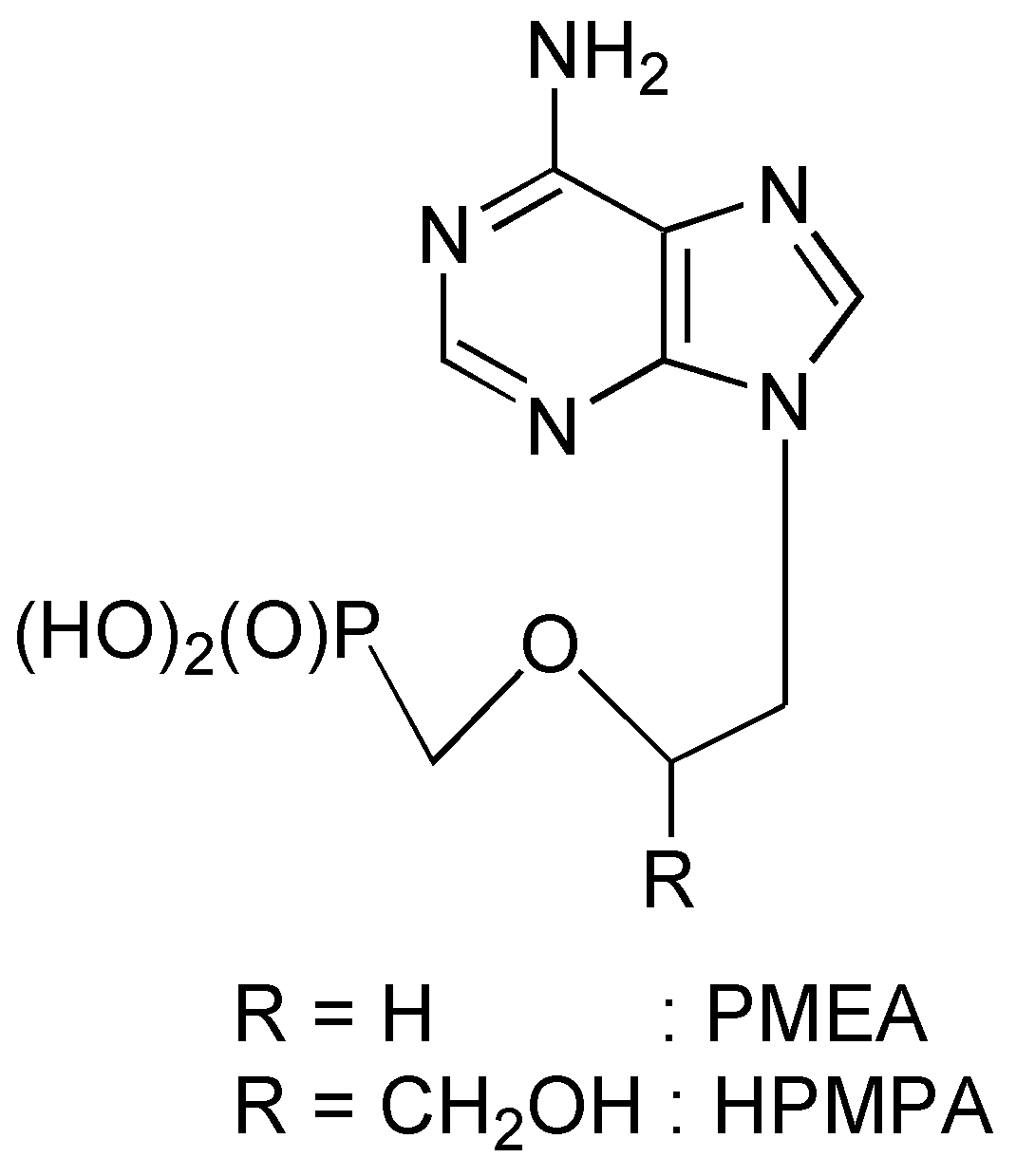
General
1H-NMR spectra were recorded using a Brucker AC 250 MHz spectrometer. Unless specified otherwise, the solvent used was DMSO-d6. Chemical shifts are reported as parts per million (δ ppm) from TMS used as internal standard. Key: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and b (broad). Ultraviolet spectral (UV) were recorded with a Cary 219 spectrometer. Mass spectra (MS) were obtained with JEOL JMS DX 300 instrument using fast atomic bombardment (FAB positive – GT). Thin layer chromatography (T.L.C) was performed on MerckKiesegel 60 F254 plates and short- wave ultraviolet light (254 nm) was used to detect the UV-absorbing spots. Column chromatography separations were obtained on silica gel (0.063 - 0.2 mm Merck). Elemental analysis were determined by the French Microanalytical Central Service.
Bis alkylating coupling method :
To a solution of 1.45 mmoles of compound
4 or
5 and potassium carbonate (1.45 mmoles) in dry DMF (15mL), 4.35 mmoles of the alkylating agent were added. The reaction mixture was allowed to stir at room temperature for several hours (see
Table 3) and the solvent was removed under reduced pressure. The residual oil was purified by silica gel column chromatography using ethylacetate/ hexane as eluant to give compounds
6a,
6b, ...,
11b as white oils.
(Z)-diethyl-2-(3-ethoxycarbonylmethyluracil-1-yl) ethylen-1-ylphosphonate (6a)
Yield: 23 %; eluant: hexane- 90% EtOAc, Rf = 0.37 (CH2Cl2/MeOH, 95/5); 1H-NMR: 7.88 (d, 1H, H6, J6, 5 = 8.1 Hz), 7.32 (dd, 1H, H2, J2, 1 = 10.9 Hz, J2, P = 41.3 Hz), 5.98 (d, 1H, H5, J5, 6 = 8.1 Hz), 5.90 (dd, 1H, H1, J1, 2 =10.9 Hz, J1, P = 9.3 Hz), 4.57 (s, 2H, CH2N-3), 4.14 (q, 2H, CO2CH2-, JCH2CH3 = 7.1 Hz), 4.01 (qd, 4H, 2 x OCH2, JCH2-P = 7.9 Hz, JCH2CH3 = 7.0 Hz), 1.22 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax = 271 nm; MS, FAB positive, m/z = 361[M+H]+; Elem. Anal. Calcd. for C14H21N2O7P : C 46.67 H 5.87 N 7.77 Found : C 46.58 H 5.97 N 7.69.
(E)-diethyl-2-(3-ethoxycarbonylmethyl uracil-1-yl) ethylen-1-ylphosphonate (6b)
Yield: 68 %; eluant: hexane- 90% EtOAc, Rf = 0.38 (CH2Cl2/MeOH, 95/5); 1H-NMR: 8.28 (d, 1H, H6, J6, 5 = 8.1 Hz), 7.69 (dd, 1H, H2, J2,1 = 16.0 Hz, J2, P = 18.1 Hz), 6.29 (dd, 1H, H1), J1, 2 = 16.0 Hz, J1, P = 10.3 Hz), 6.07 (d, 1H, H5, J5, 6 = 8.1 Hz), 4.58 (s, 2H, CH2N-3), 4.14 (q, 2H, CO2CH2-, JCH2CH3 = 7.1 Hz), 4.01 ( qd, 4H, 2 x OCH2, JCH2-P = 8.0 Hz, JCH2CH3 = 7.1 Hz), 1.22 (t, 6H, 2 x CH3, JCH2CH3 = 7.1 Hz); UV (MeOH) λmax = 277 nm; MS, FAB positive, m/z = 361 [M+H]+; Elem. Anal. Calcd. for C14H21N2O7P : C 46.67 H 5.87 N 7.77 Found: C 46.73 H 5.94 N 7.69.
(Z)-diethyl-2-(3-ethoxycarbonylmethylthymin-1-yl)ethylen-1-ylphosphonate (7a)
Yield: 17 %; eluant: hexane- 70% EtOAc, Rf = 0.40 (CH2Cl2/MeOH, 95/5); 1H-NMR: 7.86 (s, 1H, H6), 7.34 (dd, 1H, H2, J2, 1 = 11.1 Hz, J2, P = 1.5 Hz), 5.83 (dd, 1H, H1, J1, 2 = 11.1 Hz, J1, P = 9.2 Hz), 4.58 (s, 2H, CH2N-3), 4.14 (q, 2H, CO2CH2-, JCH2CH3 = 7.1 Hz), 4.01 (qd, 4H, 2 OCH2, JCH2-P = 8.0 Hz, JCH2CH3 = 7.1 Hz), 1.22 (m, 9H, 3x CH3); UV (MeOH) λmax = 278 nm; MS, FAB positive, m/z = 375 [M+H]+; Elem. Anal. Calcd. for C15H23N2O7P : C 48.13 H 6.19 N 7.48 Found : C 48.19 H 6.21 N 7.40.
(E)-diethyl-2-(3-ethoxycarbonylmethyl thymin-1-yl) ethylen-1-ylphosphonate (7b)
Yield: 68 %; eluant: hexane- 70% EtOAc, Rf = 0.38 (CH2Cl2/ MeOH, 95/ 5); 1H-NMR: 8.21 (s, 1H, H6), 7.72 (dd, 1H, H2, J2,1 = 15.9 Hz, J2, P = 18.1 Hz), 6.20 (dd, 1H, H1, J1, 2 = 15.9 Hz, J1, P = 10.2 Hz), 4.60 (s, 2H, CH2N-3), 4.14 (q, 2H, CO2CH2-, JCH2CH3 = 7.1 Hz), 4.02 (qd, 4H, 2 x OCH2, JCH2-P = 7.9 Hz, JCH2CH3 = 7.1 Hz), 1.91 (s, 3H, CH3-5), 1.23 (m, 9H, 3 x CH3); UV (MeOH) λmax = 284 nm; MS, FAB positive, m/z = 375 [M+H]+; Elem. Anal. Calcd. for C15H23N2O7P : C 48.13 H 6.19 N 7.48 Found: C 48.21 H 6.12 N 7.38.
(Z)-diethyl-2-(3-allyluracil-1-yl)ethylen-1-ylphosphonate (8a)
Yield: 21 %; eluant: hexane- 80% EtOAc, Rf = 0.42 (CH2Cl2/ MeOH, 95/5), 1H-NMR: 7.83 (d, 1H, H6, J6, 5 = 8.0 Hz), 7.33 (dd, 1H, H2, J2, 1 = 11.0 Hz, J2, P = 41.6 Hz), 5.91 (d, 1H, H5, J5, 6 = 8.0 Hz), 5.83 (m., 2H, H1=CH-), 5.10 (m, 2H, H2C=C), 4.40 (d, 2H, CH2N-3, JCH2CH = 5.2 Hz), 4.00 ( qd, 4H, 2 x OCH2, JCH2-P = 7.8 Hz, JCH2CH3 = 7.0 Hz), 1.21 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax = 270 nm; MS, FAB positive, m/z = 315 [M+H]+; Elem. Anal. Calcd. for C13H19N2O5P : C 49.68 H 6.09 N 8.91 Found : C 49.61 H 6.13 N 8.88.
(E)-diethyl-2-(3-allyluracil-1-yl)ethylen-1-ylphosphonate (8b).
Yield: 63 %; eluant: hexane- 80% Ac OEt, Rf = 0.38 (CH2Cl2/ MeOH, 95/ 5); 1H-NMR: 8.20 (d, 1H, H6, J6, 5 = 8.1 Hz), 7.74 (dd, 1H, H2, J2, 1 = 16.0 Hz, J2, P = 18.2 Hz), 6.19 (dd, 1H, H1, J1, 2 = 16.0 Hz, J1, P = 10.4 Hz), 5.99 (d, 1H, H5, J5, 6 = 8.1 Hz), 5.83 (m., 2H, H1=CH-), 5.11 (m, 2H, H2C=C), 4.41 (d, 2H, CH2N-3, JCH2CH- = 5.2 Hz), 4.01 (qd, 4H, 2 x OCH2, JCH2-P = 8.1 Hz, JCH2CH3 = 7.0 Hz), 1.25 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax = 278 nm; MS, FAB positive, m/z = 315 [M+H]+; Elem. Anal. Calcd. for C13H19N2O5P : C 49.68 H 6.09 N 8.91 Found : C 49.59 H 6.15 N 8.93.
(Z)-diethyl-2-(3-allylthymin-1-yl)ethylen-1-ylphosphonate (9a)
Yield: 17 %; eluant: hexane- 70% EtOAc, Rf = 0.47 (CH2Cl2/ MeOH, 95/ 5); 1H-NMR: 7.82 (s, 1H, H6), 7.35 (dd, 1H, H2, J2, 1 =11.1 Hz, J2, P = 41.8 Hz), 5.81 (m., 2H, H1= CH-), 5.11 (m, 2H, H2C=C), 4.42 (d, 2H, CH2N-3, JCH2CH=C = 5.2 Hz), 4.00 (qd, 4H, 2 x OCH2, JCH2-P = 7.9 Hz, JCH2CH3 = 7.0 Hz), 1.85 (s, 1H, CH3-5), 1.21 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz), UV (MeOH) λmax = 278 nm, MS, FAB positive, m/z = 329 [M+H]+, Elem. Anal. Calcd. for C14H21N2O5P : C 51.22 H 6.45 N 8.53 Found : C 51.30 H 6.49 N 8.46.
(E)-diethyl-2-(3-allylthymin-1-yl)ethylen-1-ylphosphonate (9b).
Yield: 70 %; eluant: hexane- 70% EtOAc, Rf = 0.43 (CH2Cl2/ MeOH, 95/5); 1H-NMR: 8.14 (s, 1H, H6), 7.76 (dd, 1H, H2, J2,1 = 15.9 Hz, J2, P = 18.2 Hz), 6.12 (dd, 1H, H1, J1, 2 = 15.9 Hz, J1, P = 10.5 Hz), 5.82 (m., 1H, = CH-), 5.11 (m, 2H, H2C=C), 4.43 (d, 2H, CH2N-3, JCH2CH=C = 5.2 Hz), 4.01 (qd, 4H, 2 OCH2, JCH2-P = 7.9 Hz, JCH2CH3 = 7.0 Hz), 1.89 (s, 1H, CH3-5), 1.25 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax = 282 nm; MS, FAB positive, m/z = 329 [M+H]+, Elem. Anal. Calcd. For C14H21N2O5P : C 51.22 H 6.45 N 8.53 Found : C 51.26 H 6.48 N 8.47.
(Z)-diethyl-2-(3-propargyluracil-1-yl)ethylen-1-ylphosphonate (10a)
Yield: 23 %; eluant: hexane-80% EtOAc, Rf = 0.45 (CH2Cl2/ MeOH, 95/5); 1H-NMR (CDCl3): 8.03 (d, 1H, H6 , J6, 5 = 8.2 Hz), 7.38 (dd, 1H, H2, J2,1 = 11.4 Hz, J2, P = 41.7 Hz), 5.85 (d, 1H, H5, J5, 6 = 8.2 Hz), 5.49 (dd, 1H, H1, J1, 2 = 11.4 Hz, J1, P = 7.1 Hz), 4.67 (d, 2H, CH2N-3, JCH2C≡CH = 2.5 Hz), 4.07 (qd, 4H, 2 x OCH2, JCH2-P = 7.7 Hz, JCH2CH3 = 7.0 Hz), 2.15 (t, 1H, HC≡C, JHC≡CCH2 = 2.5 Hz), 1.28 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax = 272 nm; MS, FAB positive, m/z = 312 [M+H]+; Elem. Anal. Calcd. for C13H17N2O5P : C 50.00 H 5.49 N 8.97 Found : C 49.93 H 5.53 N 8.88.
(E)-diethyl-2-(3-propargyl uracil-1-yl) ethylen-1-ylphosphonate (10b).
Yield: 67 %; eluant: hexane-80% EtOAc, Rf = 0.36 (CH2Cl2/MeOH, 95/5); 1H-NMR (CDCl3): 7.81 (dd, 1H, H2, J2,1 = 16.0 Hz, J2, P = 17.5 Hz), 7.48 ( d, 1H, H6, J6, 5 = 8.2 Hz), 5.92 (d, 1H, H5, J5, 6 = 8.2 Hz), 5.68 (dd, 1H, H1, J1, 2 = 16.0 Hz, J1, P = 9.6 Hz), 4.66 (d, 2H, CH2N-3, JCH2C≡CH = 2.5 Hz), 4.09 (qd, 4H, 2 x OCH2, JCH2-P = 7.9 Hz, JCH2CH3 = 7.1 Hz), 2.15 (t, 1H, HC≡C, JHC≡CCH2 = 2.5 Hz), 1.29 (t, 6H, 2 x CH3, JCH2CH3 = 7.1 Hz); UV (MeOH) λmax = 276 nm; MS, FAB positive, m/z = 312 [M+H]+; Elem. Anal. Calcd. for C13H17N2O5P : C 50.00 H 5.49 N 8.97 Found : C 50.05 H 5.42 N 8.92.
(Z)-diethyl-2-(3-propargylthymin-1-yl)ethylen-1-yl- phosphon-ate (11a)
Yield: 17 %; eluant: hexane- 80% EtOAc, Rf = 0.36 (CH2Cl2/MeOH, 95/5); 1H-NMR (CDCl3): 7.98 (s, 1H, H6), 7.42 (dd, 1H, H2, J2,1 = 11.5 Hz, J2, P = 42.1 Hz), 5.42 (dd, 1H, H1, J1, 2 = 11.5 Hz, J1, P = 7.1 Hz), 4.70 (d, 2H, CH2N-3, JCH2C≡CH = 2.5 Hz), 4.09 (qd, 4H, 2 x OCH2, JCH2-P = 7.9 Hz, JCH2CH3 = 7.0 Hz), 2.16 (t, 1H, HC≡C, JHC≡CCH2 = 2.5 Hz), 1.97 ( s, 3H, CH3-5), 1.31 ( t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax= 277 nm; MS, FAB positive, m/z = 326 [M+H]+; Elem. Anal. Calcd. for C14H19N2O5P : C 51.54 H 5.87 N 8.59 Found : C 51.59 H 5.95 N 8.55.
(E)-diethyl-2-(3-propargylthymin-1-yl)ethylen-1-yl-phosphonate (11b).
Yield: 68 %; eluant: hexane- 80% EtOAc, Rf = 0.39 (CH2Cl2/MeOH, 95/5); 1H-NMR (CDCl3): 7.86 (dd, 1H, H2, J2,1 = 16.0 Hz, J2, P = 17.6 Hz), 7.28 ( s, 1H, H6), 5.63 (dd, 1H, H1 , J1, 2 = 16.0 Hz, J1, P = 9.7 Hz), 4.69 (d, 2H, CH2N-3, JCH2C≡CH = 2.5 Hz), 4.09 (qd, 4H, 2 x OCH2, JCH2-P = 7.8 Hz, JCH2CH3 = 7.1 Hz), 2.16 (t, 1H, HC≡C, JHC≡CCH2 = 2.5 Hz), 1.99 (s, 3H, CH3-5), 1.32 (t, 6H, 2 x CH3, JCH2CH3 = 7.0 Hz); UV (MeOH) λmax = 280 nm; MS, FAB positive, m/z = 326 [M+H]+; Elem. Anal. Calcd. for C14H19N2O5P : C 51.54 H 5.87 N 8.59 Found : C 51.52 H 5.93 N 8.53.