Addition of Organochromium Reagents to Heteroaryl Aldehydes. Synthesis of Heteroaryl Substituted bis-Allyl Ethers and Homoallyl Ethers
Abstract
:Introduction
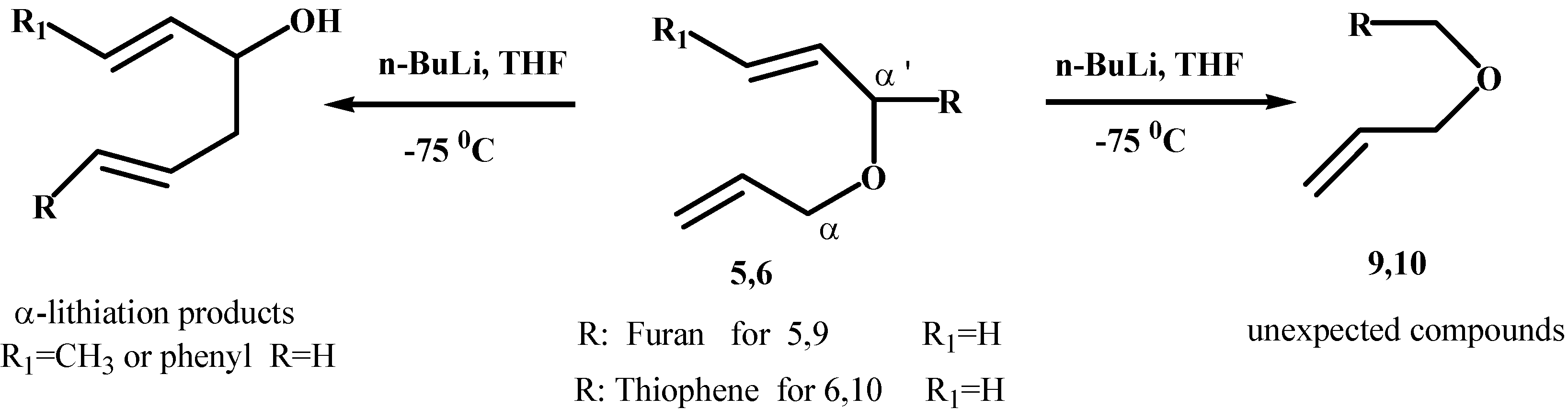
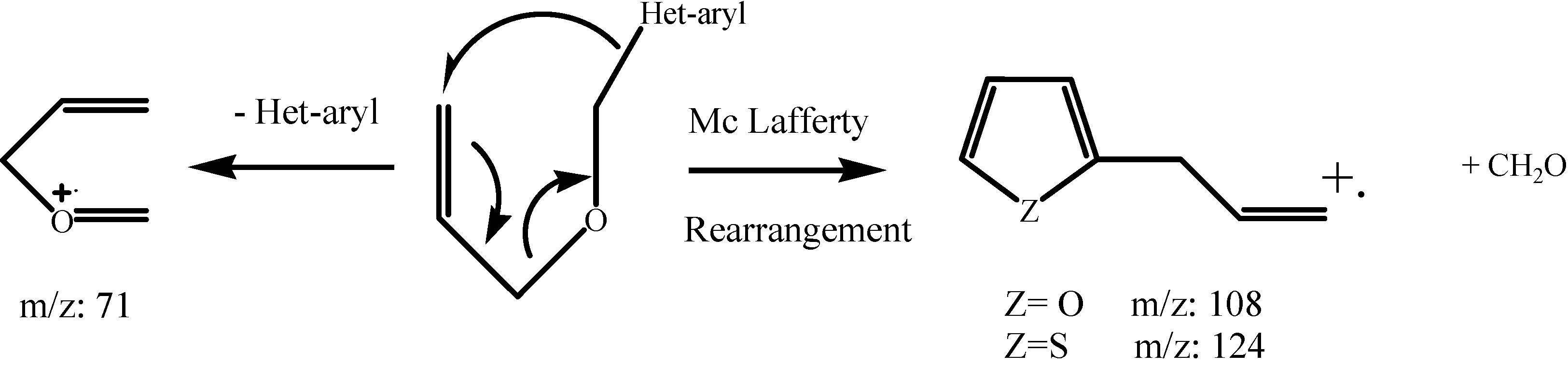
Results and Discussion

Experimental
General

Addition of Organochromium Reagents to Heteroaromatic Aldehydes: Synthesis of Hetero-aromatic Substituted Allyl Alcohols (1, 2) and Homoallyl Alcohols (3, 4)
Method A:
Method B:
General Procedure for the Synthesis of bis-Allyl Ethers and Homoallyl Ethers (5-8):
Acknowledgments
References
- Okude, Y.; Hirano, S.; Hiyama, T.; Nozaki, H. Grignard-type carbonyl addition of allyl halides by means of chromous salt chemospecific synthesis of homoallyl alcohols. J. Am. Chem. Soc. 1977, 99, 3179–3181. [Google Scholar]
- Takai, K.; Kimura, K.; Kuroda, T.; Hiyama, T.; Nozaki, H. Selective Grignard type carbonyl addition of alkenyl halides mediated by chromium (II) chloride. Tetrahedron Lett. 1983, 24, 5281–5284. [Google Scholar]
- Cintos, P. Addition of organochromium compounds to aldehyde: Nozaki-Hiyama reaction. Synthesis 1992, 248–257. [Google Scholar]
- Furstner, A. Carbon-carbon bond formations involving organochromium (III) reagents. Chem. Rev. 1999, 99, 991–1045. [Google Scholar]
- Wuts, P.G.M.; Callen, G.R. An improved procedure for the Cr (II) mediated homoallylic alcohol synthesis. Synth. Commun. 1986, 16, 1833–1837. [Google Scholar]
- Servi, S.; Acar, A. An investigation of the reactions of substituted homoallylic alcohols with various oxidation reagents. Molecules 2002, 7, 104–111. [Google Scholar]
- Nakai, T.; Mikami, K. [2,3]-Wittig sigmatropic rearrangements in organic synthesis. Chem. Rev. 1986, 86, 885–902. [Google Scholar]
- Mikami, K.; Nakai, T. Applications of the tandem [2,3]-Wittig oxy-Cope rearrangement to synthesis of exo-brevicomin and oxocrinol. The scope and limitation of the sigmatropic sequences as a synthetic method for delta, epsilon-unsaturated ketones. Chem. Lett. 1982, 9, 1349–1352. [Google Scholar]
- Mikami, K.; Kishi, N.; Nakai, T. New sigmatropic sequences based on the [2,3]-Wittig rearrangement of the bis-allylic ether system. Tetrahedron 1986, 42, 2911–2918. [Google Scholar]
- Nicholas, G.; Wai-Man, L. Stereoselective [2,3]-Wittig and tandem [2,3]-Wittig anionic oxy-cope rearrangement of bis allylic ethers: effect of substituents. Tetrahedron Lett. 1997, 38, 6445–6448. [Google Scholar]
- Zair, T.; Santelli, C.; Santelli, R.M. Palladium mediated cyclization of 1,5-hexadien-3-ols to 1-methyl-1,3-cyclopentadienes. Tetrahedron 1993, 49, 3313–3324. [Google Scholar]
- Servi, S.; Ahmetzade, M.; Coskun, M.; Cansiz, A. Use of 2-alkenyl-3-(chloromethyl) oxiranes in the synthesis of 1, 5-dien-3-ols. S. Afr. J. Chem. 2000, 53, 73–76. [Google Scholar]
- Servi, S.; Digrak, M.; Cansiz, A.; Ahmetzade, M. Synthesis of allyl-cyclopropyl alcohols and allyl-1,5-hexadien-3-ols and investigation of their antibacterial and antifungal activities. Ind. J. Chem. 2000, 39, 629–633. [Google Scholar]
- Budzikiewicz, H.; Djerassi, C.; Williams, H.D. Ethers, acetals, ketals, and orthoesters. In Mass Spectrometry of Organic Compounds; Holden-Day Inc.: San Francisco, California, USA, 1967; pp. 232–233. [Google Scholar]
- Sample Availability: Available from the authors.
© 2004 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Servi, S.; Topaloglu, C. Addition of Organochromium Reagents to Heteroaryl Aldehydes. Synthesis of Heteroaryl Substituted bis-Allyl Ethers and Homoallyl Ethers. Molecules 2004, 9, 22-28. https://doi.org/10.3390/90100022
Servi S, Topaloglu C. Addition of Organochromium Reagents to Heteroaryl Aldehydes. Synthesis of Heteroaryl Substituted bis-Allyl Ethers and Homoallyl Ethers. Molecules. 2004; 9(1):22-28. https://doi.org/10.3390/90100022
Chicago/Turabian StyleServi, S., and C. Topaloglu. 2004. "Addition of Organochromium Reagents to Heteroaryl Aldehydes. Synthesis of Heteroaryl Substituted bis-Allyl Ethers and Homoallyl Ethers" Molecules 9, no. 1: 22-28. https://doi.org/10.3390/90100022



