Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biocatalyst Characterization
2.2. Stability in the Presence of Solvents
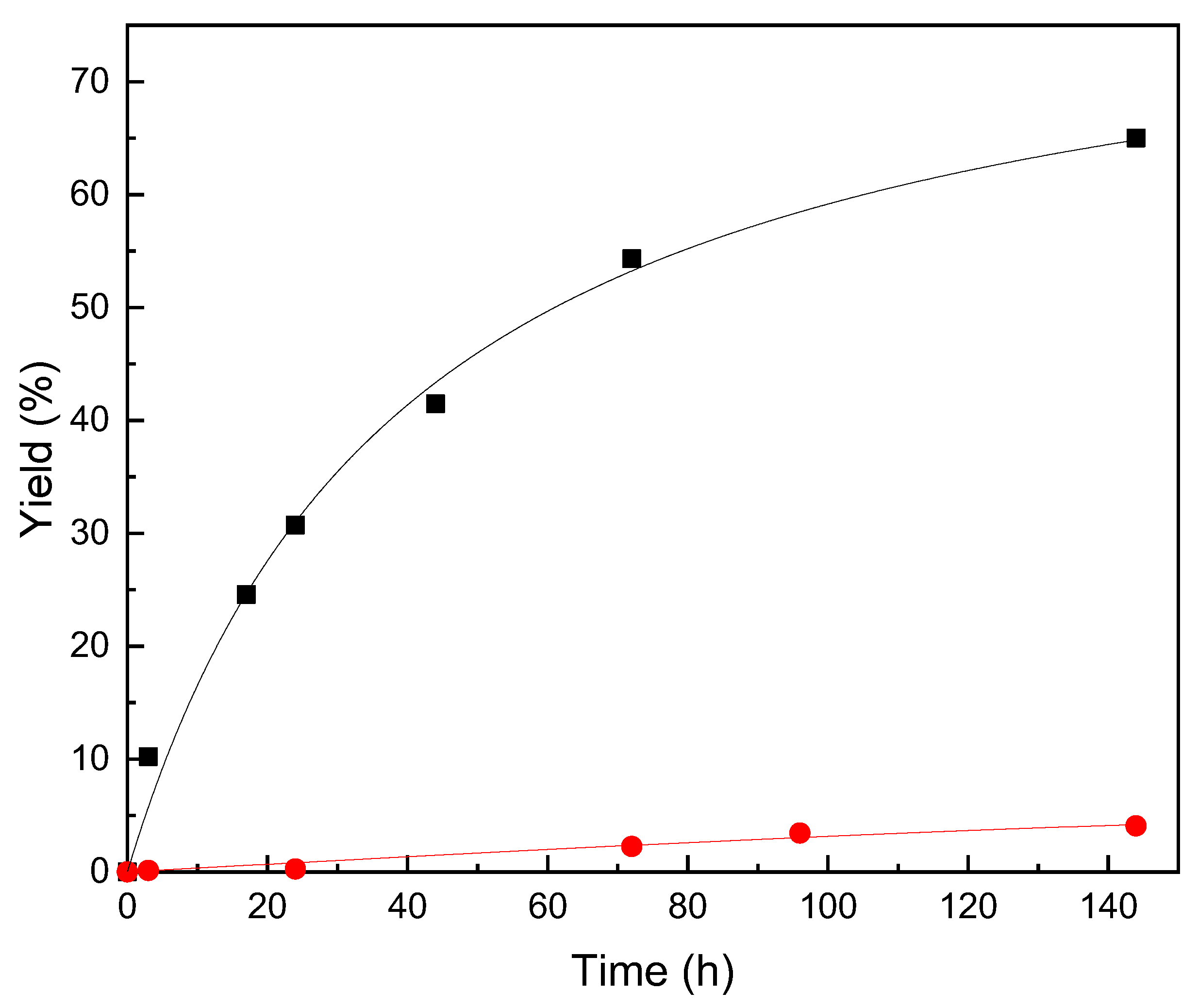
2.3. Synthesis of Ascorbyl Palmitate under Standard Conditions
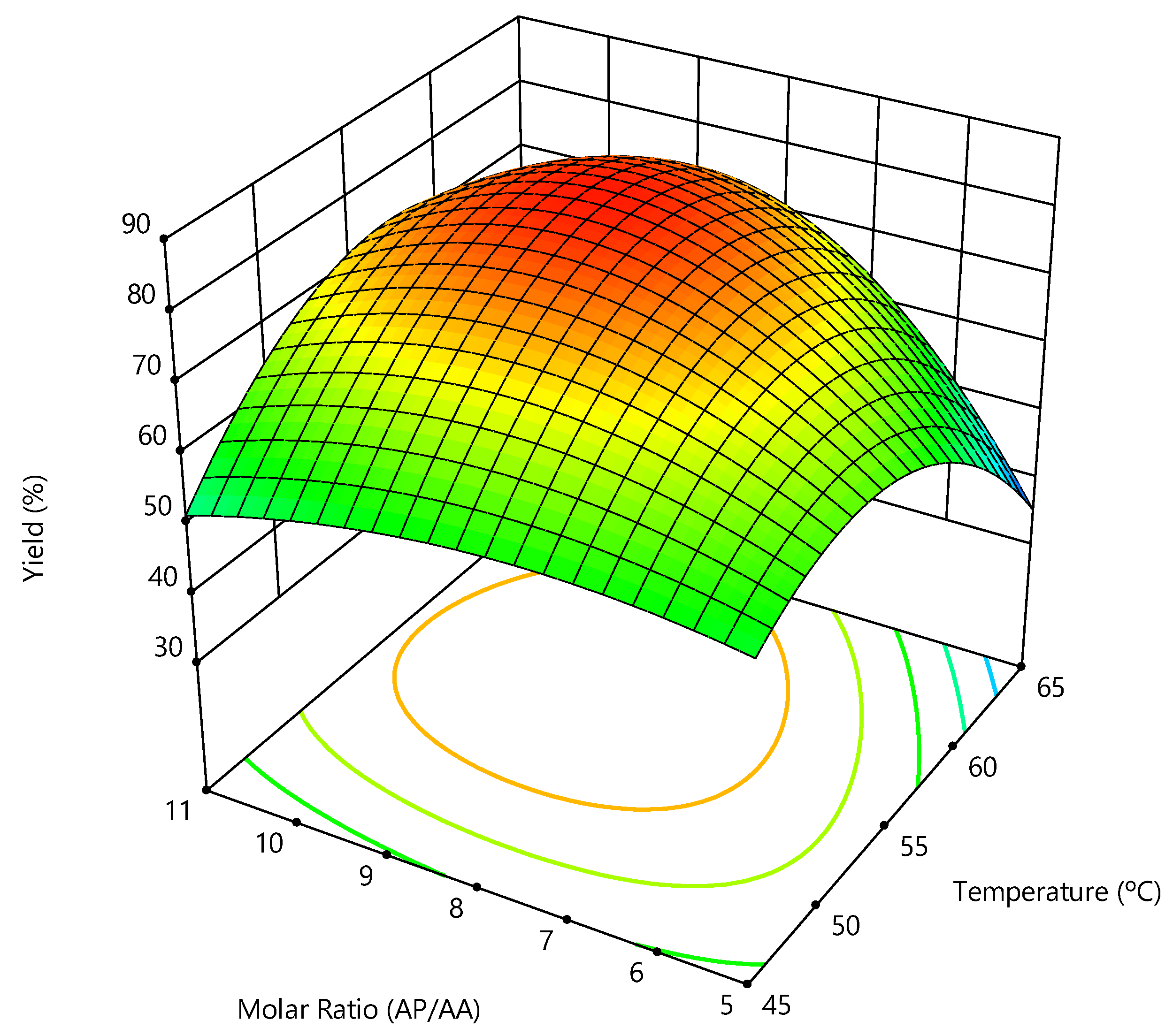
2.4. Effect of Reaction Conditions on Ascorbyl Palmitate Synthesis
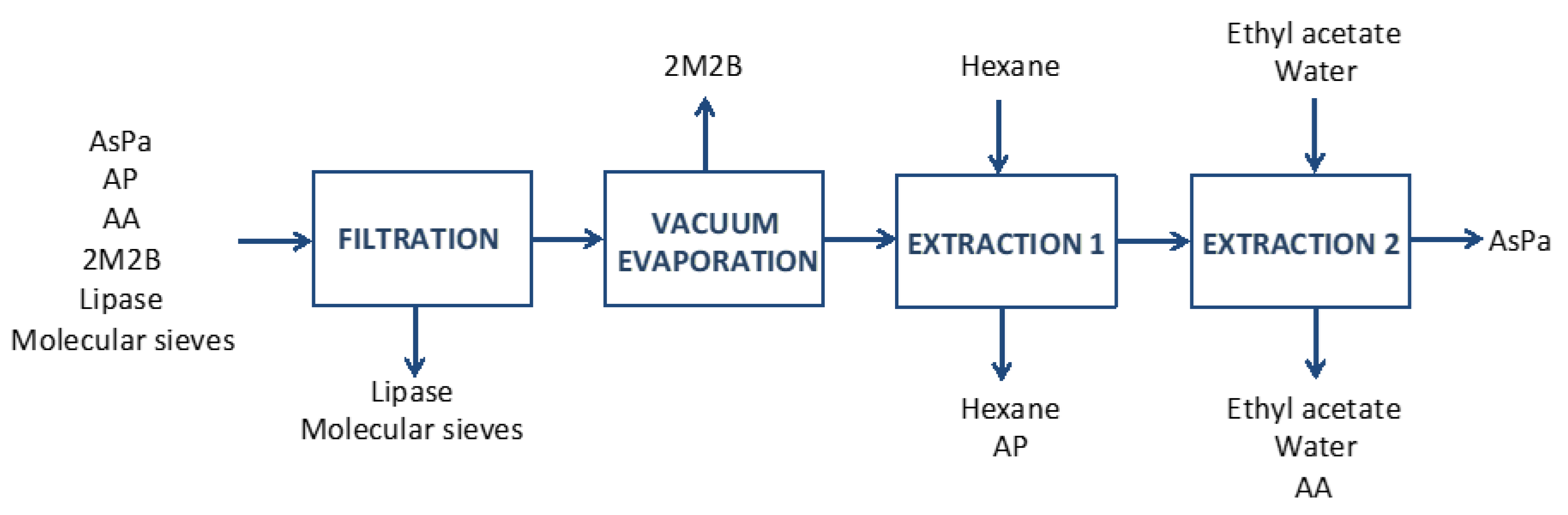
2.5. Downstream Processing
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Functionalization of Silica
3.3. Enzymatic Activity Assay
3.4. Rate of Reaction of Ascorbyl Palmitate Synthesis
3.5. Immobilization of Lipase on Silica Support
3.6. Stability in the Presence of Solvents
3.7. Ascorbyl Palmitate Synthesis
3.8. High-Performance Liquid Chromatography (HPLC) Analysis of the Reaction Products
3.9. Experimental Design
3.10. Downstream Processing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Karmee, S.K. Biocatalytic synthesis of ascorbyl esters and their biotechnological applications. Appl. Microbiol. Biotechnol. 2009, 81, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-tert-butyl-hydroxytoluene and its metabolites in foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of ascorbyl palmitate, ascorbyl dipalmitate, ascorbyl stearate, erythorbic acid, and sodium erythorbate. Int. J. Toxicol. 1999, 18, 1–26. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Tullio, M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta - Gen. Subj. 2002, 1569, 1–9. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Sandoval, G.; Plou, F.; Valero, F. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- Lerin, L.A.; Richetti, A.; Dallago, R.; Treichel, H.; Mazutti, M.A.; Oliveira, J.V.; Antunes, O.A.C.; Oestreicher, E.G.; de Oliveira, D. Enzymatic synthesis of ascorbyl palmitate in organic solvents: Process optimization and kinetic evaluation. Food Bioprocess Technol. 2012, 5, 1068–1076. [Google Scholar] [CrossRef]
- Viklund, F.; Alander, J.; Hult, K. Antioxidative properties and enzymatic synthesis of ascorbyl FA esters. J. Am. Oil Chem. Soc. 2003, 80, 795–799. [Google Scholar] [CrossRef]
- Ji, J.M. Chemical synthesis of ascorbyl palmitate in [BMIM]BF4. Adv. Mater. Res. 2011, 236–238, 1962–1965. [Google Scholar] [CrossRef]
- Humeau, C.; Girardin, M.; Coulon, D.; Miclo, A. Synthesis of 6-O-palmitoyl L-ascorbic acid catalyzed by Candida antartica lipase. Biotechnol. Lett. 1995, 17, 1091–1094. [Google Scholar] [CrossRef]
- Halling, P.J. Thermodynamic predictions for biocatalysis in nonconventional media: Theory, tests, and recommendations for experimental design and analysis. Enzym. Microb. Technol. 1994, 16, 178–206. [Google Scholar] [CrossRef]
- Marty, A.; Chulalaksananukul, W.; Willemot, R.M.; Condoret, J.S. Kinetics of lipase-catalyzed esterification in supercritical CO2. Biotechnol. Bioeng. 1992, 39, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wehtje, E.; Kaur, J.; Adlercreutz, P.; Chand, S.; Mattiasson, B. Water activity control in enzymatic esterification processes. Enzym. Microb. Technol. 1997, 21, 502–510. [Google Scholar] [CrossRef]
- Paludo, N.; Alves, J.S.; Altmann, C.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase. Ultrason. Sonochem. 2015, 22, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Dossat, V.; Combes, D.; Marty, A. Continuous enzymatic transesterification of high oleic sunflower oil in a packed bed reactor: Influence of the glycerol production. Enzym. Microb. Technol. 1999, 25, 194–200. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B: Enzym. 2002, 19, 279–286. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Bradoo, S.; Saxena, R.K.; Gupta, R. High yields of ascorbyl palmitate by thermostable lipase-mediated esterification. J. Am. Oil Chem. Soc. 1999, 76, 1291–1295. [Google Scholar] [CrossRef]
- Santibáñez, L.; Wilson, L.; Illanes, A. Synthesis of ascorbyl palmitate with immobilized lipase from Pseudomonas stutzeri. J. Am. Oil Chem. Soc. 2014, 91, 405–410. [Google Scholar] [CrossRef]
- Rios, N.S.; Pinheiro, B.B.; Pinheiro, M.P.; Bezerra, R.M.; dos Santos, J.C.S.; Barros Gonçalves, L.R. Biotechnological potential of lipases from Pseudomonas: Sources, properties and applications. Process Biochem. 2018, 75, 99–120. [Google Scholar] [CrossRef]
- Sharma, S.; Kanwar, K.; Kanwar, S.S. Ascorbyl palmitate synthesis in an organic solvent system using a Celite-immobilized commercial lipase (Lipolase 100L). 3 Biotech 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.G.; Kavadia, M.R.; Vadgama, R.N.; Odaneth, A.A.; Lali, A.M. Production of 6-O-L-ascorbyl palmitate by immobilized Candida antarctica lipase B. Appl. Biochem. Biotechnol. 2018, 184, 1168–1186. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bornscheuer, U.T.; Schmid, R.D. Lipase-catalyzed synthesis of vitamin C fatty acid esters. Biotechnol. Lett. 1999, 21, 1051–1054. [Google Scholar] [CrossRef]
- Bezbradica, D.; Stojanović, M.; Veličković, D.; Dimitrijević, A.; Carević, M.; Mihailović, M.; Milosavić, N. Kinetic model of lipase-catalyzed conversion of ascorbic acid and oleic acid to liposoluble vitamin C ester. Biochem. Eng. J. 2013, 71, 89–96. [Google Scholar] [CrossRef]
- Stojanovic, M.; Carevic, M.; Mihailovic, M.; Veličkovic, D.; Dimitrijevic, A.; Milosavic, N.; Bezbradica, D. Influence of fatty acid on lipase-catalyzed synthesis of ascorbyl esters and their free radical scavenging capacity. Biotechnol. Appl. Biochem. 2015, 62, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, J.; Lv, F.; Ye, R.; Bie, X.; Zhang, C.; Lu, Z. Enzymatic synthesis of lard-based ascorbyl esters in a packed-bed reactor: Optimization by response surface methodology and evaluation of antioxidant properties. Lwt - Food Sci. Technol. 2014, 57, 393–399. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, D.E.; Sung, H.; Lee, J.; Chang, P.S. Lipase-catalysed synthesis of erythorbyl laurate in acetonitrile. Food Chem. 2011, 129, 59–63. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Improvement of efficiency in the enzymatic synthesis of lactulose palmitate. J. Agric. Food Chem. 2015, 63, 3716–3724. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quilles, J.C.J.; Brito, R.R.; Borges, J.P.; Aragon, C.C.; Fernandez-Lorente, G.; Bocchini-Martins, D.A.; Gomes, E.; da Silva, R.; Boscolo, M.; Guisan, J.M. Modulation of the activity and selectivity of the immobilized lipases by surfactants and solvents. Biochem. Eng. J. 2015, 93, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. Rsc Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef] [Green Version]
- Saunders, P.; Brask, J. Improved immobilization supports for Candida antarctica lipase B. In Biocatalysis in Polymer Chemistry; Wiley-VCH: Weinheim, Germany, 2010; pp. 65–82. [Google Scholar]
- Poojari, Y.; Clarson, S.J. Thermal stability of Candida antarctica lipase B immobilized on macroporous acrylic resin particles in organic media. Biocatal. Agric. Biotechnol. 2013, 2, 7–11. [Google Scholar] [CrossRef]
- Arroyo, M.; Sánchez-Montero, J.M.; Sinisterra, J.V. Thermal stabilization of immobilized lipase B from Candida antarctica on different supports: Effect of water activity on enzymatic activity in organic media. Enzym. Microb. Technol. 1999, 24, 3–12. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Fredes, Y.; Chamorro, L.; Cabrera, Z. Increased selectivity of Novozym 435 in the asymmetric hydrolysis of a substrate with high hydrophobicity through the use of deep eutectic solvents and high substrate concentrations. Molecules 2019, 24, 792. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Hu, Y.; Jiang, L.; Zou, B.; Song, P.; Huang, H. Optimization of enzymatic synthesis of L-ascorbyl palmitate by solvent engineering and statistical experimental designs. Biotechnol. Bioprocess Eng. 2013, 18, 350–357. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef]
- Vescovi, V.; Giordano, R.L.C.; Mendes, A.A.; Tardioli, P.W. Immobilized lipases on functionalized silica particles as potential biocatalysts for the synthesis of fructose oleate in an organic solvent/water system. Molecules 2017, 22, 212. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Mesa, M.; Sierra, L. Synthesis of new silicas with high stable and large mesopores and macropores for biocatalysis applications. Mater. Sci. Eng. C 2012, 32, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Biocatalyst | Hydrolytic Activity | Residual Activity (A/A0) 1 | ||
|---|---|---|---|---|
| IU/gbiocatalyst | Acetone | Acetonitrile | 2M2B | |
| PS-octyl-silica | 70.44 | 0.84 | 2.24 | 0.36 |
| Novozym 435 | 34.61 | 0.20 | 0.24 | 0.82 |
| Biocatalysts | Time | Initial Rate | Yield | Productivity |
|---|---|---|---|---|
| (h) | (μmol × min−1 × mL−1) | (%) | (mgAsPa × gbiocat−1 × h−1) | |
| Novozym 435 | 144 | 411.0 | 65 | 13.6 |
| PS octyl-silica | 144 | 3.7 | 4.1 | 0.9 |
| Experiment | Temperature (°C) | AA:PA Molar Ratio | Yield (%) | Productivity (mgAsPa × gcat−1 × h−1) |
|---|---|---|---|---|
| 1 | 55 | 1:8 | 81 ± 2 | 16.8 |
| 2 | 45 | 1:5 | 65 ± 2 | 13.6 |
| 3 | 45 | 1:11 | 60 ± 2 | 12.5 |
| 4 | 65 | 1:5 | 43 ± 2 | 9.1 |
| 5 | 65 | 1:11 | 71 ± 2 | 14.7 |
| 6 | 41 | 1:8 | 33 ± 2 | 6.9 |
| 7 | 69 | 1:8 | 51 ± 2 | 10.7 |
| 8 | 55 | 1:3.8 | 37 ± 0 | 7.7 |
| 9 | 55 | 1:12.2 | 53 ± 0 | 11.0 |
| Component | Vacuum Evaporation | Extraction 1 | Extraction 2 |
|---|---|---|---|
| AsPa (g) | 1.39 | 1.3 | 1.17 |
| PA (g) | 10.4 | 0.09 | – |
| AA (g) | 0.5 | 0.5 | 0.07 |
| 2M2B (g) | – | – | – |
| Total (g) | 12.2 | 1.9 | 1.2 |
| Recovery Yield (%) | 100 | 93.5 | 90.0 |
| Purity (%) | 11.4 | 68.4 | 97.5 |
| Variable | Levels | ||||
|---|---|---|---|---|---|
| −1.41 | −1 | 0 | 1 | 1.41 | |
| Temperature (°C) | 41 | 45 | 55 | 65 | 69 |
| Molar ratio AA:PA | 1:3.8 | 1:5 | 1:8 | 1:11 | 1:12.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufiño, C.; Bernal, C.; Ottone, C.; Romero, O.; Illanes, A.; Wilson, L. Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules 2019, 24, 3227. https://doi.org/10.3390/molecules24183227
Tufiño C, Bernal C, Ottone C, Romero O, Illanes A, Wilson L. Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules. 2019; 24(18):3227. https://doi.org/10.3390/molecules24183227
Chicago/Turabian StyleTufiño, Carolina, Claudia Bernal, Carminna Ottone, Oscar Romero, Andrés Illanes, and Lorena Wilson. 2019. "Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate" Molecules 24, no. 18: 3227. https://doi.org/10.3390/molecules24183227






