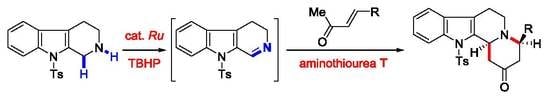
Oxidative Asymmetric Formal Aza-Diels–Alder Reactions of Tetrahydro-β-carboline with Enones in the Synthesis of Indoloquinolizidine-2-ones
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rowland, G.; Rowland, E.; Zhang, Q.; Antilla, J. Stereoselective aza-Diels–Alder reactions. Curr. Org. Chem. 2006, 10, 981–1005. [Google Scholar] [CrossRef]
- Girling, P.R.; Kiyoi, T.; Whiting, A. Mannich-Michael versus formal aza-Diels–Alder approaches to piperidine derivatives. Org. Biomol. Chem. 2011, 9, 3105–3121. [Google Scholar] [CrossRef] [PubMed]
- Memeo, M.G.; Quadrelli, P. Iminium ions as dienophiles in aza-Diels–Alder reactions: A closer look. Chem. Eur. J. 2012, 18, 12554–12582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, R. Recent developments in catalytic asymmetric inverse-electron-demand Diels–Alder reaction. Chem. Rev. 2013, 113, 5515–5546. [Google Scholar] [CrossRef] [PubMed]
- Masson, G.; Lalli, C.; Benohoud, M.; Dagousset, G. Catalytic enantioselective [4 + 2]-cycloaddition: A strategy to access aza-hexacycles. Chem. Soc. Rev. 2013, 42, 902–923. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Fochi, M.; Caruana, L. Catalytic Asymmetric Aza-Diels–Alder Reactions: The Povarov Cycloaddition Reaction. Synthesis 2013, 46, 135–157. [Google Scholar]
- Sarmah, M.; Prajapati, D. Aza-Diels–Alder reaction: An efficient approach for construction of heterocycles. Curr. Org. Chem. 2014, 18, 1586–1620. [Google Scholar] [CrossRef]
- Wojaczynska, E.; Wojaczynski, J.; Kleniewska, K.; Dorsz, M.; Olszewski, T.K. 2-Azanorbornane—A versatile chiral aza-Diels–Alder cycloadduct: Preparation, applications in stereoselective synthesis and biological activity. Org. Biomol. Chem. 2015, 13, 6116–6148. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.H.; Green, N.J.; Xu, S.Z. Application of the aza-Diels–Alder reaction in the synthesis of natural products. Org. Biomol. Chem. 2017, 15, 3105–3129. [Google Scholar] [CrossRef] [PubMed]
- Stockigt, J.; Antonchick, A.P.; Wu, F.; Waldmann, H. The Pictet-Spengler reaction in nature and in organic chemistry. Angew. Chem. Int. Ed. 2011, 50, 8538–8564. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, M.; Rozwadowska, M.D. Asymmetric synthesis of isoquinoline alkaloids. Chem. Rev. 2004, 104, 3341–3370. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.D.; Cook, J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Cordell, G. The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: New York, NY, USA, 2008; Volume 65, ISBN 978-0-12-374296-4. [Google Scholar]
- Baxter, E.W.; Mariano, P.S. Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Springer: New York, NY, USA, 1992; Volume 8, pp. 197–319. [Google Scholar]
- Li, G.; Nakamura, H. Synthesis of 2-indolyltetrahydroquinolines by zinc(II)-catalyzed intramolecular hydroarylation-redox cross-dehydrogenative coupling of N-propargylanilines with indoles. Angew. Chem. Int. Ed. 2016, 55, 6757–6760. [Google Scholar]
- Itoh, T.; Yokoya, M.; Miyauchi, K.; Nagata, K.; Ohsawa, A. Total synthesis of ent-dihydrocorynantheol by using a proline-catalyzed asymmetric addition reaction. Org. Lett. 2006, 8, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Yokoya, M.; Miyauchi, K.; Nagata, K.; Ohsawa, A. Proline-catalyzed asymmetric addition reaction of 9-tosyl-3,4-dihydro-beta-carboline with ketones. Org. Lett. 2003, 5, 4301–4304. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.P.; McGowan, M.A.; Rajapaksa, N.S.; Jacobsen, E.N. Enantioselective formal aza-Diels–Alder reactions of enones with cyclic imines catalyzed by primary aminothioureas. J. Am. Chem. Soc. 2013, 135, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksa, N.S.; McGowan, M.A.; Rienzo, M.; Jacobsen, E.N. Enantioselective total synthesis of (+)-reserpine. Org. Lett. 2013, 15, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Sanchawala, A.; Seidel, D. Dual C–H functionalization of N-aryl amines: Synthesis of polycyclic amines via an oxidative Povarov approach. Org. Lett. 2014, 16, 2756–2759. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Li, D.; Li, W.; Yu, W.; Bian, F. The reaction of tertiary anilines with maleimides under visible light redox catalysis. Adv. Synth. Catal. 2012, 354, 3561–3567. [Google Scholar] [CrossRef]
- Nishino, M.; Hirano, K.; Satoh, T.; Miura, M. Copper-catalyzed oxidative direct cyclization of N-methylanilines with electron-deficient alkenes using molecular oxygen. J. Org. Chem. 2011, 76, 6447–6451. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, X.; Zhang, Y. CuBr-catalyzed reaction of N,N-dimethylanilines and silyl enol ethers: An alternative route to beta-arylamino ketones. Org. Lett. 2009, 11, 3730–3733. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, D.-F.; Chen, S.-S.; Zhu, Y.-F. Synthesis of polycyclic amines through mild metal-free tandem cross-dehydrogenative coupling/intramolecular hydroarylation of N-aryltetrahydroisoquinolines and crotonaldehyde. Eur. J. Org. Chem. 2015, 2015, 468–473. [Google Scholar] [CrossRef]
- Tang, J.; Grampp, G.; Liu, Y.; Wang, B.X.; Tao, F.F.; Wang, L.J.; Liang, X.Z.; Xiao, H.Q.; Shen, Y.M. Visible light mediated cyclization of tertiary anilines with maleimides using nickel(II) oxide surface-modified titanium dioxide catalyst. J. Org. Chem. 2015, 80, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Kawade, R.K.; Huple, D.B.; Lin, R.J.; Liu, R.S. Cu-catalyzed oxidative Povarov reactions between N-alkyl N-methylanilines and saturated oxa- and thiacycles. Chem. Commun. 2015, 51, 6625–6628. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Q.; Li, C.G.; Liu, M.Q.; Cao, J.; Luo, Y.C.; Xu, P.F. Dual C–H functionalization of N-aryl tetrahydroisoquinolines: A highly diastereoselective synthesis of dibenzo[a,f]quinolizines via visible-light induced oxidation and inverse electron-demand aza-Diels–Alder reaction. Chem. Commun. 2016, 52, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Matsumoto, S.; Ogiwara, Y. Cobalt-catalyzed oxidative annulation of aromatic tertiary amines with electron-deficient maleimides leading to tetrahydroquinoline derivatives. Tetrahedron Lett. 2016, 57, 5449–5452. [Google Scholar] [CrossRef]
- Huo, C.; Chen, F.; Quan, Z.; Dong, J.; Wang, Y. Cobalt-catalyzed aerobic oxidative Povarov reaction of tertiary anilines with dihydrofuran for the synthesis of hexahydrofuroquinolines. Tetrahedron Lett. 2016, 57, 5127–5131. [Google Scholar] [CrossRef]
- Zhao, M.-N.; Yu, L.; Hui, R.-R.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Iron-catalyzed dehydrogenative [4 + 2] cycloaddition of tertiary anilines and enamides for the synthesis of tetrahydroquinolines with amido-substituted quaternary carbon centers. ACS Catal. 2016, 6, 3473–3477. [Google Scholar] [CrossRef]
- Nicholls, T.P.; Constable, G.E.; Robertson, J.C.; Gardiner, M.G.; Bissember, A.C. Brønsted acid cocatalysis in copper(I)-photocatalyzed α-amino C–H bond functionalization. ACS Catal. 2015, 6, 451–457. [Google Scholar] [CrossRef]
- Yadav, A.K.; Yadav, L.D.S. Visible light photoredox catalysis with N-hydroxyphthalimide for [4 + 2] cyclization between N-methylanilines and maleimides. Tetrahedron Lett. 2017, 58, 552–555. [Google Scholar] [CrossRef]
- Guo, J.T.; Yang, D.C.; Guan, Z.; He, Y.H. Chlorophyll-catalyzed visible-light-mediated synthesis of tetrahydroquinolines from N,N-dimethylanilines and maleimides. J. Org. Chem. 2017, 82, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Guo, J.D.; Lei, T.; Chen, B.; Tung, C.H.; Wu, L.Z. Oxidative cyclization synthesis of tetrahydroquinolines and reductive hydrogenation of maleimides under redox-neutral conditions. Org. Lett. 2018, 20, 2916–2920. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Peng, X.X.; Chen, F.; Han, B. TEMPO-mediated aza-Diels–Alder reaction: Synthesis of tetrahydropyridazines using ketohydrazones and olefins. Org. Lett. 2016, 18, 2070–2073. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, H.-J.; Li, F.; Zhu, C.-F.; Luo, Y.-F.; Wu, X.; Kantchev, E.A.B. Ruthenium-catalyzed oxidative formal aza-Diels–Alder reaction: Enantioselective synthesis of benzo[a]quinolizine-2-ones. Adv. Synth. Catal. 2017, 359, 3095–3101. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative coupling between two hydrocarbons: An update of recent C–H functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Hartwig, J.F. Catalytic silylation of unactivated C–H bonds. Chem. Rev. 2015, 115, 8946–8975. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.-I.; Komiya, N. Ruthenium-catalyzed oxidation for organic synthesis. In Modern Oxidation Methods, 2nd ed.; Bäeckvall, J.-E., Ed.; Wiley-VCH: Weinheim, Germany, 2010; p. 241. [Google Scholar]
- Ishizuka, T.; Kotani, H.; Kojima, T. Characteristics and reactivity of ruthenium-oxo complexes. Dalton. Trans. 2016, 45, 16727–16750. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Zhang, D. Ruthenium catalyzed biomimetic oxidation in organic synthesis inspired by cytochrome P-450. Chem. Soc. Rev. 2008, 37, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Viviano, M.; Glasnov, T.N.; Reichart, B.; Tekautz, G.; Kappe, C.O. A scalable two-step continuous flow synthesis of nabumetone and related 4-aryl-2-butanones. Org. Process Res. Dev. 2011, 15, 858–870. [Google Scholar] [CrossRef]
- Cochrane, E.J.; Hassall, L.A.; Coldham, I. Preparation of 1-substituted tetrahydro-beta-carbolines by lithiation-substitution. J. Org. Chem. 2015, 80, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.C.; Lennox, W.J.; Stock, J.R.; Ellingboe, J.W.; Mazandarani, H.; Smith, D.L.; Zhang, G.; Tawa, G.J.; Schechter, L.E. Conformationally constrained N1-arylsulfonyltryptamine derivatives as 5-HT6 receptor antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 4780–4785. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 6 and 7 are available from the authors. |

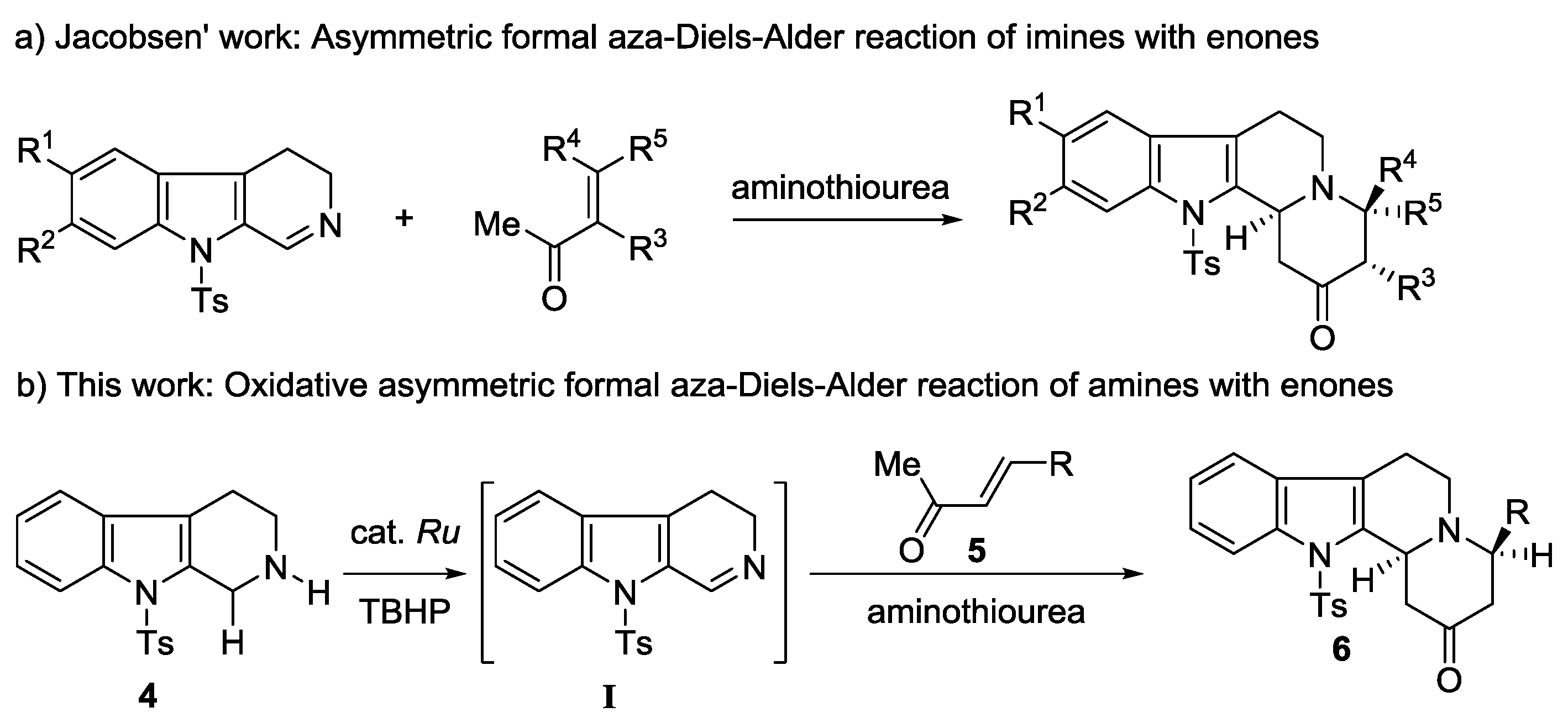


| Entry | Metal Salt | Acid (mol %) | Oxidant | Solvent | Yield ** (%) | dr *** (6aa:7aa) | ee **** (%) (6aa/7aa) |
|---|---|---|---|---|---|---|---|
| 1 | – | CH3COOH (30) | TBHP | toluene | – | – | – |
| 2 | Cu(OAc)2 | CH3COOH (30) | TBHP | toluene | 22 | 1.8:1 | 15/14 |
| 3 | RuCl3·xH2O | CH3COOH (30) | TBHP | toluene | 44 | 3.4:1 | 27/35 |
| 4 | [RuCl2(p-cymene)]2 | CH3COOH (30) | TBHP | toluene | 50 | 1.8:1 | 60/59 |
| 5 | RuCl2(PPh3)3 | CH3COOH (30) | TBHP | toluene | 37 | 1.3:1 | 69/66 |
| 6 | RuCl2(PPh3)3 | CH3COOH (15) | TBHP | toluene | 40 | 2:1 | 87/83 |
| 7 | RuCl2(PPh3)3 | PhCOOH (15) | TBHP | toluene | 32 | 1:1 | 79/76 |
| 8 | RuCl2(PPh3)3 | o-F-PhCOOH (15) | TBHP | toluene | 30 | 2.8:1 | 71/71 |
| 9 | RuCl2(PPh3)3 | – | TBHP | toluene | 24 | 2.2:1 | 51/51 |
| 10 | RuCl2(PPh3)3 | CH3COOH (15) | PhI=O | toluene | – | – | – |
| 11 | RuCl2(PPh3)3 | CH3COOH (15) | DDQ | toluene | – | – | – |
| 12 | RuCl2(PPh3)3 | CH3COOH (15) | H2O2 | toluene | trace | – | – |
| 13 | RuCl2(PPh3)3 | CH3COOH (15) | TBHP | THF | 17 | 1.8:1 | 89/89 |
| 14 | RuCl2(PPh3)3 | CH3COOH (15) | TBHP | CHCl3 | 7 | 6:1 | 64/70 |
| 15 | RuCl2(PPh3)3 | CH3COOH (15) | TBHP | CH3CN | 11 | 10:1 | 75/72 |
| 16 | RuCl2(PPh3)3 | CH3COOH (15) | TBHP | CH2Cl2 | 21 | 8:1 | 70/70 |
| 17 ^ | RuCl2(PPh3)3 | CH3COOH (15) | TBHP | toluene | 73 | 1.8:1 | 94/90 |

| Entry | 5 | R | t (h) | 6 | Yield ** (%) | dr *** (6:7) | ee **** (%) (6/7) |
|---|---|---|---|---|---|---|---|
| 1 | 5b | C6H5 | 48 | 6ab | 31 | 1:1 | 96/91 |
| 2 | 5c | o-MeC6H4 | 72 | 6ac | 36 | 1.8:1 | 82/95 |
| 3 | 5d | m-MeC6H4 | 72 | 6ad | 41 | 1:1 | 88/92 |
| 4 | 5e | p-MeC6H4 | 72 | 6ae | 61 | 1.5:1 | 94/96 |
| 5 | 5f | p-ClC6H4 | 72 | 6af | 34 | 1:1 | 93/91 |
| 6 | 5g | p-BrC6H4 | 48 | 6ag | 65 | 1.2:1 | 85/87 |
| 7 | 5h | p-CF3C6H4 | 36 | 6ah | 35 | 1.6:1 | 86/93 |
| 8 | 5i | m-NO2C6H4 | 72 | 6ai | 37 | 1:1 | 94/85 |
| 9 | 5j | C6F5 | 48 | 6aj | 57 | >10:1 | 86 |
| 10 | 5k | 2,3-Cl2C6H3 | 72 | 6ak | 45 | >10:1 | 92 |
| 11 | 5l | 2,4-(NO2)2C6H3 | 72 | 6al | 45 | >10:1 | 85 |
| 12 | 5m | 2-thienyl | 60 | 6am | 48 | 1.3:1 | 89/93 |
| 13 | 5n | 2-furyl | 48 | 6an | 42 | 1:1 | 96/95 |
| 14 | 5o | CH=CMe2 | 96 | 6ao | 24 | >10:1 | 85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhao, S.-B.; Zheng, L.-L.; Li, Y.-G. Oxidative Asymmetric Formal Aza-Diels–Alder Reactions of Tetrahydro-β-carboline with Enones in the Synthesis of Indoloquinolizidine-2-ones. Molecules 2018, 23, 2228. https://doi.org/10.3390/molecules23092228
Wu X, Zhao S-B, Zheng L-L, Li Y-G. Oxidative Asymmetric Formal Aza-Diels–Alder Reactions of Tetrahydro-β-carboline with Enones in the Synthesis of Indoloquinolizidine-2-ones. Molecules. 2018; 23(9):2228. https://doi.org/10.3390/molecules23092228
Chicago/Turabian StyleWu, Xiang, Shi-Bao Zhao, Lang-Lang Zheng, and You-Gui Li. 2018. "Oxidative Asymmetric Formal Aza-Diels–Alder Reactions of Tetrahydro-β-carboline with Enones in the Synthesis of Indoloquinolizidine-2-ones" Molecules 23, no. 9: 2228. https://doi.org/10.3390/molecules23092228