Structural Elucidation of Z- and E- Isomers of 5-Alkyl-4-ethoxycarbonyl-5-(4`-chlorophenyl)-3-oxa-4-pentenoic Acids
Abstract
:Introduction
Results and Discussion
Structure and Configuration of the Hemiesters
Experimental
General
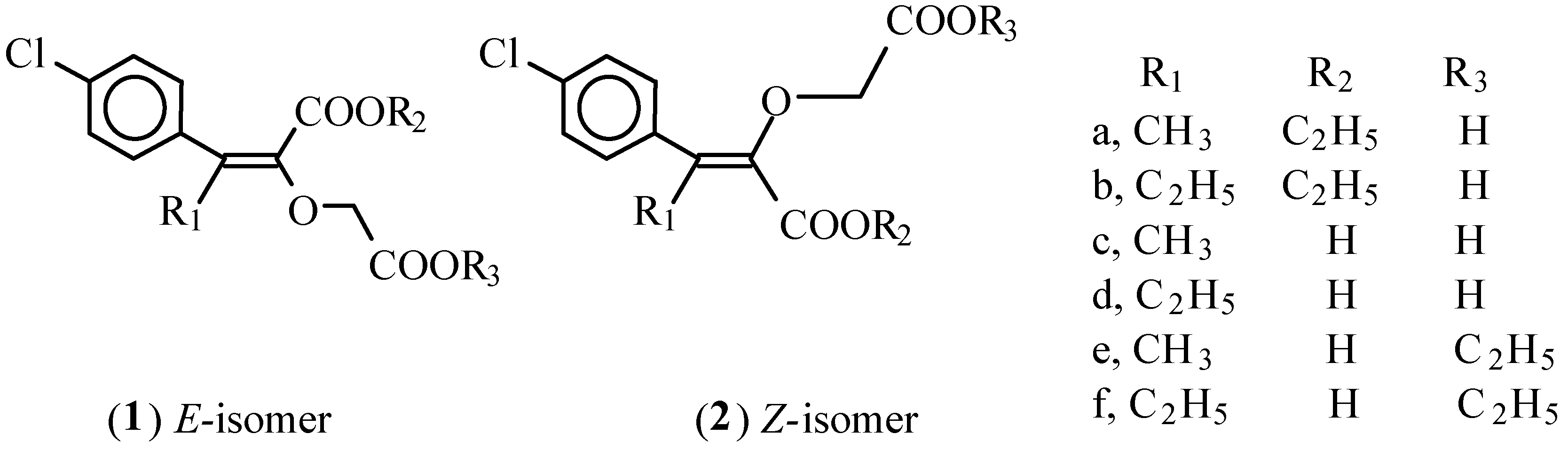
Z- and E-isomers of 5-alkyl-4-ethoxycarbonyl-5-[4‵-chlorophenyl]-3-oxa-4-pentenoic acids 1a,b and 2a,b
Saponification of the hemiesters
Formation of cyclic anhydrides 3a-d
Action of anhydrous aluminium chloride upon the cyclic anhydrides 3a,b
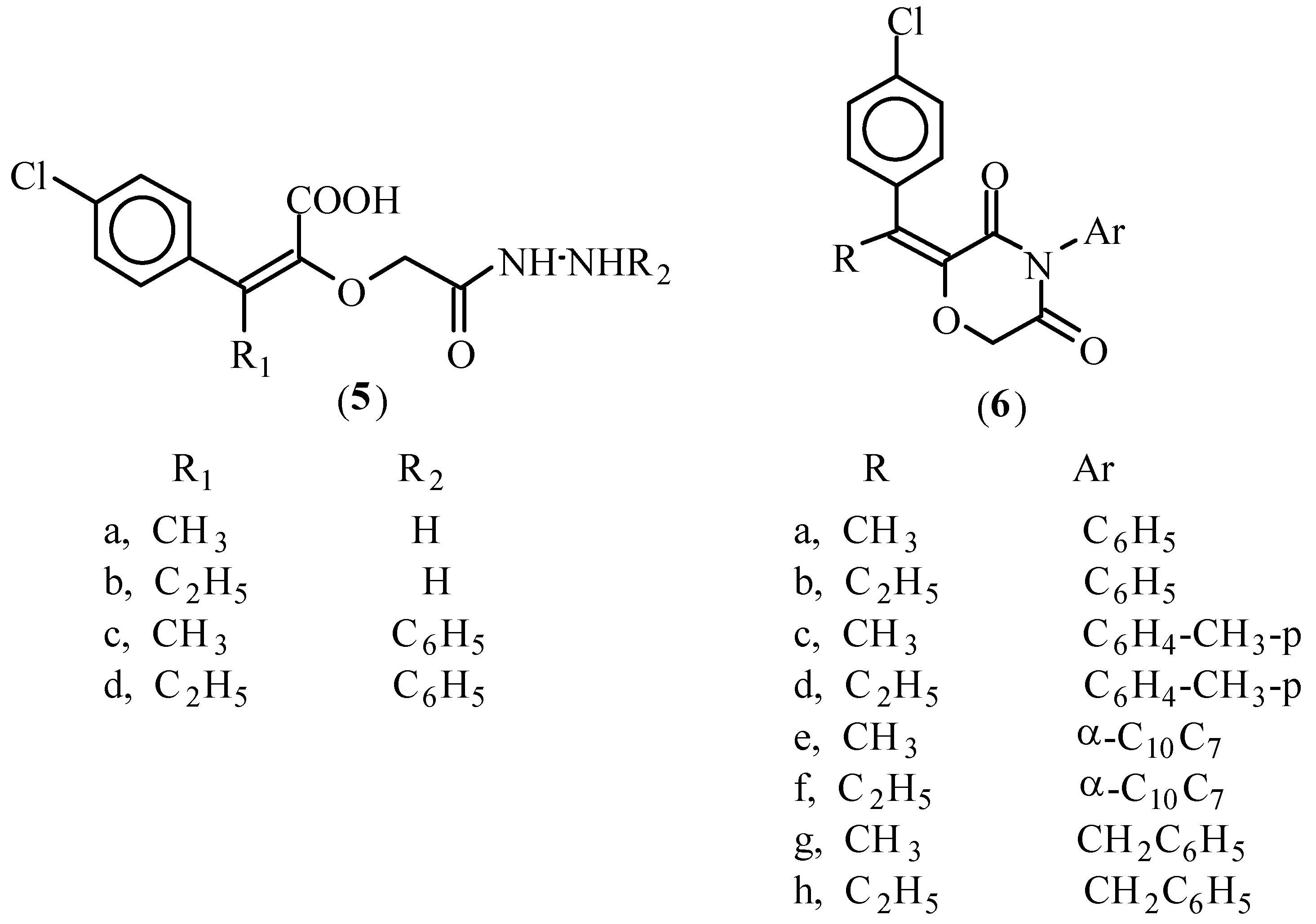
Formation of hydrazide derivatives 5a-d
Formation of 4-aryl-2-(2'-alkyl-2'-(4-chlorophenyl)methylene-3,5-dioxo-2,3,5,6-tetrahydro-1,4-[4H] oxazine derivatives 6a-h
Reaction of 3b with aromatic hydrocarbons: formation of 7a-d
Ethanolysis of anhydrides 3a and 3b
References and Notes
- Madkour, H.M.F.; Salem, M.A.I.; Abdel-Rahman, T.M.; Azab, M.E. Heterocycles 1994, 38, 57.
- El-Hashash, M.A.; Madkour, H.M.F.; Amine, M.S. Pak. J. Sci. Ind. Res. 1991, 34, 288.
- Mahmoud, M.R. Ind. J. Chem. 1994, 33B, 1028–1032.
- Mahmoud, M.R. J. Chem. Soc. Pak. 1989, 11, 144–150.
- El-Newaihy, M.F.; Salem, M.R.; Enayat, E.I.; El-Bassiony, F.A. Aust. J. Chem. 1979, 32, 1159.
- Bellamy, L.J. The Infrared Spectra of Complex Molecules,3rd Edn.; Chapman & Hall: London, 1975; Vol. 1. [Google Scholar]
- Abdel-Hamid, H.A.; Enayat, E.I.; Mahmoud, M.R. J. Chem. Soc. Pak. 1990, 12, 128–133.
- Mahmoud, M.R.; El-Nagdy, S.; El-Bassiouny, F.A. J. Chem. Soc. Pak. 1988, 10, 261–267.
- Samples Availability: Not available.



| Compound | M.p (°C) & Solvent | IR (cm-1) | |
| νC=O | νOH | ||
| 1c | 235 | 1700-1698 | br. 3100-3500 |
| Benzene | |||
| 1d | 230-2 | 1705-1688 | br. 3110-3460 |
| Light petroleum1 | |||
| 2c | 218 | 1700-1690 | br.3080-3500 |
| Light petroleum1 | |||
| 2d | 207-9 | 1710-1695 | br.3180-3480 |
| Light petroleum1 | |||
| 3a | 173.5 | 1780-1742 | |
| Light petroleum | |||
| 3b | 176.8 | 1772-1753 | |
| Benzene | |||
| 3c | 167-9 | 1780-1750 | |
| Light petroleum1 | |||
| 3d | 135-7 | 1775-1742 | |
| Light petroleum1 | |||
| Compound | M.p (°C) & Solvent | IR (cm-1) | |
| νC=O | νNH,OH | ||
| 5a | 190-2 | 1705-1660 | 3510-3210 |
| Benzene | |||
| 5b | 210-2 | 1696-1656 | 3450-3205 |
| Methanol | |||
| 5c | 122-3 | 1692-1678 | 3480-3250 |
| Light petroleum | |||
| 5d | 220-2 | 1700-1662 | 3510-3200 |
| Benzene | |||
| 6a | 140-2 | 1782-1705 | |
| Light petroleum | |||
| 6b | 165-7 | 1776-1700 | |
| Benzene | |||
| 6c | 205-7 | 1780-1705 | |
| Methanol | |||
| 6d | 200 | 1772-1690 | |
| Methanol | |||
| 6e | 240-2 | 1785-1710 | |
| Benzene | |||
| 6f | 205-7 | 1760-1700 | |
| Methanol | |||
| 6g | 172-3 | 1782-1710 | |
| Benzene | |||
| 6h | 196-7 | 1777-1700 | |
| Methanol | |||
| Compound | M.p (°C) & Solvent | IR (cm-1) | |
| νC=O | νNH,OH | ||
| 7a | >280 | 1702-1696 | br. 3400 |
| Ethanol | |||
| 7b | 260-2 | 1700-1686 | br. 3210-3450 |
| Benzene-methanol | |||
| 7c | >300 | 1700-1698 | br. 3430 |
| Benzene-methanol | |||
| 7d | 250-2 | 17072-1688 | br. 3510 |
| Benzene-methanol | |||
| 1e | 117-9 | 1732-105 | br. 3500-3180 |
| Light petroleum | |||
| 1f | 220-3 | 1728-1700 | br. 3480-3120 |
| Benzene | |||
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Madkour, H.M.F. Structural Elucidation of Z- and E- Isomers of 5-Alkyl-4-ethoxycarbonyl-5-(4`-chlorophenyl)-3-oxa-4-pentenoic Acids. Molecules 2000, 5, 737-745. https://doi.org/10.3390/50500737
Madkour HMF. Structural Elucidation of Z- and E- Isomers of 5-Alkyl-4-ethoxycarbonyl-5-(4`-chlorophenyl)-3-oxa-4-pentenoic Acids. Molecules. 2000; 5(5):737-745. https://doi.org/10.3390/50500737
Chicago/Turabian StyleMadkour, H. M. F. 2000. "Structural Elucidation of Z- and E- Isomers of 5-Alkyl-4-ethoxycarbonyl-5-(4`-chlorophenyl)-3-oxa-4-pentenoic Acids" Molecules 5, no. 5: 737-745. https://doi.org/10.3390/50500737




