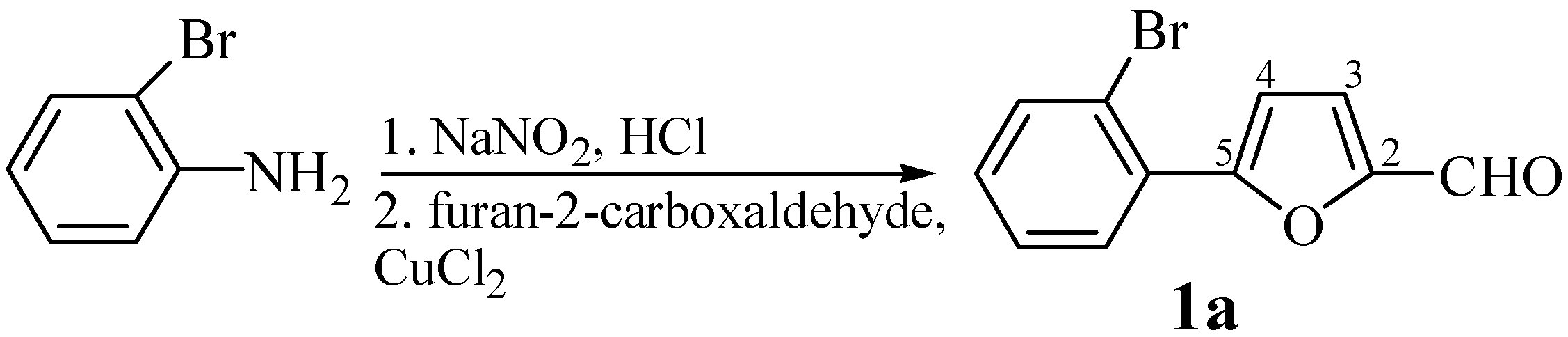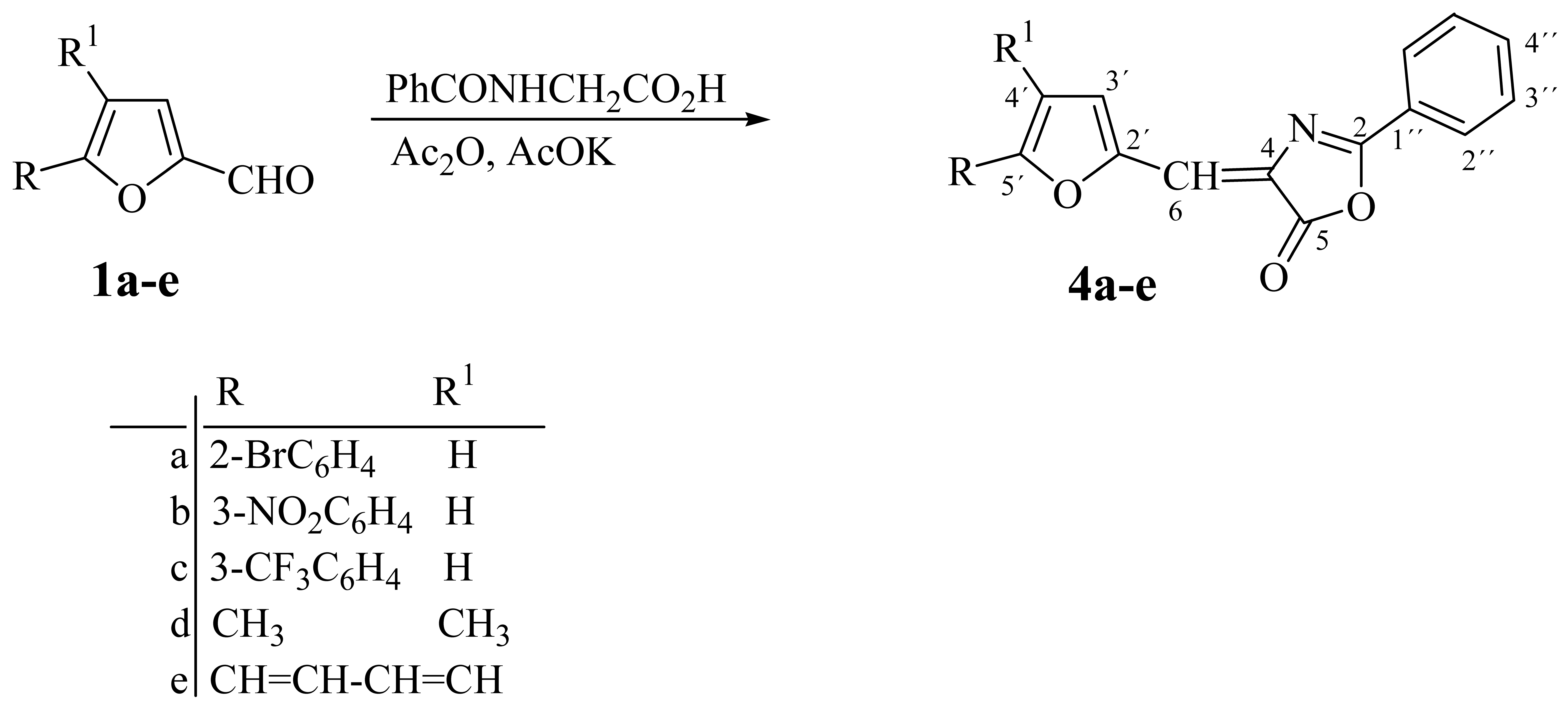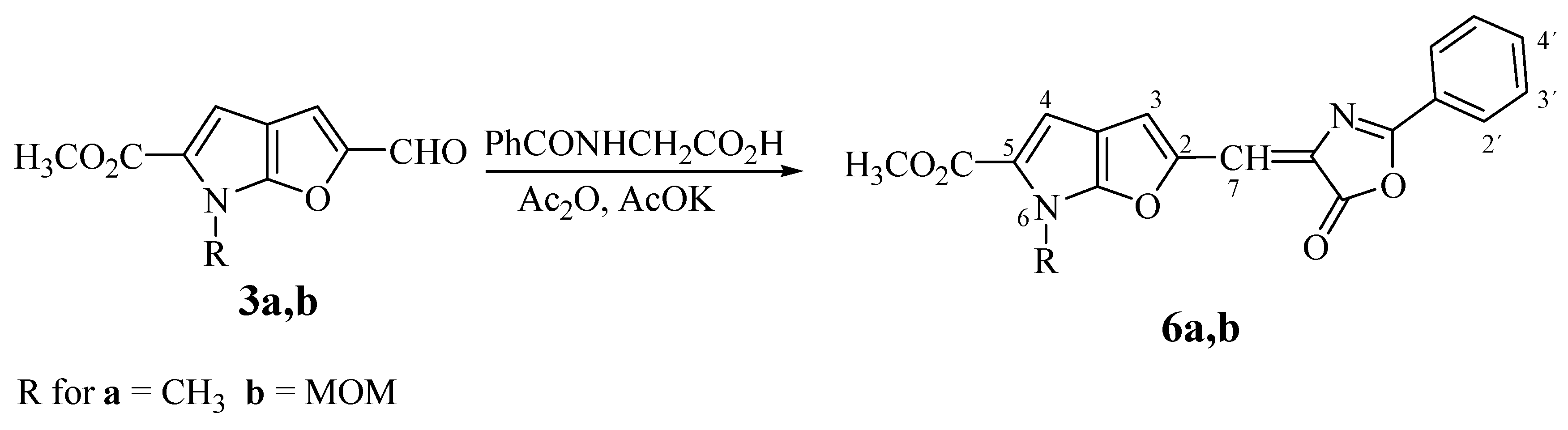(4E)-2-Phenyl-4-{[5-(2-bromophenyl)-2-furyl]methylene}-1,3-oxazol-5(4H)-one (4a)
A mixture of 5-(2-bromophenyl)furan-2-carboxaldehyde (1a, 0.4 g, 2 mmol), hippuric acid (0.4 g, 2.4 mmol) and potassium acetate (0.3 g, 3 mmol) in acetic anhydride (10 mL) was refluxed with stirring for 15 min. (reaction progress was monitored by TLC using 3:1 isohexane-ethyl acetate as eluent). The mixture was then cooled down and neutralised by addition of solid potassium carbonate. The solid product was separated by filtration, dried and purified by crystallisation. Yield: 83%; m.p. 168-171°C (ethanol); For C20H12BrNO3 (394.2) calculated: 60.93% C, 3.07% H, 20.27% Br, 3.55% N; found: 60.79% C, 3.12% H, 20.16% Br, 3.60% N; IR νmax/cm-1 1791 (C=O), 1650 (C=C), 1327 (C-O furan), 1245 (C-O lactone), 1024 (C-H furan); UV: 419, (3.70); 1H-NMR δH: 8.19-7.70 (m, 5H, H-2´´, H-3´´, H-4´´, H-5´´, H-6´´), 8.05-7.68 (m, 4H, ArH), 7.76 (d, 1H, J 3´,4´ 3.7 Hz, H-4´), 7.55 (d, 1H, J 3´,4´ 3.7 Hz, H-3´), 7.31 (s, 1H, H-6); 13C-NMR δC: 166.68 (C-5), 162.54 (C-2), 154.67 (C-5´), 149.82 (C-2´), 133.64 (C-4´´), 130.21 (C-4), 129.4 (C-3´´), 128.6 (C-4´), 127.98 (C-2´´), 125.22 (C-1´´), 122.2 (C-3´), 116.88 (C-6), 134.86, 130.68, 129.88, 119.48, 114.61.
According to this same procedure the following compounds were prepared:
(4E)-4-{[5-(3-Nitrophenyl)-2-furyl]methylene}-2-phenyl-1,3-oxazol-5(4H)-one (4b). Reaction time: 30 min. Yield: 70%; m.p. 223-227°C (ethanol); For C20H12N2O5 (360.3) calculated: 66.67% C, 3.36% H, 7.77% N; found: 66,64% C, 3.29% H, 7,72% N; IR νmax/cm-1 1783 (C=O), 1654 (C=C), 1347 (C-O furan), 1243 (C-O lactone), 1028 (C-H furan); UV: 444, (3.77); 1H-NMR δH: 8.32-7.82 (m, 4H, ArH), 8.25-7.70 (m, 5H, H-2´´, H-3´´, H-4´´, H-5´´, H-6´´), 7.69 (d, 1H, J 3´,4´ 3.7 Hz, H-4´), 7.60 (d, 1H, J 3´,4´ 3.7 Hz, H-3´), 7.34 (s, 1H, H-6); 13C-NMR δC: 166.34 (C-5), 162.43 (C-2), 154.62 (C-5´), 150.45 (C-2´), 133.53 (C-4´´), 130.42 (C-4), 129.25 (C-3´´), 127.89 (C-2´´), 125.14 (C-1´´), 123.12 (C-4´), 116.51 (C-6), 149.59, 130.82, 130.42, 125.12, 118.57.
(4E)-2-Phenyl-4-({5-[3-(trifluromethyl)phenyl]-2-furyl}methylene)-1,3-oxazol-5(4H)-one (4c). The reaction time: 15 min. Yield: 80%; m.p. 172-174°C (ethanol); For C21H12F3NO3 (383.3) calculated: 65.80% C, 3.16% H, 3.65% N; found: 65.69% C, 3.22% H, 3.72% N; IR νmax/cm-1 1785 (C=O), 1651 (C=C), 1331 (C-O furan), 1242 (C-O lactone), 1030 (C-H furan); UV: 419, (3.70). 1H-NMR δH: 8.32-8.19 (m, 4H, ArH), 7.82-7.66 (m, 5H, H-2´´, H-3´´, H-4´´, H-5´´, H-6´´), 7.71(d, 1H, J 3´,4´ 3.6 Hz, H-4´), 7.58 (d, 1H, J 3´,4´ 3.6 Hz, H-3´), 7.34 (s, 1H, H-6); 13C-NMR δC: 166.47 (C-5), 162.34 (C-2), 155.31 (C-5´), 150.32 (C-2´), 133.57 (C-4), 130.5 (C-4´´), 129.96 (C-3´´), 129.29 (C-2´´), 125.19 (C-1´´), 128.18 (C-4´), 123.01 (C-3´), 120.7 (q, CF3), 116.62 (C-6), 130.41, 130.26, 129.83, 125.81, 112.02, 111.69.
(4E)-4-[4,5-Dimethyl-2-furyl)methylene]-2-phenyl-1,3-oxazol-5(4H)-one (4d). The reaction time: 60 min. Yield: 62%; m.p. 149-151°C (ethanol); For C16H13NO3 (267.3) calculated: 71.90% C, 4.90% H, 5.24% N; found: 74.60% C, 3.88% H, 4.74% N; IR νmax/cm-1: 1789 (C=O (E)), 1767 (C=O (Z)), 1641 (C=C), 1329 (C-O furan), 1254 (C-O lactone), 1020 (C-H furan); UV 336, (3.32); 1H-NMR δH: 8.07 (d, 2H, J2´´,3´´ 7.35 Hz, H-2´´, H-6´´), 7.74 (t, 1H, J3´´,4´´ 7.29 Hz, H-4´´), 7.60 (t, 2H, H-3´´, H-5´´), 7.50 (s, 1H, H-3´), 7.07 (s, 1H, H-6), 2.3 (s, 3H, CH3), 2.02 (s, 3H, CH3); 13C-NMR δC: 167 (C-5), 161.4 (C-2), 155 (C-5´), 147.8 (C-2´), 133.4 (C-4´´), 129.5 (C-3´´, C-5´´), 128.2 (C-4), 127.9 (C-2´´, C-6´´), 125.5 (C-1´´), 124.8 (C-3´), 120.2 (C-4´), 117.8 (C-6), 12.3 (CH3), 9.7 (CH3).
(4E)-4-(1-Benzofuran-2-ylmethylene)-2-phenyl-1,3-oxazol-5(4H)-one (4e). The reaction time: 20 min. Yield: 67%; m.p. 183-186°C (ethanol); For C18H11NO3 (289.3) calculated: 74.73% C, 3.83% H, 4.84% N; found: 74.60% C, 3.88% H, 4.72% N; IR νmax/cm-1: 1793 (C=O), 1644 (C=C), 1329 (C-O furan), 1255 (C-O lactone), 1000 (C-H furan); UV: 331, (3.76); 1H-NMR δH: 8.17 (d, 2H, J2´´,3´´ 7.78 Hz, H-2´, H-6´´), 8.03 (s, 1H, H-3´), 7.74 (t, 1H, J3´´,4´´ 7.4 Hz, H-4´´), 7.65 (t, 2H, H-3´´, H-5´´), 7.32 (s, 1H, H-6), 7.81-7.34 (m, 4H, ArH); 13C-NMR δC: 166.5 (C-5), 163.8 (C-2), 155.8 (C-5´), 151.7 (C-2´), 134.3 (C-4´´), 133.7 (C-4), 129.7 (C-3´´, C-5´´), 128.7 (C-4´), 128.5 (C-2´´, C-6´´), 125.3 (C-1´´), 117.5 (C-6), 116 (C-3´); 127.8, 124.3, 123.0, 111.9.
Methyl 2-[(E)-(5-oxo-2-phenyl-1,3-oxazol-5(4H)-ylidene)methyl]-4H-furo[3,2-b]pyrrole-5-carboxylate (5a). The reaction time: 60 min. Yield: 53%; m.p. 305-311°C (ethanol); For C18H12N2O5 (336.3) calculated: 64.29% C, 3.60% H, 8.33% N; found: 64.39% C, 3.52% H, 8.22% N; UV: 463, (3.41); 1H-NMR. δH: 12.1 (br s, 1H, NH), 8.11 (d, 2H, J2´,3´ 8.0 Hz, H-2´, H-6´), 7.71 (t, 1H, J3´,4´ 8.0 Hz, J4´,5´ 8.0 Hz, H-4´), 7.64 (d, 2H, J2´,3´ 8.0 Hz, J5´,6´ 8.0 Hz, H-3´, H-5´), 7.59 (s, 1H, H-7), 7.23 (s, 1H, H-3), 6.87 (s, 1H, H-6), 3.84 (s, 3H, CO2CH3).
Ethyl 2-[(E)-(5-oxo-2-phenyl-1,3-oxazol-5(4H)-ylidene)methyl]-4H-furo[3,2-b]pyrrole-5-carboxylate (5b). The reaction time: 60 min. Yield: 74%; m.p. 295-300°C (ethanol); For C19H14N2O5 (350.3) calculated: 65.14% C, 4.03% H, 8.00% N; found: 64.94% C, 4.13% H, 7.95% N; UV: 463, (3.44); 1H-NMR δH: 12.05 (br s, 1H, NH), 8.11 (d, 2H, J2´,3´ 8.0 Hz, H-2´, H-6´), 7.72 (t, 1H, J3´,4´ 8.0 Hz, J4´,5´8.0 Hz, H-4´), 7.64 (t, 2H, J2´,3´ 8.0 Hz, J5´,6´ 8.0 Hz, H-3´, H-5´), 7.60 (s, 1H, H-7), 7.23 (s, 1H, H-3), 6.84 (s, 1H, H-6), 4.32 (q, 2H, CO2CH2CH3 ), 1.32 (t, 3H, CO2CH2CH3 ).
Ethyl 4-Methyl-2-[(E)-(5-oxo-2-phenyl-1,3-oxazol-5(4H)-ylidene)methyl]furo[3,2-b]pyrrole-5-carboxy-late (5c). The reaction time: 30 min. Yield: 90%; m.p. 174-177°C (ethanol); For C20H16N2O5 (364.4) calculated: 63.93% C, 4.43% H, 7.69% N; found: 63.88% C, 4.52% H, 7.84% N; UV: 463, (3.38); 1H-NMR δH: 8.17 (d, 2H, J2´,3´ 8.0 Hz, H-2´, H-6´), 7.89 (s, 1H, H-7), 7.70 (t, 1H, J3´,4´ 8.0 Hz, J4´,5´ 8.0 Hz, H-4´), 7.63 (t, 2H, J2´,3´ 8.0 Hz, J5´,6´ 8.0 Hz, H-3´, H-5´), 7.22 (s, 1H, H-3), 6.87 (s, 1H, H-6), 4.28 (q, 2H, CO2CH2CH3 ), 4.00 (s, 3H, NCH3), 1,32 (t, 3H, CO2CH2CH3 ).
Ethyl 4-Benzyl-2-[(E)-(5-oxo-2-phenyl-1,3-oxazol-5(4H)-ylidene)methyl]furo[3,2-b]pyrrole-5-carboxylate (5d). The reaction time: 30 min. Yield: 81%; m.p. 175-179°C (ethanol); For C26H20N2O5 (440.4) calculated: 70.90% C, 4.58% H, 76.36% N; found: 71.03% C, 4.52% H, 76.28% N; UV: 468, (3.89); 1H-NMR δH: 8.12 (d, 2H, J2´,3´ 8.0 Hz, H-2´, H-6´), 7.72 (t, 1H, J3´,4´ 8.0 Hz, J4´,5´ 8.0 Hz, H-4´), 7.65 (t, 2H, J2´,3´ 8.0 Hz, J5´,6´ 8.0 Hz, H-3´, H-5´), 7.58 (s, 1H, H-7), 7,21-7,39 (m, 5H, ArH), 7.19 (s, 1H, H-3), 6.97 (s, 1H, H-6), 5.75 (s, 2H, NCH2), 4.24 (q, 2H, CO2CH2CH3 ), 1.26 (t, 3H, CO2CH2CH3)
Methyl 6-Methyl-2-[(E)-(5-oxo-2-phenyl-1,3-oxazol-5(4H)-ylidene)methyl]furo[2,3-b]pyrrole-5-car-boxylate (6a). The reaction time: 120 min. Yield: 66%; m.p. 255-260°C (ethanol); For C19H14N2O5 (350.3) calculated: 65.14% C, 4.03% H, 8.00% N; found: 65.04% C, 4.26% H, 8.05% N; UV: 453, (3.78); 1H-NMR δH: 8.13 (d, 2H, J2´,3´ 8.0 Hz, H-2´, H-6´), 7.80 (s, 1H, H-7), 7.70 (t, 1H, J3´,4´ 8.0 Hz, J4´,5´ 8.0 Hz, H-4´), 7.63 (t, 2H, , J2´,3´ 8.0 Hz, J5´,6´ 8.0 Hz, H-3´, H-5´), 7.27 (s, 1H, H-3), 7.01 (s, 1H, H-4), 3.97 (s, 3H, NCH3), 3.81 (s, 3H, CO2CH3).
Methyl 6-Methoxymethyl-2-[(E)-(5-oxo-2-phenyl-1,3-oxazol-5(4H)-ylidene)methyl]furo[2,3-b]-pyrrole-5-carboxylate (6b). The reaction time: 60 min. Yield: 70%; m.p. 165-168°C (ethanol); For C20H16N2O6 (380.3) calculated: 63.16% C, 4.24% H, 7.37% N; found: 63.01% C, 4.17% H, 7.28% N; UV: 444 (3.50); 1H-NMR δH: 8.12 (d, 2H, J2´,3´ 8,0 Hz, H-2´, H-6´), 7.80 (s, 1H, H-7), 7.71 (t, 1H, J3´,4´ 8.0 Hz, J4´,5´ 8.0 Hz, H-4´), 7.63 (t, 2H, J2´,3´ 8.0 Hz, J5´,6´ 8.0 Hz, H-3´, H-5´), 7.26 (s, 1H, H-3), 7.11 (s, 1H, H-4), 5.79 (s, 2H, NCH2), 3.81 (s, 3H, CO2CH3 ), 3.27 (s, 3H, OCH3).
Compounds 4a-e were also prepared using microwave irradiation according to the following procedure:
A mixture of substituted furan-2-carboxaldehyde
1a-e (1 mmol), hippuric acid (1.2 mmol) and potassium acetate (a catalytic amount) in acetic anhydride (0.03 mmol, 3mL) was irradiated in a microwave tube for 1-2 min. at 350W (reaction progress monitored by TLC with 3:1 isohexane-ethyl acetate as eluent). After cooling down, the solid product was separated by filtration, dried and purified by crystallisation. The results of “classical” and microwave conditions are shown in
Table 1.