Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress
Abstract
1. Introduction
2. Results
2.1. The Effect of Epicatechin on Spermatozoa Exposed to Ferrous Ascorbate
2.1.1. Spermatozoa Motility
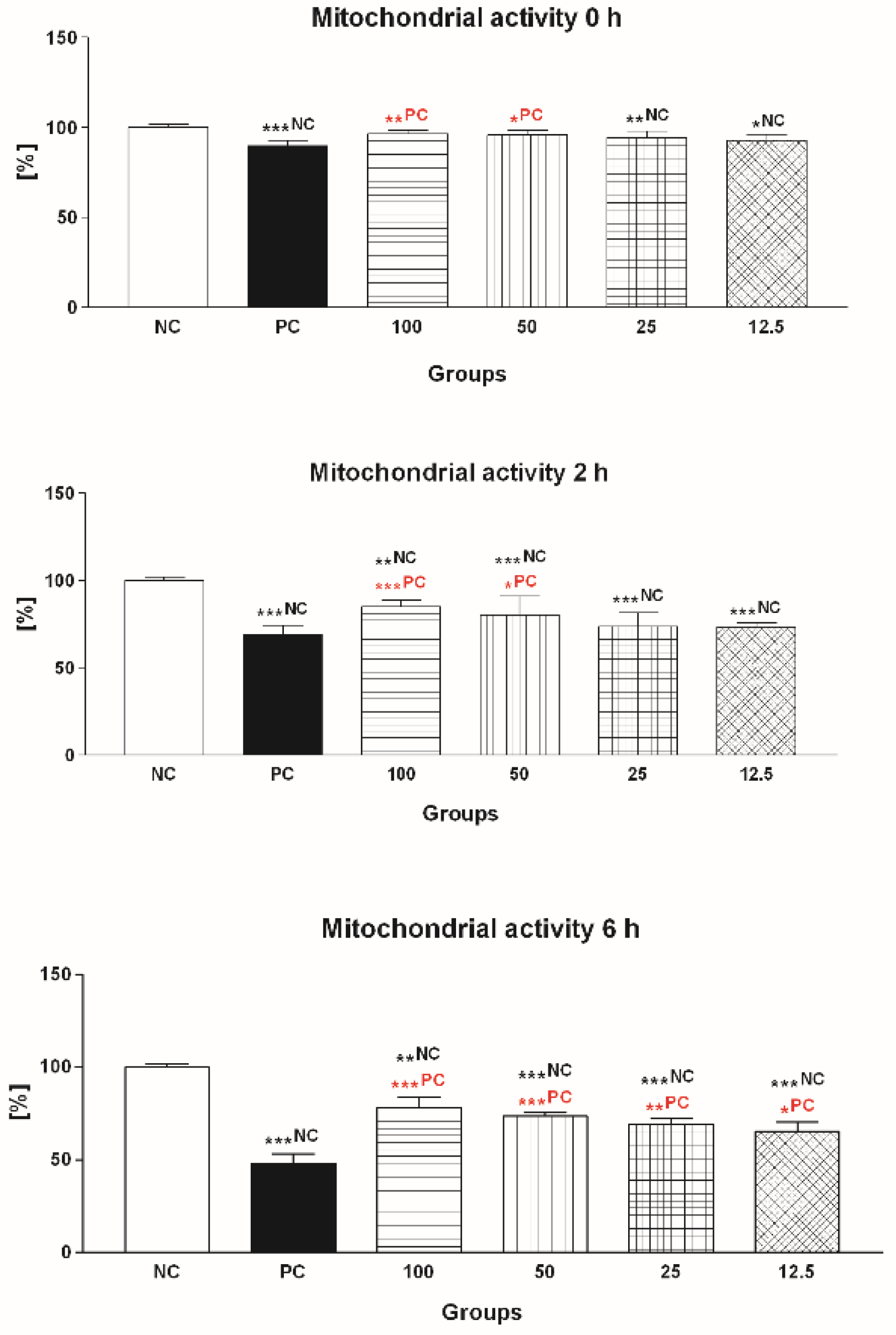
2.1.2. Mitochondrial Activity
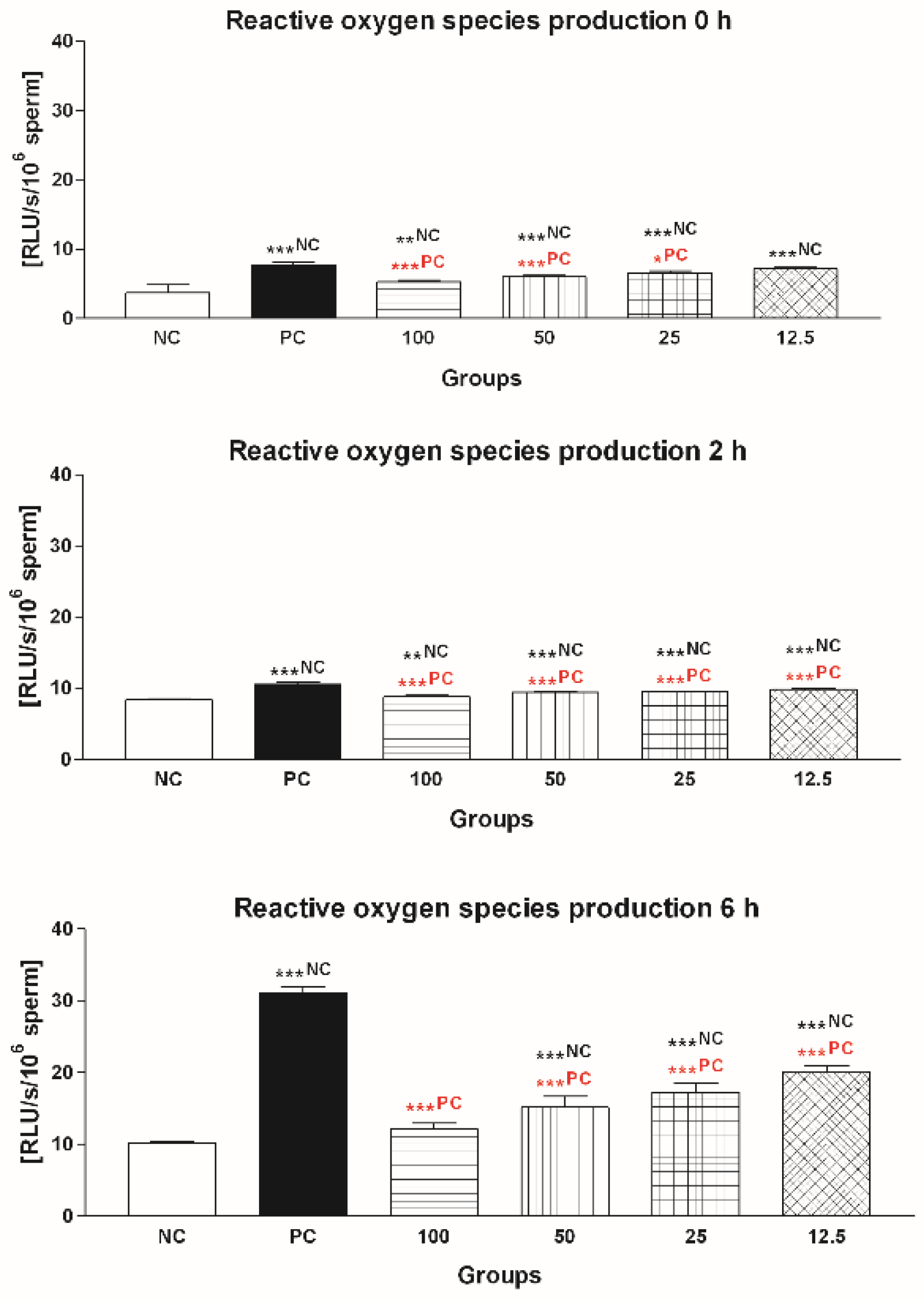
2.1.3. Reactive Oxygen Species (ROS) Production
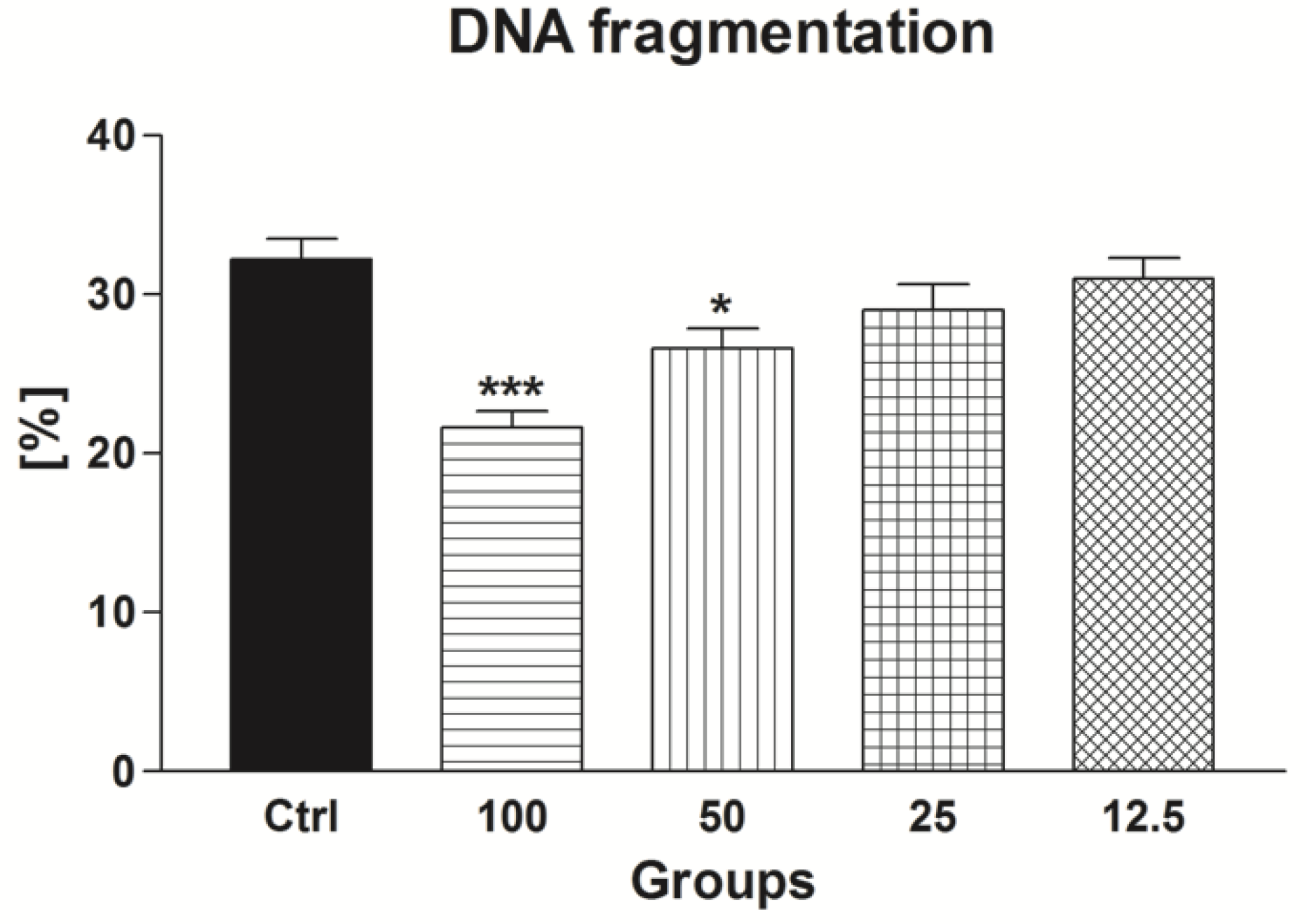
2.1.4. Sperm DNA Fragmentation
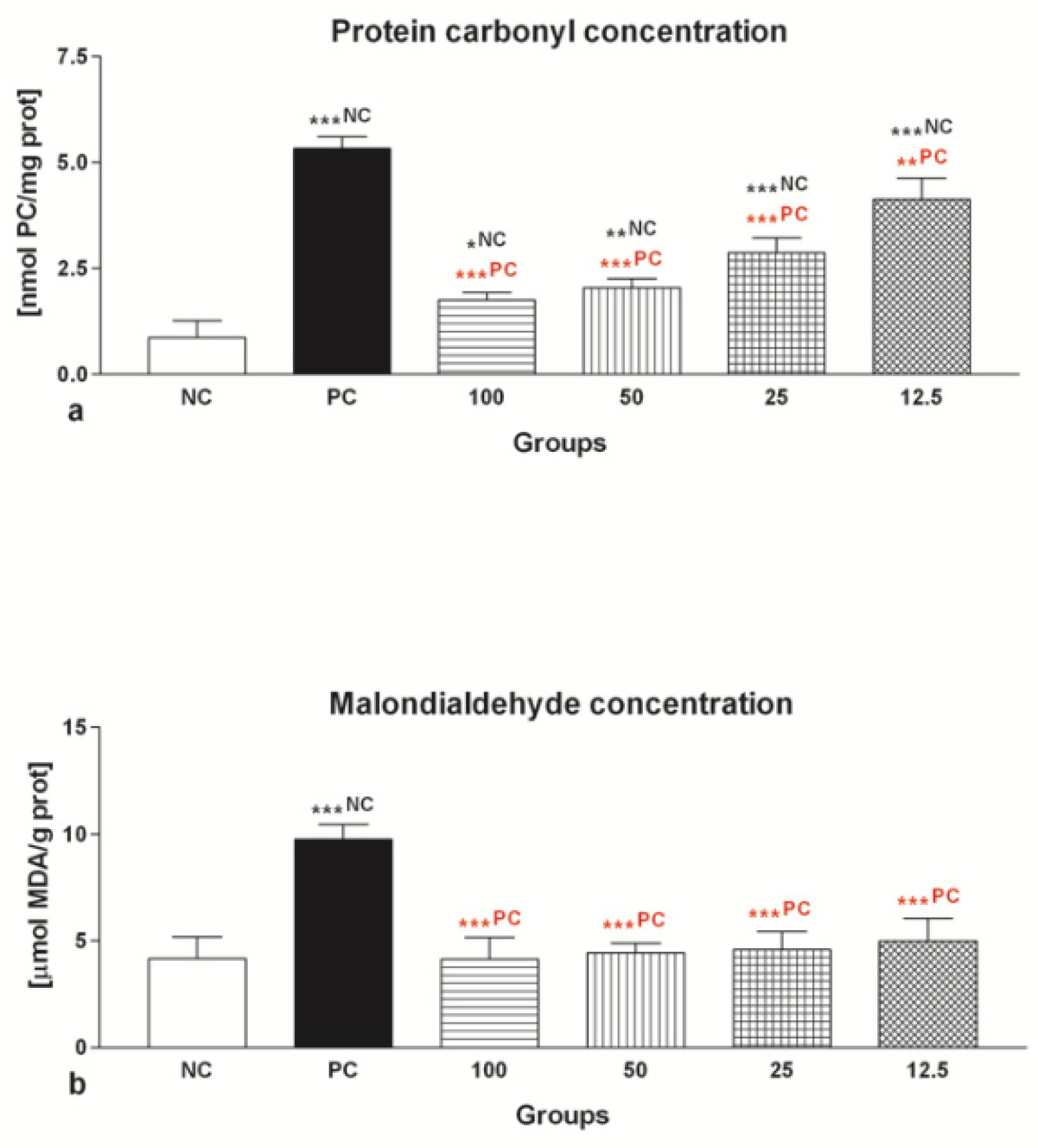
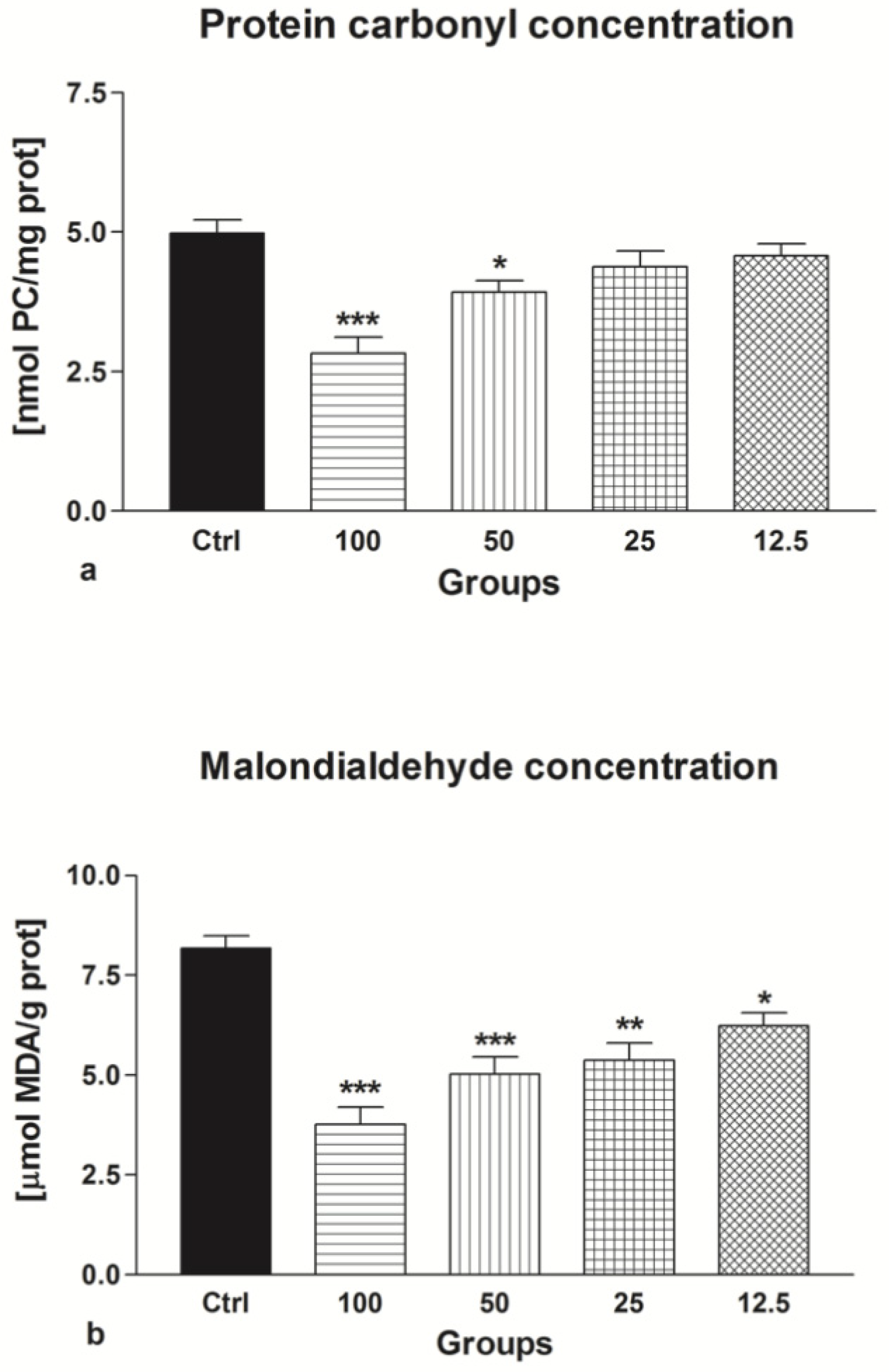
2.1.5. Oxidative Damage
2.2. The Effect of Epicatechin on Cryopreserved Spermatozoa
2.2.1. Spermatozoa Motility
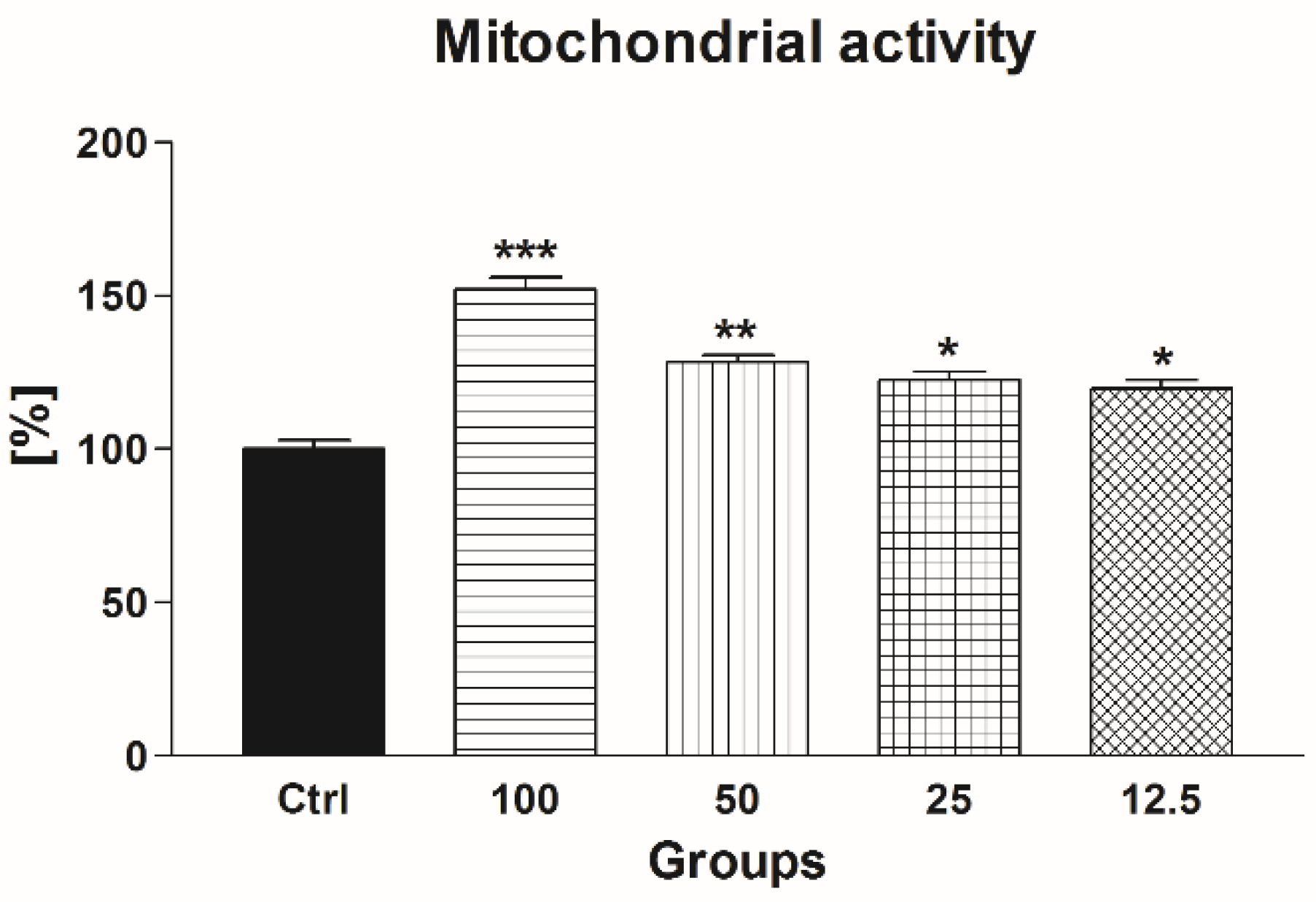
2.2.2. Mitochondrial Activity
2.2.3. Reactive Oxygen Species (ROS) Production
2.2.4. Sperm DNA Fragmentation
2.2.5. Oxidative Damage
3. Discussion
4. Materials and Methods
4.1. Exposure to Ferrous Ascorbate
4.2. Sperm Cryopreservation
4.3. Spermatozoa Motility Assessment
4.4. Sperm Mitochondrial Activity
4.5. Reactive Oxygen Species (ROS) Generation
4.6. Sperm DNA Fragmentation
4.7. Oxidative Damage Assessment
4.8. Statistical Analysis
- Negative control (NC) was compared to the positive control (PC)
- Experimental groups were compared to both controls.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maxwell, W.M.; Stohjanov, T. Liquid storage of ram semen in the absence or presence of some antioxidants. Reprod. Fertil. Dev. 1996, 8, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Sano, T. Select antioxidants improve the function of extended boar semen stored at 10 °C. Theriogenology 2005, 63, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Wingate, J.K.; De Iuliis, G.N.; Koppers, A.J.; McLaughlin, E.A. Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. J. Clin. Endocrinol. Metab. 2006, 91, 4154–4163. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Makker, K.; Desai, N.R.; Agarwal, A. Impact of oxidative stress on IVF. Expert Rev. Obstet. Gynecol. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence-based review. Int. J. Reprod. Biomed. (Yazd) 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Tahvilzadeh, M.; Hajimahmoodi, M.; Toliyat, T.; Karimi, M.; Rahimi, R. An evidence-based approach to medicinal plants for the treatment of sperm abnormalities in traditional Persian medicine. Andrologia 2016, 48, 860–879. [Google Scholar] [CrossRef]
- Arts, I.C.; van De Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food Chem. 2000, 48, 1752–1757. [Google Scholar] [CrossRef]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural properties of green tea catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid. Med. Cell. Longev. 2018, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ye, J.; Leonard, S.; Ding, M.; Vallyathan, V.; Castranova, V.; Rojanasakul, Y.; Dong, Z. Antioxidant properties of (-)-epicatechin-3-gallate and its inhibition of Cr (VI)-induced DNA damage and Cr (IV)-or TPA-stimulated NF-κB activation. Mol. Cell. Biochem. 2002, 206, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Awoniyi, D.O.; Aboua, Y.G.; Marnewick, J.; Brooks, N. The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress-induced rats. Phytother. Res. 2012, 26, 1231–1239. [Google Scholar] [CrossRef]
- Abshenas, J.; Babaei, H.; Zare, M.H.; Allahbakhshi, A.; Sharififar, F. The effects of green tea (Camellia sinensis) extract on mouse semen quality after scrotal heat stress. Vet. Res. Forum 2011, 6, 242–247. [Google Scholar]
- Greifova, H.; Tvrda, E.; Jambor, T.; Lukac, N. Dose- and time-dependent effects of epicatechin on bovine spermatozoa in vitro. JMBFS 2018, 2, 235–239. [Google Scholar]
- Silva, E.C.B.; Arruda, L.C.P.; Vieira, J.I.T.; Soares, P.C.; Guerra, M.M.P. (+)-Catechin and (-)-epigallocatechin gallate: Are these promising antioxidant therapies for frozen goat semen? Arq. Bras. Med. Vet. Zootec 2019, 71, 521–528. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Wu, Z.-B.; Zhao, J.; Zhang, S.; Li, W. Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 2015, 114, 122333. [Google Scholar] [CrossRef]
- Zanchi, M.M.; Manfredini, V.; Dos Santos Brum, D.; Vargas, L.M.; Spiazzi, C.C.; Soares, M.B.; Izaguirry, A.P.; Santos, F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015, 2, 252–260. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human sperm quality and metal toxicants: Protective effects of some flavonoids on male reproductive function. Int. J. Fertil. Steril 2016, 10, 215–223. [Google Scholar] [PubMed]
- Mojica-Villegas, M.A.; Izquierdo-Vega, J.A.; Chamorro Cevallos, G.; Sanchez-Guiterrez, M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients 2014, 6, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Libová, Ľ.; Lukáč, N. Protective effects of quercetin on selected oxidative biomarkers in bovine spermatozoa subjected to ferrous ascorbate. Reprod. Domest. Anim. 2016, 51, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.K.; Bilaspuri, G.S. Antioxidant effect of vitamin E on motility, viability and lipid peroxidation of cattle spermatozoa under oxidative stress. Anim. Sci. Pap. Rep. 2009, 27, 5–14. [Google Scholar]
- Aitken, R.J. The Amoroso Lecture. The human spermatozoon-a cell in crisis? J. Reprod. Fertil. 1999, 115, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Kňažická, Z.; Bárdos, L.; Massányi, P.; Lukáč, N. Impact of oxidative stress on male fertility—A review. Acta Vet. Hung. 2011, 59, 465–484. [Google Scholar] [CrossRef]
- Chaki, S.P.; Misro, M.M. Assessment of human sperm function after hydrogen peroxide exposure. development of a vaginal contraceptive. Contraception 2002, 66, 187–192. [Google Scholar] [CrossRef]
- Evdokimov, V.V.; Barinova, K.V.; Turovetskii, V.B.; Muronetz, V.I.; Schmalhausen, E.V. Low concentrations of hydrogen peroxide activate the antioxidant defense system in human sperm cells. Biochemistry (Moscow) 2015, 80, 1178–1185. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, T.; Zhao, J.; Xu, B. Interactive enhancements of ascorbic acid and iron in hydroxyl radical generation in quinone redox cycling. Environ. Sci. Technol. 2012, 46, 10302–10309. [Google Scholar] [CrossRef]
- Tvrda, E.; Peer, R.; Sikka, S.C.; Agarwal, A. Iron and copper in male reproduction: A double-edged sword. J. Assist. Reprod. Genet. 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Aisen, E.; Fernández-Santos, M.R.; Esteso, M.C.; Maroto-Morales, A.; García-Álvarez, O.; Garde, J.J. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa. Reproduction 2009, 137, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [PubMed]
- Hosen, M.B.; Islam, M.R.; Begum, F.; Kabir, Y.; Howlader, M.Z. Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran. J. Reprod. Med. 2015, 13, 525–532. [Google Scholar] [PubMed]
- Colagar, A.H.; Karimi, F.; Jorsaraei, S.G.A. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno-teratospermic men. Iran. Red. Crescent Med. J. 2013, 15, 780–785. [Google Scholar]
- Davila, M.P.; Muñoz, P.M.; Bolaños, J.M.; Stout, T.A.; Gadella, B.M.; Tapia, J.A.; da Silva, C.B.; Ferrusola, C.O.; Peña, F.J. Mitochondrial ATP is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction 2016, 152, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Mullarky, E.; Cantley, L.C. Diverting glycolysis to combat oxidative stress. In Innovative Medicine, 1st ed.; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–23. [Google Scholar]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and anticarcinogenic activities of tea polyphenols. Arch. Toxicol. 2009, 83, 11–21. [Google Scholar] [CrossRef]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Purdy, P.H.; Ericsson, S.A.; Dodson, R.E.; Sternes, K.L.; Garner, D.L. Effects of flavonoids, silibinin and catechin, on the motility of extended cooled caprine sperm. Small Rumin. Res. 2004, 55, 239–243. [Google Scholar] [CrossRef]
- Boonsorn, T.; Kongbuntad, W.; Narkkong, N.; Aengwanich, W. Effects of catechin addition to extender on sperm quality and lipid peroxidation in boar semen. Am. Eurasian J. Sustain Agric. 2010, 7, 283–288. [Google Scholar]
- Jansen, R.P.S.; Burton, G.J. Mitochondrial dysfunction in reproduction. Mitochondrion 2004, 4, 577–600. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Fiducia, M.; Lunghi, R.; Marchetti, L.; Palumbo, A.; Rizzo, F.; Koverech, A.; Lenzi, A.; Gandini, L. Effects of a dietary supplement on chronic pelvic pain syndrome (Category IIIA), leukocytospermia and semen parameters. Andrologia 2012, 44, 672–678. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Wittayarat, M.; Ito, A.; Kimura, T.; Namula, Z.; Luu, V.V.; Do, L.T.K.; Otoi, T. Effects of green tea polyphenol on the quality of canine semen after long-term storage at 5 °C. Reprod. Biol. 2013, 13, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Uekusa, Y.; Kamihira, M.; Nakayama, T. Dynamic behavior of tea catechins interacting with lipid membranes as determined by NMR spectroscopy. J. Agric. Food Chem. 2007, 55, 9986–9992. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Hayashi, F. Factors affecting sperm quality before and after mating of calopterygid damselflies. PLoS ONE 2010, 5, e9904. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gale, I.; Gil, L.; Malo, C.; González, N.; Martínez, F. Effect of Camellia sinensis supplementation and increasing holding time on quality of cryopreserved boar semen. Andrologia 2015, 47, 505–512. [Google Scholar] [CrossRef]
- Sapanidou, V.G.; Margaritis, I.; Siahos, N.; Arsenopoulos, K.; Dragatidou, E.; Taitzoglou, I.A.; Zervos, I.A.; Theodoridis, A.; Tsantarliotou, M.P. Antioxidant effect of a polyphenol-rich grape pomace extract on motility, viability and lipid peroxidation of thawed bovine spermatozoa. J. Biol. Res. (Thessalon.) 2014, 3, 19. [Google Scholar] [CrossRef]
- Kim, J.E.; Shin, M.H.; Chung, J.H. Epigallocatechin-3-gallate prevents heat shock-induced MMP-1 expression by inhibiting AP-1 activity in human dermal fibroblasts. Arch. Dermatol. Res. 2013, 305, 595–602. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Oh, S.; Kim, S.W.; Kim, J.-W.; Kim, Y.S.; Woo, J.-T. Prooxidative effects of green tea polyphenol (−)-epigallocatethin-3-gallate on the HIT-T15 pancreatic beta cell line. Cell Biol. Toxicol. 2010, 26, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Hou, Z.; Lambert, J.D.; Yang, C.S. Redox properties of tea polyphenols and related biological activities. Antioxid. Redox Signal. 2005, 7, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Greifová, H.; Mackovich, A.; Hashim, F.; Lukáč, N. Curcumin offers antioxidant protection to cryopreserved bovine semen. Czech J. Anim. Sci. 2018, 63, 247–255. [Google Scholar] [CrossRef]
- Gosálvez, J.; López-Fernández, C.; Fernández, J.L.; Gouraud, A.; Holt, W.V. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev. 2011, 78, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvrda, E.; Straka, P.; Galbavy, D.; Ivanic, P. Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules 2019, 24, 3226. https://doi.org/10.3390/molecules24183226
Tvrda E, Straka P, Galbavy D, Ivanic P. Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules. 2019; 24(18):3226. https://doi.org/10.3390/molecules24183226
Chicago/Turabian StyleTvrda, Eva, Peter Straka, Drahomir Galbavy, and Peter Ivanic. 2019. "Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress" Molecules 24, no. 18: 3226. https://doi.org/10.3390/molecules24183226
APA StyleTvrda, E., Straka, P., Galbavy, D., & Ivanic, P. (2019). Epicatechin Provides Antioxidant Protection to Bovine Spermatozoa Subjected to Induced Oxidative Stress. Molecules, 24(18), 3226. https://doi.org/10.3390/molecules24183226





