Heck Transformations of Biological Compounds Catalyzed by Phosphine-Free Palladium
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reactants
3.2. General Procedures
3.2.1. Heck Reaction (Method A)
3.2.2. Heck-Type Reaction (Method B)
3.2.3. Heck-Type Reaction (Method C)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Dounay, A.B.; Overman, L.E. The Asymmetric Intramolecular Heck Reaction in Natural Product Total Synthesis. Chem. Rev. 2003, 103, 2945–2964. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-Catalyzed Cross−Coupling Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent Applications of Palladium−Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- Tarnowicz, S.; Alsalahi, W.; Mieczynska, E.; Trzeciak, A.M. Heck arylation of allyl alcohol catalyzed by Pd(0) nanoparticles. Tetrahedron 2017, 73, 5605–5612. [Google Scholar] [CrossRef]
- Heravi, M.M.; Fazeli, A. Recent Advances in the Application of the Heck Reaction in the Synthesis of Heterocyclic Compounds. Heterocycles 2010, 81, 1979–2026. [Google Scholar] [CrossRef]
- Mazuela, J.; Tolstoy, P.; Pamies, O.; Andersson, P.G.; Dieguez, M. Phosphite-oxazole/imidazole ligands in asymmetric intermolecular Heck reaction. Org. Biomol. Chem. 2011, 9, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Enquist, P.-A.; Lindh, J.; Larhed, M. Open-air oxidative Heck reactions at room temperature. Green Chem. 2006, 8, 338–343. [Google Scholar] [CrossRef]
- Du, X.L.; Suguro, M.; Hirabayashi, K.; Mori, A.; Nishikata, T.; Hagiwara, N.; Kawata, K.; Okeda, T.; Wang, H.F.; Fugami, K.; et al. Mizoroki−Heck Type Reaction of Organoboron Reagents with Alkenes and Alkynes. A Pd(II)-Catalyzed Pathway with Cu(OAc)2 as an Oxidant. Org. Lett. 2001, 3, 3313–3316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Park, C.-M. Stereoselective Synthesis of Highly Substituted Enamides by an Oxidative Heck Reaction. Angew. Chem. Int. Ed. 2011, 50, 7333–7336. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, K.; Kawasaki, J.; Hiroya, K.; Kondo, Y.; Doi, T. Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C–H amidation sequence: A novel route to 4-aryl-2-quinolinones. Chem. Commun. 2012, 48, 4332–4334. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Bianchi, G.; Po, R.; Swager, M.; Pasini, D. Domino Direct Arylation and Cross-Aldol for Rapid Construction of Extended Polycyclic π-Scaffolds. J. Am. Chem. Soc. 2017, 139, 8788–8791. [Google Scholar] [CrossRef] [PubMed]
- Nitti, A.; Po, R.; Bianchi, G.; Pasini, D. Direct Arylation Strategies in the Synthesis of π-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2017, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Lapczynski, A.; Api, A.M. Review Fragrance material review on cinnamyl alcohol. Food Chem. Toxicol. 2005, 43, 837–866. [Google Scholar] [CrossRef] [PubMed]
- Calo, V.; Nacci, A.; Monopoli, A.; Ferola, V. Palladium−Catalyzed Heck Arylations of Allyl Alcohols in Ionic Liquids: Remarkable Base Effect on the Selectivity. J. Org. Chem. 2007, 72, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Yusub, C.T.; Hossian, A.; Manna, M.K.; Jana, R. Chemo-, Regio- and Stereoselective Heck-Matsuda Arylation of Allylic Alcohols under Mild Conditions. Org. Biomol. Chem. 2015, 13, 4841–4845. [Google Scholar] [CrossRef]
- Ambrogio, I.; Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Sgalla, S. Regio- and Stereoselective Heck α-Arylation of Cinnamyl Alcohols. Synlett 2009, 4, 620–624. [Google Scholar] [CrossRef]
- Mino, T.; Kogure, T.; Abe, T.; Koizumi, T.; Fujita, T.; Sakamoto, M. Palladium-Catalyzed Allylic Arylation of Allylic Ethers with Arylboronic Acids Using Hydrazone Ligands. Eur. J. Org. Chem. 2013, 1501–1505. [Google Scholar] [CrossRef]
- Maralhas, A.; Monteiro, A.; Martins, C.; Kranendonk, M.; Laires, A.; Rueff, J.; Rodrigues, A.S. Genotoxicity and endoreduplication inducing activity of the food flavouring eugenol. Mutagenesis 2006, 21, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, T.S. The Multiple Faces of Eugenol. A Versatile Starting Material and Building Block for Organic and Bio−Organic Synthesis and a Convenient Precursor toward Bio−Based Fine Chemicals. J. Braz. Chem. Soc. 2015, 26, 1055–1085. [Google Scholar] [CrossRef] [Green Version]
- Abdel Bar, F.M.; Khanfar, M.A.; Elnagar, A.Y.; Badria, F.A.; Zaghloul, A.M.; Ahmad, K.F.; Sylvester, P.W.; El Sayed, K.A. Design and pharmacophore modeling of biaryl methyl eugenol analogs as breast cancer invasion inhibitors. Bioorg. Med. Chem. 2010, 18, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Penn, L.; Shpruhman, A.; Gelman, D. Enantio− and Regioselective Heck−Type Reaction of Arylboronic Acids with 2,3-Dihydrofuran. J. Org. Chem. 2007, 72, 3875–3879. [Google Scholar] [CrossRef] [PubMed]
- Dallmeir, K.; Carlini, E.A. Anesthetic, hypothermic, myorelaxant and anticonvulsant effects of synthetic eugenoderivates and natural analogues. Pharmacology 1981, 22, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Sawant, D.; Wagh, Y.; Kushal, B.; Panda, A.; Bhanage, B. Palladium polyether diphosphinite complex anchored in polyethylene glycol as an efficient homogeneous recyclable catalyst for the Heck reactions. Tetrahedron Lett. 2011, 52, 2390–2393. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Api, A.M. Review Fragrance material review on linalool. Food Chem. Toxicol. 2003, 41, 943–964. [Google Scholar] [CrossRef]
- Raguso, R.A.; Pichersky, E. New perspectives in pollination biology: Floral fragrances A day in the life of a linalool molecule: Chemical communication in a plant-pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Species Biol. 1999, 14, 95–120. [Google Scholar] [CrossRef]
- Berthiol, F.; Doucet, H.; Santelli, M. Synthesis of β-aryl ketones by tetraphosphine/palladium catalysed Heck reactions of 2- or 3-substituted allylic alcohols with aryl bromides. Tetrahedron 2006, 62, 4372–4383. [Google Scholar] [CrossRef]
- Berthiol, F.; Doucet, H.; Santelli, M. Heck reactions of aryl halides with alk-1-en-3-ol derivatives catalysed by a tetraphosphine-palladium complex. Appl. Organometal. Chem. 2006, 20, 855–868. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Cotugno, A. Palladium-nanoparticle-catalysed Ullmann reactions in ionic liquids with aldehydes as the reductants: Scope and mechanism. Chem. Eur. J. 2009, 15, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

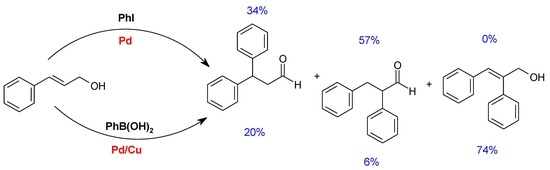
| Entry | Catalyst | Base | DMF:H2O | T (°C) | Time (h) | PhI Conversion (%) | Yield (%) a | ||
|---|---|---|---|---|---|---|---|---|---|
| 1E | 1Z | 1b | |||||||
| 1 | Pd(OAc)2 | K2CO3 | DMF | 100 | 3 | 100 | 81 | 16 | 3 |
| 2 | DMF | 100 | 1 | 95 | 78 | 15 | 2 | ||
| 3 | 4:1 | 100 | 3 | 100 | 84 | 14 | 2 | ||
| 4 | 4:1 | 100 | 2 | 100 | 85 | 15 | 0 | ||
| 5 | 4:1 | 100 | 1 | 100 | 85 | 13 | 1 | ||
| 6 | 2.5:2.5 | 100 | 2 | 100 | 79 | 21 | 0 | ||
| 7 | 2.5:2.5 | 100 | 1 | 93 | 69 | 23 | 1 | ||
| 9 | PdCl2(CH3CN)2 | DMF | 100 | 1 | 79 | 74 | 5 | 0 | |
| 10 | Pd(OAc)2 | 4:1 | 100 | 40 min | 74 | 65 | 7 | 2 | |
| 11 | 4:1 | 80 | 3 | 90 | 80 | 10 | 0 | ||
| 12 | 4:1 | 60 | 3 | 74 | 68 | 6 | 0 | ||
| Entry | Catalyst | Base | DMF:H2O | T (°C) | Time (h) | PhI Conversion (%) | Yield (%) a | ||
|---|---|---|---|---|---|---|---|---|---|
| 2E | 2Z | 2b | |||||||
| 1 | Pd(OAc)2 | K2CO3 | DMF | 80 | 3 | 71 | 36 | 30 | 5 |
| 2 | DMF | 100 | 3 | 96 | 47 | 42 | 7 | ||
| 4 | DMF | 100 | 1 | 77 | 40 | 31 | 6 | ||
| 5 | 2.5:2.5 | 100 | 1 | 41 | 24 | 14 | 3 | ||
| 3 | 4:1 | 100 | 3 | 61 | 35 | 22 | 4 | ||
| 6 | 4:1 | 100 | 1 | 15 | 11 | 4 | 0 | ||
| 7 | 4:1 | 80 | 3 | 37 | 28 | 9 | 0 | ||
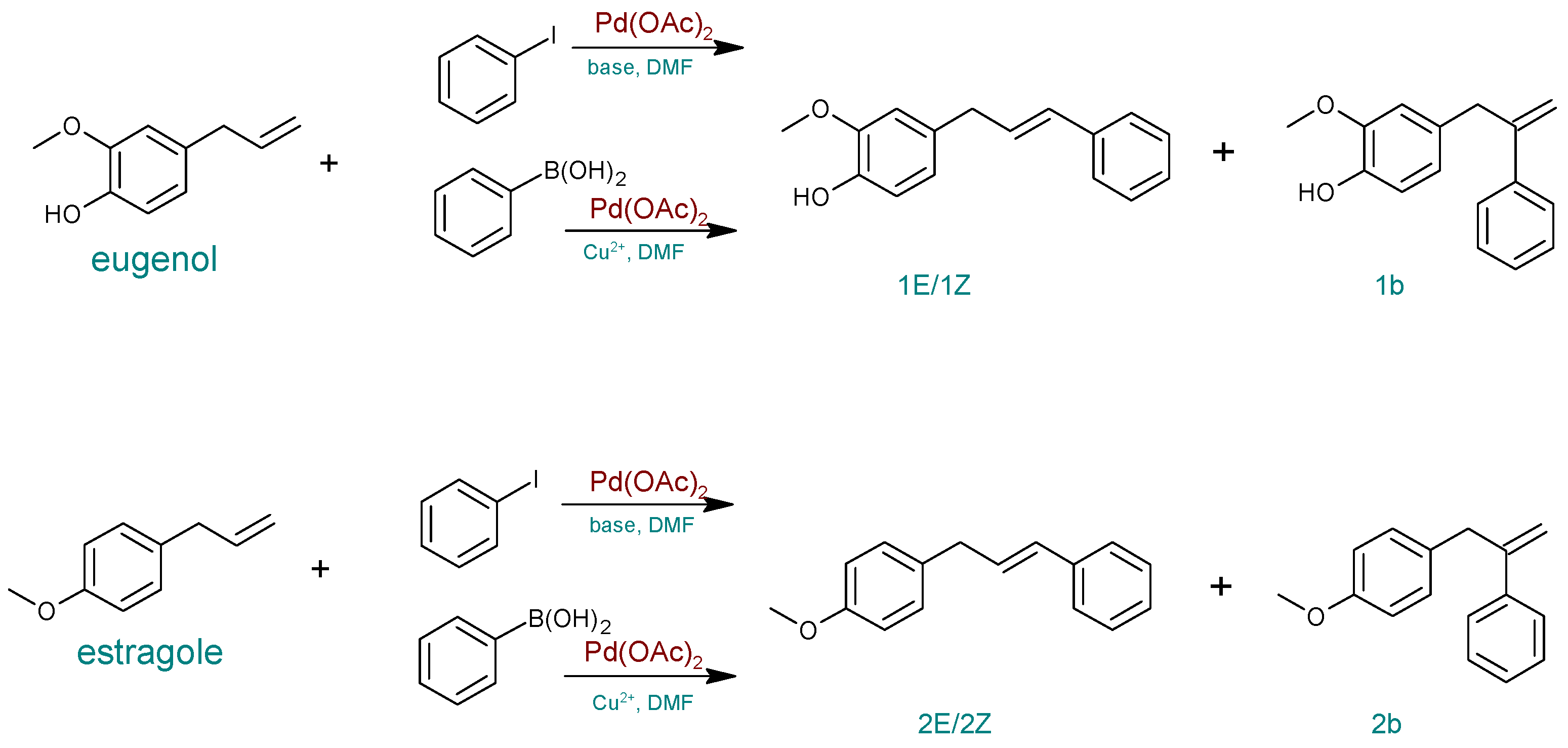
| Entry | Catalyst | Base | Modification | T (°C) | Time (h) | Eugenol Conversion (%) | Yield (%) a | ||
|---|---|---|---|---|---|---|---|---|---|
| 1E | 1Z | 1b | |||||||
| 1 | Pd(OAc)2 | KOH | Air | 50 | 3 | 0 | 0 | 0 | 0 |
| 2 | Na2CO3 | Air | 50 | 3 | 0 | 0 | 0 | 0 | |
| 3 | K2CO3 | Air | 50 | 3 | 0 | 0 | 0 | 0 | |
| 3 | PdCl2cod | ˗ | Cu(OAc)2 | 120 | 4 | 55 | 21 | 21 | 7 |
| 4 | ˗ | 100 | 4 | 25 | 9 | 6 | 10 | ||
| 5 | ˗ | Cu(OAc)2.·H2O | 100 | 4 | 53 | 26 | 17 | 9 | |
| 6 | ˗ | Cu(OAc)2 | 120 | 4 | 57 | 26 | 25 | 6 | |
| 7 | Pd(OAc)2 | ˗ | 100 | 3 | 81 | 51 | 26 | 4 | |
| 8 | ˗ | 100 | 4 | 92 | 46 | 39 | 4 | ||
| Entry | Catalyst | Base | Modification | T (°C) | Time (h) | Estragole Conversion (%) | Yield (%) a | ||
|---|---|---|---|---|---|---|---|---|---|
| 2E | 2Z | 2b | |||||||
| 1 | Pd(OAc)2 | KOH | Air | 50 | 3 | 0 | 0 | 0 | 0 |
| 2 | Na2CO3 | Air | 50 | 3 | 0 | 0 | 0 | 0 | |
| 3 | PdCl2cod | ˗ | Cu(OAc)2 | 120 | 4 | 64 | 34 | 28 | 2 |
| 4 | ˗ | 100 | 4 | 34 | 22 | 12 | 0 | ||
| 5 | ˗ | Cu(OAc)2.·H2O | 100 | 4 | 59 | 32 | 24 | 3 | |
| 6 | Pd(OAc)2 | ˗ | Cu(OAc)2 | 100 | 2.5 | 89 | 46 | 39 | 4 |
| 7 | ˗ | 100 | 3 | 90 | 49 | 37 | 4 | ||
| 8 | ˗ | 100 | 4 | 92 | 47 | 41 | 4 | ||
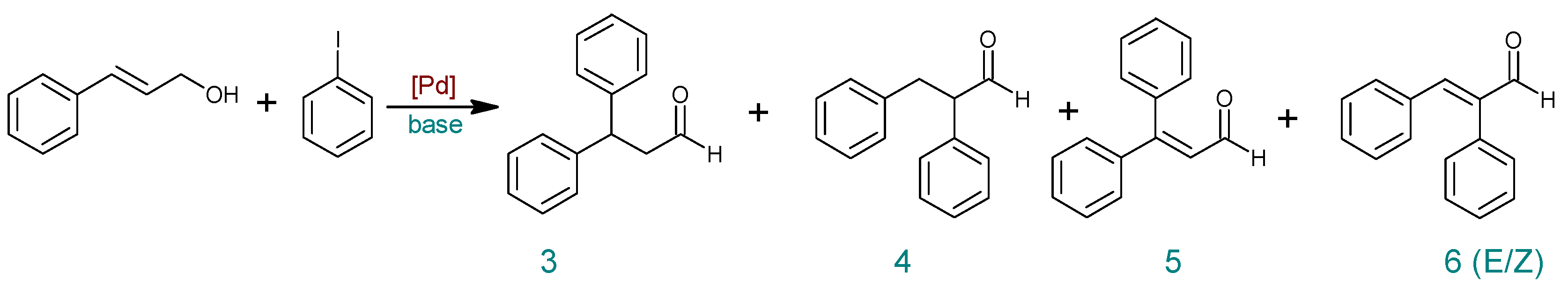
| Entry | Catalyst | Base | Modification | Time (h) | PhI Conversion (%) | Yield (%) b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bif. | 3 | 4 | 5 | 6 | ||||||
| 1 | PdCl2cod | NaOAc | ˗ | 6 | 68 | 15 | 13 | 29 | 7 | 2 |
| 2 | PdCl2cod | TBAB a | 6 | 91 | 5 | 28 | 52 | 3 | 3 | |
| 3 | PdCl2cod | DMF:H2O (1:1) | 6 | 93 | ˗ | 34 | 57 | ˗ | ˗ | |
| 4 | PdCl2cod | DMF:H2O (4:1) | 6 | 93 | 19 | 14 | 53 | ˗ | ˗ | |
| 5 | PdCl2cod | K2HPO4 | ˗ | 6 | 30 | ˗ | 11 | 19 | ˗ | ˗ |
| 6 | PdCl2cod | DMF:H2O (1:1) | 6 | 56 | 10 | 18 | 28 | ˗ | ˗ | |
| 7 | 24 | 96 | 2 | 8 | 72 | ˗ | 11 | |||
| 8 | PdCl2cod | DMF:H2O (1:4) | 6 | 68 | 10 | 13 | 40 | ˗ | ˗ | |
| 9 | PdCl2cod | Et3N | ˗ | 6 | 68 | 24 | 22 | 18 | ˗ | ˗ |
| 10 | DMF:H2O (1:1) | 6 | 67 | 5 | 26 | 36 | ˗ | ˗ | ||
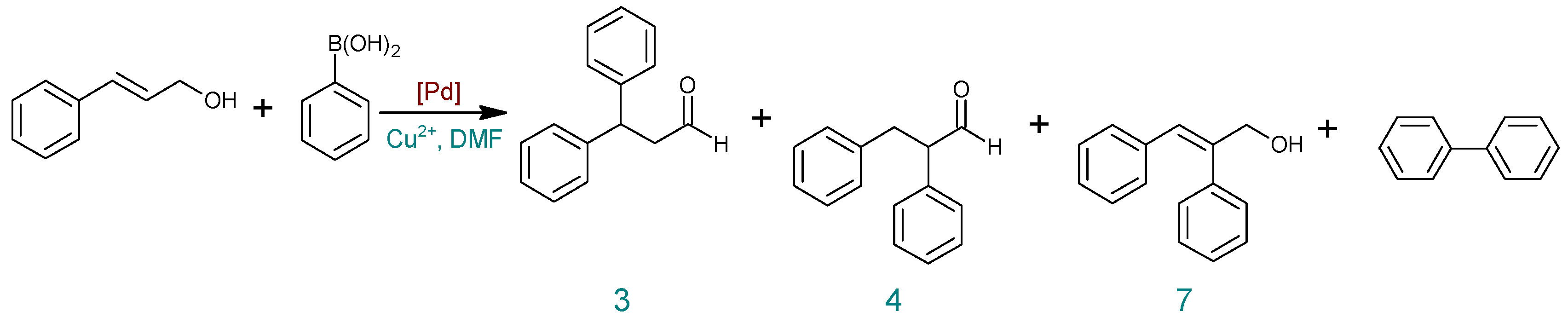
| Entry | Catalyst | Salt Cu2+ | T (°C) | Time (h) | Cinnamyl Alcohol Conversion (%) | Yield (%) b | ||
|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 7 | ||||||
| 1 | Pd(OAc)2 | Air | 100 | 4 | 0 | 0 | 0 | 0 |
| 2 | Cu(OAc)2 | 100 | 6 | 100 | 37 | 14 | 49 | |
| 3 | 100 | 4 | 100 | 40 | 16 | 44 | ||
| 4 | 50 | 1 | 90 | 18 | 6 | 63 | ||
| 5 | Pd2(dba)3 | 50 | 4 | 100 | 19 | 6 | 75 | |
| 6 | 50 | 1 | 100 | 20 | 6 | 74 | ||
| 7 a | 50 | 1 | 63 | 13 | 5 | 45 | ||
| 8 | 50 | 0.5 | 90 | 25 | 8 | 56 | ||
| Entry | Catalyst | Base | Modification | Time (h) | PhI Conversion (%) | Yield (%) a | |
|---|---|---|---|---|---|---|---|
| 8 | 9 | ||||||
| 1 | PdCl2cod | NaOAc | ˗ | 3 | 47 | 33 | 14 |
| 2 | DMF:H2O (4:1) | 3 | 95 | 66 | 29 | ||
| 3 | K2HPO4 | ˗ | 3 | 42 | 21 | 21 | |
| 4 | DMF:H2O (4:1) | 3 | 65 | 48 | 17 | ||
| 5 | Et3N | ˗ | 3 | 42 | 29 | 12 | |
| 6 | DMF:H2O (4:1) | 3 | 61 | 45 | 12 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarnowicz-Ligus, S.; Trzeciak, A.M. Heck Transformations of Biological Compounds Catalyzed by Phosphine-Free Palladium. Molecules 2018, 23, 2227. https://doi.org/10.3390/molecules23092227
Tarnowicz-Ligus S, Trzeciak AM. Heck Transformations of Biological Compounds Catalyzed by Phosphine-Free Palladium. Molecules. 2018; 23(9):2227. https://doi.org/10.3390/molecules23092227
Chicago/Turabian StyleTarnowicz-Ligus, Stanisława, and Anna M. Trzeciak. 2018. "Heck Transformations of Biological Compounds Catalyzed by Phosphine-Free Palladium" Molecules 23, no. 9: 2227. https://doi.org/10.3390/molecules23092227