 168–172. [Google Scholar]
- Yokota, H; Tsunashima, K; Iizuka, K; Okamoto, H. Direct electron beam patterning and molecular beam epitaxy growth of InAs: Site definition of quantum dots. J. Vac. Sci. Technol. B 2008, 26, 1097–1099. [Google Scholar]
- Zhu, CQ; Wang, P; Wang, X; Li, Y. Facile phosphine-free synthesis of CdSe/ZnS core/shell nanocrystals without precursor injection. Nanoscale Res. Lett 2008, 3, 213–220. [Google Scholar]
- Oluwafemi, SO; Revaprasadu, N; Ramirez, AJ. A novel one-pot route for the synthesis of water-soluble cadmium selenide nanoparticles. J. Cryst. Growth 2008, 310, 3230–3234. [Google Scholar]
- Gu, ZY; Zou, L; Fang, Z; Zhu, WH; Zhong, XH. One-pot synthesis of highly luminescent CdTe/CdS core/shell nanocrystals in aqueous phase. Nanotechnology 2008, 19, 7. [Google Scholar]
- Ma, Q; Song, TY; Yuan, P; Wang, C; Su, XG. QDs-labeled microspheres for the adsorption of rabbit immunoglobulin G and fluoroimmunoassay. Colloid Surf. B-Biointerfaces 2008, 64, 248–254. [Google Scholar]
- Wang, XY; Ma, Q; Li, B; Li, YB; Su, XG. The preparation of CdTe nanoparticles and CdTe nanoparticle-label led microspheres for biological applications. Luminescence 2007, 22, 1–8. [Google Scholar]
- Murcia, MJ; Shaw, DL; Long, EC; Naumann, CA. Fluorescence correlation spectroscopy of CdSe/ZnS quantum dot optical bioimaging probes with ultra-thin biocompatible coatings. Opt. Commun 2008, 281, 1771–1780. [Google Scholar]
- Jorge, P; Martins, MA; Trindade, T; Santos, JL; Farahi, F. Optical fiber sensing using quantum dots. Sensors 2007, 7, 3489–3534. [Google Scholar]
- Wang, J; Xu, J; Goodman, MD; Chen, Y; Cai, M; Shinar, J; Lin, ZQ. A simple biphasic route to water soluble dithiocarbamate functionalized quantum dots. J. Mater. Chem 2008, 18, 3270–3274. [Google Scholar]
- Wang, HQ; Zhang, HL; Li, XQ; Wang, JH; Huang, ZL; Zhao, YD. Solubilization and bioconjugation of QDs and their application in cell imaging. J. Biomed. Mater. Res. Part A 2008, 86A, 833–841. [Google Scholar]
- Koole, R; van Schooneveld, MM; Hilhorst, J; Donega, CD; t Hart, DC; van Blaaderen, A; Vanmaekelbergh, D; Meijerink, A. On the incorporation mechanism of hydrophobic quantum dots in silica spheres by a reverse microemulsion method. Chem. Mat 2008, 20, 2503–2512. [Google Scholar]
- Liu, W; He, ZK; Liang, JG; Zhu, YL; Xu, HB; Yang, XL. Preparation and characterization of novel fluorescent nanocomposite particles: CdSe/ZnS core-shell quantum dots loaded solid lipid nanoparticles. J. Biomed. Mater. Res. 2008, 84A, 1018–1025. [Google Scholar]
- Zhang, BB; Cheng, J; Li, DN; Liu, XH; Ma, GP; Chang, J. A novel method to make hydrophilic quantum dots and its application on biodetection. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater 2008, 149, 87–92. [Google Scholar]
- Djenizian, T; Balaur, E; Schmuki, P. Direct immobilization of DNA on diamond-like carbon nanodots. Nanotechnology 2006, 17, 2004–2007. [Google Scholar]
- Henini, M. Properties and applications of quantum dot heterostructures grown by molecular beam epitaxy. Nanoscale Res. Lett 2006, 1, 32–45. [Google Scholar]
- Dai, CA; Wu, YL; Lee, YH; Chang, CJ; Su, WF. Fabrication of 2D ordered structure of self-assembled block copolymers containing gold nanoparticles. J. Cryst. Growth 2006, 288, 128–136. [Google Scholar]
- Eberl, K; Lipinski, MO; Manz, YM; Winter, W; Jin-Phillipp, NY; Schmidt, OG. Self-Assembling Quantum Dots for Optoelectronic Devices on Si and GaAs. 11th International Winterschool on New Developments in Solid State Physics, Mauterndorf, Austria, 21–25, February, 2000; Elsevier Science: Bv: Mauterndorf, Austria, 2000; pp. 164–174. [Google Scholar]
- Wang, ZM; Holmes, K; Mazur, YI; Ramsey, KA; Salamo, GJ. Self-organization of quantum-dot pairs by high-temperature droplet epitaxy. Nanoscale Res. Lett 2006, 1, 57–61. [Google Scholar]
- Chu, SZ; Inoue, S; Wada, K; Hishita, S; Kurashima, K. Self-organized nanoporous anodic titania films and ordered titania nanodots/nanorods on glass. Adv. Func. Mat 2005, 15, 1343–1349. [Google Scholar]
- Xiao, P; Garcia, BB; Guo, Q; Liu, DW; Cao, GZ. TiO2 nanotube arrays fabricated by anodization in different electrolytes for biosensing. Electrochem. Commun 2007, 9, 2441–2447. [Google Scholar]
- Chen, PL; Kuo, CT; Pan, FM; Tsai, TG. Preparation and phase transformation of highly ordered TiO2 nanodot arrays on sapphire substrates. Appl. Phys. Lett 2004, 84, 3888–3890. [Google Scholar]
- Naicker, PK; Cummings, PT; Zhang, HZ; Banfield, JF. Characterization of titanium dioxide nanoparticles using molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 15243–15249. [Google Scholar]
- Peng, HW; Li, JB; Li, SS; Xia, JB. First-principles study on rutile TiO2 quantum dots. J. Phys. Chem. C 2008, 112, 13964–13969. [Google Scholar]
- Luo, M; Cheng, K; Weng, WJ; Song, CL; Du, P; Shen, G; Xu, G; Han, GR. Preparation of high-density TiO2 nanodots on Si substrate by a novel method. Mater. Lett 2008, 62, 1965–1968. [Google Scholar]
- Bao, SJ; Li, CM; Zang, JF; Cui, XQ; Qiao, Y; Guo, J. New nanostructured TiO2 for direct electrochemistry and glucose sensor applications. Adv. Func. Mater 2008, 18, 591–599. [Google Scholar]
- Hazdra, P; Voves, J; Oswald, J; Kuldova, K; Hospodkova, A; Hulicius, E; Pangrac, J. Optical Characterisation of MOVPE Grown Vertically Correlated InAs/GaAs Quantum Dots. Conference on European Nano Systems (ENS 2006), Paris, FRANCE, 14–15 December 2006; Elsevier Sci. Ltd.: Paris, France, 2006; pp. 1070–1074. [Google Scholar]
- Gu, Y; Kuskovsky, IL; Fung, J; Robinson, R; Herman, IP; Neumark, GF; Zhou, X; Guo, SP; Tamargo, MC. Determination of size and composition of optically active CdZnSe/ZnBeSe quantum dots. Appl. Phys. Lett 2003, 83, 3779–3781. [Google Scholar]
- Rameshwar, T; Samal, S; Lee, S; Kim, S; Cho, J; Kim, IS. Determination of the size of water-soluble nanoparticles and quantum dots by field-flow fractionation. J. Nanosci. Nanotechnol 2006, 6, 2461–2467. [Google Scholar]
- Hapke-Wurst, I; Zeitler, U; Schumacher, HW; Haug, RJ; Pierz, K; Ahlers, FJ. Size determination of InAs quantum dots using magneto-tunnelling experiments. Semicond. Sci. Technol 1999, 14, L41–L43. [Google Scholar]
- Yamauchi, T; Matsuba, Y; Ohyama, Y; Tabuchi, M; Nakamura, A. Quantum Size Effects of InAs- and InGaAs-Quantum Dots Studied by Scanning Tunneling Microscopy/Spectroscopy. In Inst Pure Applied Physics, International Symposium on Formation, Physics and Device Application of Quantum Dot Structures (QDS 2000), Sapporo, Japan, 10–14 September 2000; Sapporo, Japan; pp. 2069–2072.
- Lees, EE; Gunzburg, MJ; Nguyen, TL; Howlett, GJ; Rothacker, J; Nice, EC; Clayton, AHA; Mulvaney, P. Experimental determination of quantum dot size distributions, ligand packing densities, and bioconjugation using analytical ultracentrifugation. Nano Lett 2008, 8, 2883–2890. [Google Scholar]


© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum Dots — Characterization, Preparation and Usage in Biological Systems. Int. J. Mol. Sci. 2009, 10, 656-673. https://doi.org/10.3390/ijms10020656
Drbohlavova J, Adam V, Kizek R, Hubalek J. Quantum Dots — Characterization, Preparation and Usage in Biological Systems. International Journal of Molecular Sciences. 2009; 10(2):656-673. https://doi.org/10.3390/ijms10020656
Chicago/Turabian StyleDrbohlavova, Jana, Vojtech Adam, Rene Kizek, and Jaromir Hubalek. 2009. "Quantum Dots — Characterization, Preparation and Usage in Biological Systems" International Journal of Molecular Sciences 10, no. 2: 656-673. https://doi.org/10.3390/ijms10020656
APA StyleDrbohlavova, J., Adam, V., Kizek, R., & Hubalek, J. (2009). Quantum Dots — Characterization, Preparation and Usage in Biological Systems. International Journal of Molecular Sciences, 10(2), 656-673. https://doi.org/10.3390/ijms10020656







