Introduction
One of the domains of free radical chemistry is industrial polymerization of alkenes. At present, emulsion polymerization has become increasingly important because of replacement of organic solu- tions by more environment-friendly aqueous dispersions [
1]. On the other hand, however, despite synthetic chemists’ skepticism, it was also proved that the radical reaction can lead to carbon-carbon bond formation in chemoselective, regioselective, and even stereoselective ways [
2]. For said radical reactions to take place, it is necessary to synthesize water soluble initiators. The aim of this work is to prepare water soluble azo initiators from 2,2’-azodiisobutyronitrile (AIBN), the initiators having sur- face activity necessary for stabilization of the latex particles [
1].
Chemical modifications of AIBN must be based on reactions that can occur below 30°C to prevent decomposition of the starting AIBN. The most appropriate reaction seems to be the Pinner synthesis [
3,
4] consisting in reaction of the starting nitrile with excess anhydrous alcohol and hydrogen chloride to give the respective iminoester hydrochloride. The latter can be subsequently hydrolyzed to ester. The ester, which contains a benzene ring, can then be sulfonated in an anhydrous medium (
Scheme 1). Another possible modification of the nitrile group consists in its transformation into an amide group
via a Ritter reaction [
5].
Results and Discussion
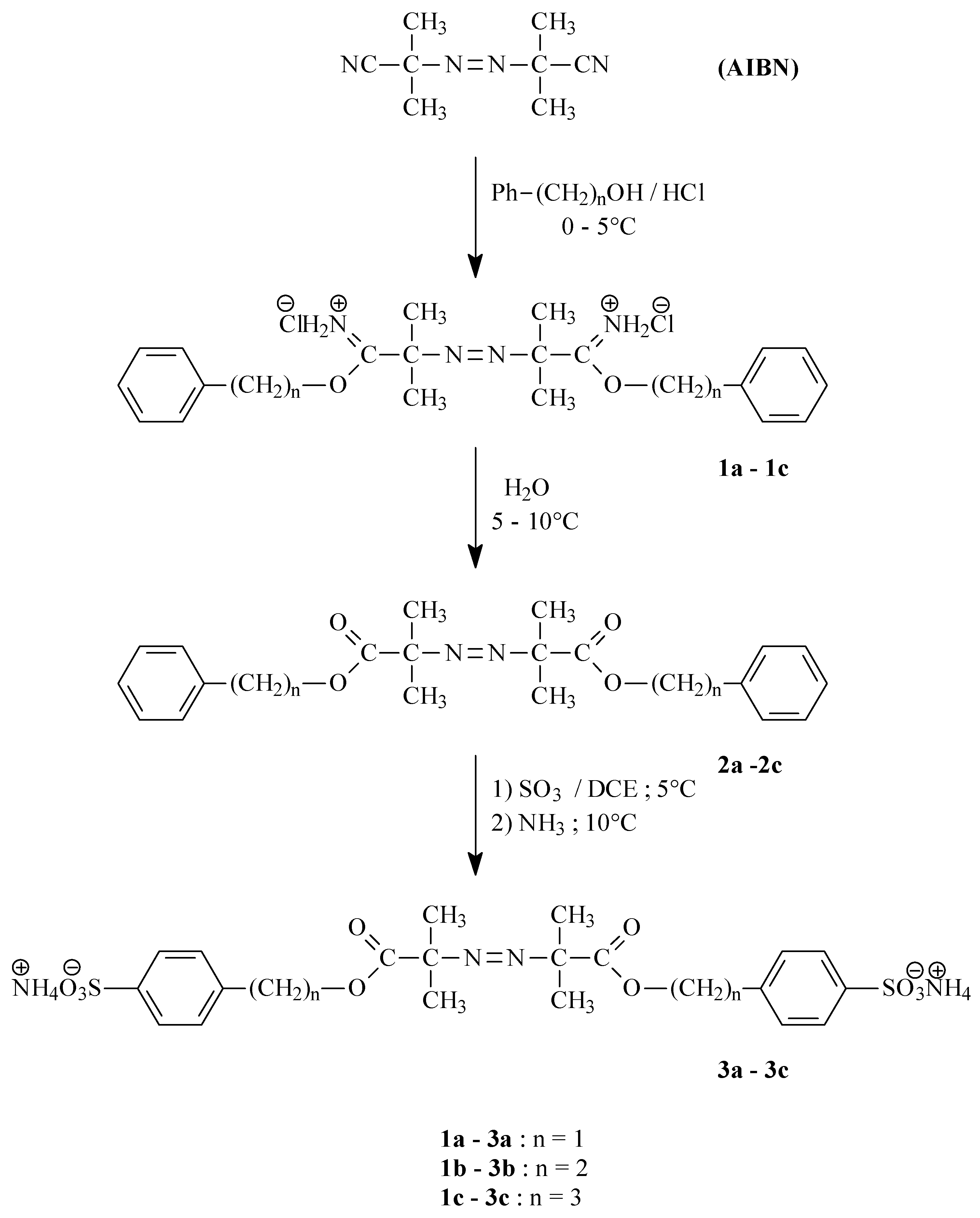
Bisiminoester hydrochlorides
1a-
1c were prepared by reaction of 1 mol AIBN with 15 mol of the corresponding alcohol (benzyl alcohol, 2-phenyl-1-ethyl alcohol, 3-phenyl-1-propyl alcohol) saturated with gaseous hydrogen chloride at 0-5°C. The separated crystalline hydrochlorides
1a-
1c were col- lected by filtration and immediately hydrolyzed to give the corresponding esters
2a-
2c. The yield of 2,2’-azodiisobutyrate
2a was only 15%, 2,2’-azodiisobutyramide and benzyl chloride being isolated as the side products. Our finding corresponds with the earlier described easy nucleophilic substitution of stabilized carbocations with chloride ion, where the Pinner reaction of imidates from branched alco- hols gives the respective amides and chloroderivatives [
6]. The yields of bis(2-phenylethyl) 2,2’- azodiisobutyrate (
2b) and bis(3-phenylpropyl) 2,2’-azodiisobutyrate were 77 and 75%, respectively.
The esters thus prepared (
2a-
2c) were subsequently sulfonated in a chemo- and regioselective way,
viz. at the 4-position of the benzene ring, by treatment with sulfur trioxide in 1,2-dichlorethane at 5°C. After removing excess sulfur trioxide, the resulting sulfonic acids (
3a-
3c) were transformed to their ammonium salts: diammonium 2,2’-azobis[p-(2-methylpropanoyloxymethyl)benzene-sulfonate] (
3a; yield 67%), diammoniun 2,2’-azobis[p-(β-(2-methylpropanoyloxy)ethyl)benzene-sulfonate] (
3b; yield 87%) and diammonium 2,2’-azobis[p-(χ-(2-methylpropanoyloxy)propyl)-benzenesulfonate](
3c; yield 52%). The other AIBN modification chosen made use of the Ritter reaction, consisting in the transfor- mation of a suitable olefin or tertiary alcohol by treatment with sulfuric acid into the respective carbo- cation, which is subsequently attacked by the free electron pair at the nitrogen atom of a nitrile group and finally producesa an
N-alkyl amide [
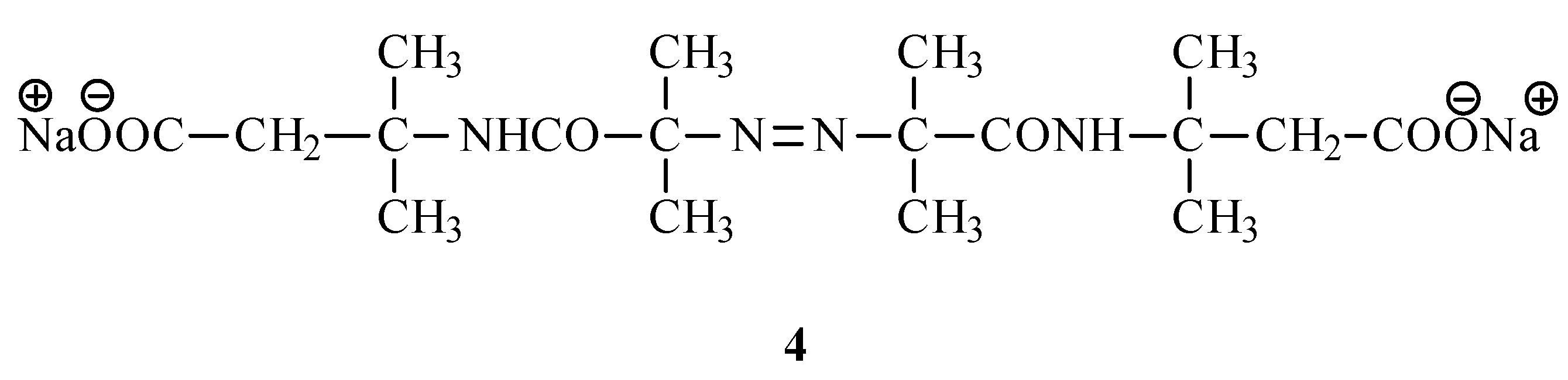
5]. In order to introduce the solubilizing group in the same reaction step, we adopted 3-methyl-2-butenoic acid as the source of the carbocation. After neutraliza- tion and recrystallization from water, we isolated disodium 6,6’-azobis(3,3,6-trimethyl-4-aza-5- oxopentanoate) (
4) (
Figure 1).
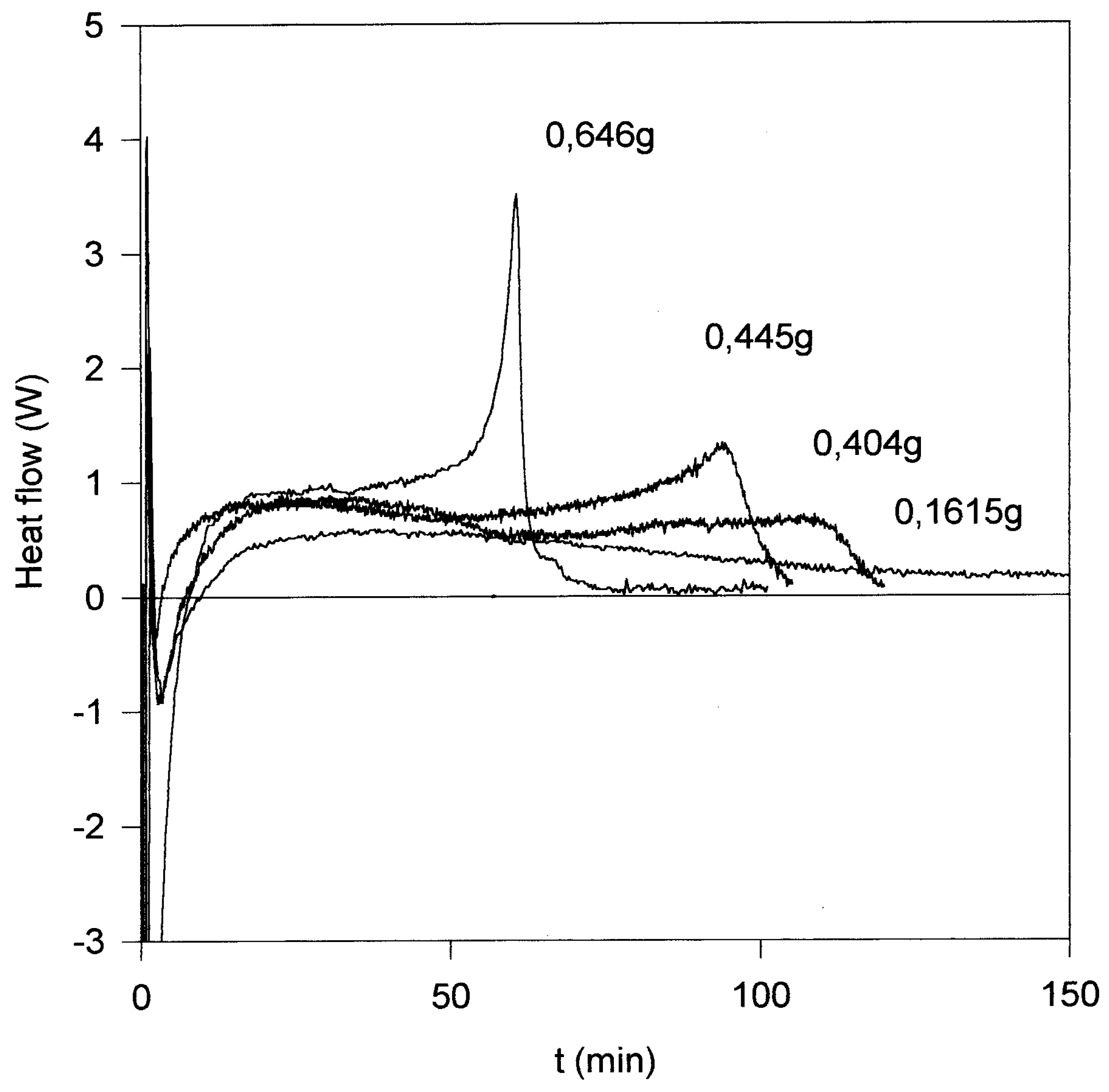
The emulsion polymerization of styrene was studied in more detail with the help of a RM - 2S reac- tion calorimeter. For instance, the phenetyl derivative
3b (n = 2) has been used to prepare mono- disperse poly(styrene) latexes in the 200 nm size range. The rate of polymerization can be controlled by the initiator concentration. The figure shows the heat flow- time curves for varying amounts of
3b. It is clearly seen that with increasing amount of
3b a distinct gel peak appears in the last stage of the polymerization. The overall enthalpy of polymerization was determined to be (58.50 ± 0.05) kJ/mol which corresponds to the literature value [
8].
Experimental
General
Unless otherwise stated, all the chemicals used were obtained from Aldrich and used without further purification. Melting points were measured on a Kofler hot-stage instrument and are uncorrected. The 1H and 13C NMR spectra were measured on an AMX 360 Bruker spectrometer at 360.14 and 90.57 MHz, respectively at 25°C in hexadeuteriodimethyl sulfoxide (DMSO-d6) or in deuterium oxide (D2O). In the cases where DMSO-d6 was used the chemical shifts are referred to the middle signal of the solvent multiplet [δ (1H) 2.55 and δ(13C) 39.6]. The internal reference used for the deuterium oxide solutions was sodium 4,4-dimethyl-4-silapentane-1-sulfonate (DSS).
General procedure of the reaction of the AIBN with alcohols
A mixture of AIBN (65.6 g, 0.4 mol) and an appropriate alcohol (6 mol) was saturated with HCl gas at 3°C for 4 h (until no crystals occur). The mixture was stirred at 0°C for 24 h. After further cooling to – 15°C the corresponding iminoesters 1a, 1b or 1c were separated by filtration and washed with cold acetone. Dry iminoesters were decomposed with 4 L of ice-water mixture. After hydrolysis (overnight) the crude esters 2a, 2b or 2c were recrystallized from acetone at 25°C.
Dibenzyl-2,2’-azodiisobutyrate (2a)
Yield 15%, m.p. 44 - 46°C (dec.); TLC : silica gel plates, ethyl acetate, RF = 0.47; 1H NMR (DMSO-d6) δ: 1.35 (s; 6H, CH3); 5.09 (s; 2H, CH2); 7.30 (m; 5H-arom). 13C NMR (DMSO-d6) δ 22.5 (CH3); 66.1 (CH2); 75.0 (Cq); 127.0 (CH-arom); 136.1 (Cq-arom); 171.9 (C=O). Calculated for C22H26N2O4 (382.5): 69.09%C, 6.85%H, 7.32%N. Found: 69.14%C, 6.81%H, 7.46%N.
2,2’-Azodiisobutyramide (isolated as a by product) m.p. 80- 82°C (dec.); TLC: silica gel plates, ethyl acetate, RF = 0.12; 1H NMR (DMSO-d6) d: 1.34 (s; 3H, CH3); 7.20 (d; 2H, NH2). Calculated for C8H16N4O2 (200.2): 47.99%C, 8.05%H, 27.98%N. Found: 48.12%C, 8.08%H, 28.10%N.
Bis(2-phenylethyl)-2,2’-azodiisobutyrate (2b)
Yield 77%, m.p. 48 - 49°C (dec.); TLC: silica gel plates, ethyl acetate, RF = 0.63; 1H NMR (DMSO-d6) δ: 1.62 (s; 6H, CH3); 2.84 (t; 2H, CH2Ph); 4.24 (t; 2H, CH2O); 7.23 (m; 5H-arom). 13C NMR (DMSO-d6) δ 22.5 (CH3); 35.1 (CH2Ph); 66.1 (CH2O); 76.0 (Cq); 128.0 (CH-arom); 138.5 (Cq-arom); 173.1 (C=O). Calculated for C24H30N2O4 (410.5): 70.22%C, 7.37%H, 6.82%N. Found: 70.34%C, 7.41%H, 6.95%N.
Bis(3-phenylpropyl)-2,2’-azodiisobutyrate (2c)
Yield 75%, m.p. 29 - 31°C; TLC: silica gel plates, ethyl acetate, RF = 0.68; 1H NMR (DMSO-d6) δ: 1.38 (s; 6H, CH3); 1.81 (m; 2H, CH2); 2.59 (t; 2H, CH2Ph); 4.02 (t; 2H, CH2O); 7.21 (m; 5H-arom). 13C NMR (DMSO-d6) δ 23.1 (CH3); 31.1 (CH2); 32.1 (CH2Ph); 64.6 (CH2O); 76.0 (Cq); 128.1 (CH-arom); 142.1 (Cq-arom); 172.9 (C=O). Calculated for C26H34N2O4 (438.6): 71.21%C, 7.81%H, 6.39%N. Found: 71.28%C, 7.77% H, 6.46%N.
General procedure of sulfonation of esters 2a, 2b and 2c
A solution of the ester (0.024 mol) in 1,2-dichloroethane (50 g) was added dropwise with stirring to a solution of freshly distilled sulfur trioxide (20 g, 0.25 mol) in 1,2-dichloroethane (70 g ) at 3-5°C for 30 min. After the was complete addition the solutions were stirred for one hour at 10°C to give a two- phase reaction mixture. After separation the top layer was removed and the bottom phase was washed 8 times with 1,2-dichloroethane (50 g). The bottom layer was neutralized with an aqueous solution of ammonia (22%) with cooling to 15°C and vigorous mixing. The precipitated diammonium salts 3a, 3b or 3c were isolated by filtration and purified by crystallization from methanol-water mixtures (25°C).
Diammonium 2,2’-azobis[p-(2-methylpropanoyloxymethyl)benzenesulfonate] (3a)
Yield 67%, 1H NMR (D2O) δ:1.18 (s; 6H, CH3); 5.18 (s; 2H CH2); 7.38 (d; J = 7.7 Hz, 2H-arom), 7.71 (d; J = 7.7 Hz, 2H-arom). 13C NMR (D2O) δ 22.1(CH3); 60.8 (CH2); 75.6 (qC; quaternary carbon); 128.3 (CH-arom); 131.4 (CH-arom); 142.1 (qC -arom); 142.9 (qC-arom); 175.3 (C=O). Calculated for C22H32N4O10S2 (576.6): 45.82%C, 5.59%H, 9.72%N, 11.12%S. Found: 45.89%C, 5.57%H, 9.87%N, 11.28%S.
Diammonium 2,2’-azobis[p-(μ-(2-methylpropanoyloxy)ethyl)benzenesulfonate] (3b)
Yield 87%, 1H NMR (D2O) δ: 1.21 (s; 6H, CH3); 2.97 (t; 2H, CH2-Ph); 4.35 (t; 2H, CH2O); 7.32 (d; J = 7.8 Hz, 2H-arom); 7.68 (d; J = 7.8 Hz, 2H-arom). 13C NMR (D2O) δ 22.2 (CH3); 34.8 (CH2Ph); 66.2 (CH2O); 75.9 (qC); 126.2 (CH-arom); 130.1 (CH-arom); 141.5 (qC-arom); 142.6 (qC-arom); 175.2 (C=O). Calculated for C24H36N4O10S2 (604.7): 47.67%C, 6.00%H, 9.27%N, 10.60%S. Found: 47.74%H, 5.98%H, 9.32%N, 10.73%S.
Diammonium 2,2’-azobis[p-(ξ-(2-methylpropanoyloxy)propyl)benzenesulfonate] (3c)
Yield 52%, 1H NMR (D2O) δ: 1.30 (s; 6H, CH3); 1.92 (m; 2H, CH2), 2.67 (t; 2H, CH2Ph); 4.24 (t; 2H, CH2O); 7.35 (d; J = 7.8 Hz, 2H-arom); 7.75 (d; J = 7.8 Hz, 2H-arom). 13C NMR (D2O) δ 22.1 (CH3); 35.1 (CH2); 36.4 (CH2); 66.5 (CH2O); 75.8 (qC); 126.8 (CH-arom); 130.3 (CH-arom); 141.4 (qC-arom); 142.8 (qC-arom); 175.3 (C=O). Calculated for C26H40N4O10S2 (632.7): 49.35%C, 6.37%H, 8.85% N, 10.13%S. Found : 49.29%C, 6.38%H, 8.93%N, 10.26%S.
Disodium 6,6’-azobis(3,3,6-trimethyl-4-aza-5-oxoheptanoate) (4)
A mixture of AIBN (4.1 g, 0.025 mol), 3-methyl-2-butenoic acid (10 g, 0.10 mol) and glacial acetic (50 mL) acid was treated with H2SO4 (98%, 50 mL) added over 60 min. The temperature was kept at 30°C. The mixture was allowed to stand at room temperature overnight and then poured onto ice (200g). The precipitated acid was isolated by filtration and neutralized with a solution of NaOH. Recrystalization from water (25°C) gave colorless crystals (2.5g; 23%). 1H NMR (D2O) δ 1.44 (s; 6H, CH3); 1.55 (s; 6H, CH3); 2.66 (s; 2H, CH2); 13C NMR (D2O) δ 19.8 (CH3); 23.4 (CH3); 46.9 (qC); 50.1 (CH2); 72.4 (qC); 173.2 (CONH); 177.4 (COO). Calculated for C18H30N4O6Na2 (444.4): 48.65%C, 6.80%H, 12.61%N. Found: 48.68%C, 6.97%H, 12.74%N.
The emulsion polymerization of styrene
A mixture of styrene (10 g) and water (95 g) was heated with stirring in a reaction calorimeter (RM - 2S, heat flow/ heat balance system; ChemiSens AB, Lund, Sweden). At a temperature of 90°C a so- lution of 3b (0.646 g; 0445 g; 0.404 g; 0.1615 g) in water (5 g) was injected into the calorimeter. The heat-flow-time dependence was recorded. The particle size of the latex obtained after polymerization (150 min) was analyzed by electron microscopy (Rast’s electron microscope DSM 940A, Zeiss).