Novel 3-Methyl-2-alkylthio Benzothiazolyl-Based Ionic Liquids: Synthesis, Characterization, and Antibiotic Activity
Abstract
:1. Introduction
2. Results and Discussion
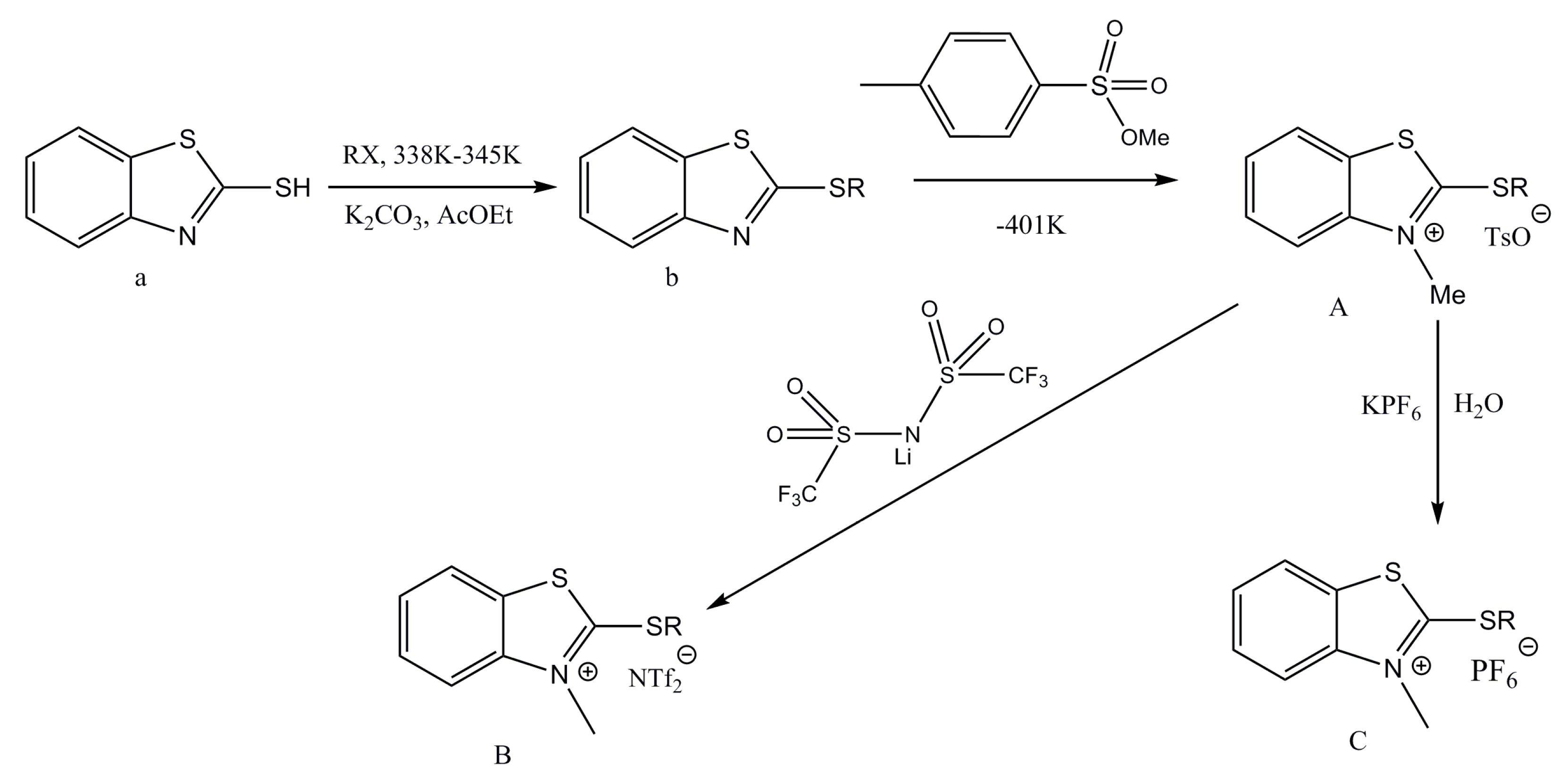
2.1. Synthesis Method and Conditions
2.2. Spectral Data and Analysis
2.3. Physical State and Melting Points of Synthesized ILs
2.4. Solubility Investigation
2.5. Thermal Stability
2.6. Antibiotic Activity
3. Materials and Methods
3.1. Reagents and Methods
3.2. Synthesis of 3-Methyl-2-alkylthio Benzothiazolyl-Based Ionic Liquids
3.3. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, S.W.; Nile, S.H.; Chikhalia, K.H.; Patel, R.V. Ionic liquid mediated tandem synthesis of bioactive quinoline based thiophene/thiazole linked multi-heterocomponentugi adducts. Curr. Org. Chem. 2013, 17, 1125–1129. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V.; Velez, C.; Zayas, B. Imidazolium-derived ionic salts induce inhibition of cancerous cell growth through apoptosis. Med. Chem. Commun. 2014, 5, 1404–1409. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kumar, S.; Singh, I.; Saxena, K.K.; Kumar, A. Synthesis, characterization and biological activity of various substituted benzothiazole derivatives. Dig. J. Nanomater. Biostruct. 2010, 5, 67–76. [Google Scholar]
- Huang, Q.; Mao, J.; Wan, B.; Wang, Y.; Brun, R.; Franzblau, S.G.; Kozikowski, A.P. Searching for new cures for tuberculosis: Design, synthesis, and biological evaluation of 2-methylbenzothiazoles. Med. Chem. 2009, 52, 6757–6767. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Zhao, S.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of a series of benzoxazole/benzothiazole-containing 2,3-dihydrobenzo-[1,4]dioxine derivatives as potential antidepressants. Med. Chem. Lett. 2014, 24, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Kang, T.; Wang, Y.; Zhang, X.; Zhu, X. Synthesis of novel benzothiazolium ionic liquids and research on their catalytic esterification for ricinoleic acid. Chin. J. Org. Chem. 2016, 36, 1104–1107. [Google Scholar] [CrossRef]
- Ghanem, O.B.; Shah, S.N.; Lévêque, J.M.; Mutalib, M.; Elharbawi, M.; Khan, A.S.; Alnarabijia, M.S.; Al-Absig, H.R.H.; Ullahhi, Z. Study of the antimicrobial activity of cyclic cation-based ionic liquids via experimental and group contribution QSAR model. Chemosphere 2018, 195, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Seter, M.; Thomson, M.J.; Stoimenovski, J.; Macfarlane, D.R.; Forsyth, M. Dual active ionic liquids and organic salts for inhibition of microbially influenced corrosion. Chem. Commun. 2012, 48, 5983–5985. [Google Scholar] [CrossRef] [PubMed]
- Deive, F.J.; Rodríguez, A.; Varela, A.; Rodrígues, C.; Leitão, M.C.; Houbraken, J.A.M.P.; Pereiro, A.B.; Longo, M.A.; Sanroman, M.A.; Samson, R.A.; et al. Impact of ionic liquids on extreme microbial biotypes from soil. Green Chem. 2011, 13, 687–696. [Google Scholar] [CrossRef]
- Pernak, J.; Syguda, A.; Mirska, I.; Pernak, A.; Nawrot, J.; Pradzyńska, A.; Griffin, S.T.; Rogers, R.D. Choline-derivative-based ionic liquids. Chem. Eur. J. 2010, 3, 6817–6827. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Feder-Kubis, J.; Cieniecka-Rosonkiewicz, A.; Fischmeister, C.; Griffin, S.T.; Rogers, R.D. Synthesis and properties of chiral imidazolium ionic liquids with a (1, 2, 5)-(−)-menthoxymethyl substituent. New J. Chem. 2007, 31, 879–892. [Google Scholar] [CrossRef]
- Hodyna, D.; Bardeau, J.F.; Metelytsia, L.; Riabov, S.; Kobrina, L.; Laptiy, S.; Kalashnikova, L.; Parkhommenko, V.; Tarasyuk, O.; Rogalsky, S. Efficient antimicrobial activity and reduced toxicity of 1-dodecyl-3-methylimidazolium tetrafluoroborate ionic liquid/β-cyclodextrin complex. Chem. Eng. J. 2016, 284, 1136–1145. [Google Scholar] [CrossRef]
- Ranke, J.; Bottin-Weber, M.U.; Stock, F.; Stolte, S.; Arning, J.; Störmann, R.; Jastorff, B. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotox. Environ. Saf. 2007, 67, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Scammells, P.J.; Scott, J.L.; Singer, R.D. Ionic liquids: The neglected issues. Aust. J. Chem. 2005, 58, 155–169. [Google Scholar] [CrossRef]
- Wells, A.S.; Coombe, V.T. On the freshwater ecotoxicity and biodegradation properties of some common ionic liquids. Org. Process. Res. Dev. 2006, 10, 794–798. [Google Scholar] [CrossRef]
- Cho, C.W.; Pham, T.P.T.; Jeon, Y.C.; Vijayaraghavan, K.; Choe, W.S.; Yun, Y.S. Toxicity of imidazolium salt with anion bromide to a phytoplankton selenastrumcapricornutum: Effect of alkyl-chain length. Green Chem. 2007, 69, 1003–1007. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, Y.W.; Wang, X.M.; Wang, Z.D.; Yao, S.; Song, H. Synthesis of novel ionic liquid with benzothiazolium and research on their physical chemical property. Chin. J. Synth. Chem. 2012, 1, 28–31. [Google Scholar] [CrossRef]
- Daidone, G.; Maggio, B.; Schillaci, D. Salicylanilide and its heterocyclic analogues. A comparative study of their antimicrobial activity. Pharmazie 1990, 45, 441–442. [Google Scholar] [PubMed]
- Zhang, L; Peng, X.M.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Comprehensive Review in Current Developments of Imidazole-Based Medicinal Chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef] [PubMed]
- Messali, M. Eco-friendly synthesis of a new class of pyridinium-based ionic liquids with attractive antimicrobial activity. Molecules 2015, 20, 14936–14949. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Yuan, T.J.; Yang, Y.; Chen, J.M. Novel copper/PEG-400 catalyst systems for chemoselective S- and N-arylation of 2-mercaptobenzothiazole. Tetrahedron 2014, 70, 9652–9660. [Google Scholar] [CrossRef]
- Zhou, X.S.; Liu, J.B.; Luo, W.F.; Zhang, Y.W.; Song, H. Novel Brønsted-acidic ionic liquids based on benzothiazoliumcations as catalysts for the acetalization reactions. J. Serb. Chem. Soc. 2011, 76, 1607–1615. [Google Scholar] [CrossRef]
- Tawfik, S.M. Simple one step synthesis of gemini cationic surfactant-based ionic liquids: Physicochemical, surface properties and biological activity. J. Mol. Liq. 2015, 209, 320–326. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids: A taste of the future. Nat. Mater. 2003, 2, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, C.; Chen, R.; Li, J.; Li, J. Novel ionic liquid-type gemini surfactants: Synthesis, surface property and antimicrobial activity. Colloids Surf. A 2012, 395, 116–124. [Google Scholar] [CrossRef]
- Al-mohammed, N.N.; Alias, A.; Abdullah, Z. Bis-imidazolium and benzimidazolium based gemini-type ionic liquids structure: Synthesis and antibacterial evaluation. RSC Adv. 2015, 5, 92602–92617. [Google Scholar] [CrossRef]
- Xu, W.; Wang, L.M.; Nieman, R.A.; Angell, C.A. Ionic liquids of chelated orthoborates as model ionic glassformers. J. Phys. Chem. B. 2003, 107, 11749–11756. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.S.S.; Carvalho, P.J.; Gardas, R.L.; Fernandes, A.M.; Marrucho, I.M.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Mutual solubilities of water and hydrophobic ionic liquids. J. Phys. Chem. B. 2007, 111, 13082–13089. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Lethesh, K.C.; Mutalib, M.I.A.; Pilus, R.B.M. Evaluation of thermophysical properties of imidazolium-based phenolate ionic liquids. Ind. Eng. Chem. Res. 2015, 54, 3697–3705. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Czekański, L.; Almeida, T.S.D.; Mota, J.P.; Rijo, P.; Araújo, M.E.M. Synthesis of benzoazole ionic liquids and evaluation of their antimicrobial activity. Biomed. Biopharm. Res. 2014, 11, 227–235. [Google Scholar] [CrossRef]
- Hiremath, G.S.; Kulkarni, R.D.; Naik, B.D. Evaluation of minimal inhibitory concentration of two new materials using tube dilution method: An in vitro study. J. Conserv. Dent. 2015, 18, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Sobaszkiewicza, K.; Mirska, I. Anti-microbial activities of ionic liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Ryu, H.; Lee, H.; Iwata, S.; Choi, S.; Kim, M.K.; Kim, Y.R.; Maruta, S.; Kim, S.M.; Jeon, T.J. Investigation of ion channel activities of gramicidin a in the presence of ionic liquids using model cell membranes. Sci. Rep. 2015, 5, 11935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.X.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Micobiol. 2009, 136, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Dienstag, J.; Nue, H.C. In vitro studies of tobramycin and aminoglycoside antibiotic. Antimicrob. Agents Chemother. 1972, 1, 41–45. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds A1–A5, B1–B5 and C1–C3 are available from the authors. |
| Entry | ILs Structure | ILs abbr. | M.W. (HR-MS) | Yield/% | Reaction Time/h |
|---|---|---|---|---|---|
| A1 |  | [3-Me-2-S-C1-MBT][OTs] | 367.5064 | 97 | 6 |
| A2 |  | [3-Me-2-S-C2-MBT][OTs] | 381.5328 | 95 | 6 |
| A3 |  | [3-Me-2-S-C4-MBT][OTs] | 409.5856 | 85 | 6 |
| A4 |  | [3-Me-2-S-C6-MBT][OTs] | 437.6389 | 72 | 6.5 |
| A5 |  | [3-Me-2-S-C8-MBT][OTs] | 465.6920 | 68 | 7 |
| B1 |  | [3-Me-2-S-C1-MBT][NTf2] | 476.4586 | 95 | 12 |
| B2 |  | [3-Me-2-S-C2-MBT][NTf2] | 490.4852 | 93 | 12 |
| B3 |  | [3-Me-2-S-C4-MBT][NTf2] | 518.5380 | 95 | 13 |
| B4 |  | [3-Me-2-S-C6-MBT][NTf2] | 546.5915 | 99 | 13 |
| B5 |  | [3-Me-2-S-C8-MBT][NTf2] | 574.6445 | 96 | 13 |
| C1 |  | [3-Me-2-S-C2-MBT][PF6] | 355.3031 | 90 | 11 |
| C2 |  | [3-Me-2-S-C6-MBT][PF6] | 411.4093 | 94 | 11 |
| C3 |  | [3-Me-2-S-Bn-MBT][PF6] | 417.3726 | 93 | 11 |
| Entry | State under Room Temperature | Entry | Melting Point/°C | Entry | Melting Point/°C |
|---|---|---|---|---|---|
| A1 | Brown viscous liquid | B1 | 78.9–79.9 | C1 | 146.4–147.3 |
| A2 | Brown viscous liquid | B2 | 58.1–58.8 | C2 | 206.6–207.0 |
| A3 | Brown viscous liquid | B3 | 55.1–56.0 | C3 | 171.3–171.9 |
| A4 | Brown viscous liquid | B4 | 49.9–50.1 | ||
| A5 | Brown semi-solid | B5 | 78.9–79.5 |
| No. | Water | Alcohol | Acetone | Tetrahydrofuran | Ethyl Acetate | Chloroform | Toluene | n-Hexane |
|---|---|---|---|---|---|---|---|---|
| ε | 80.1 | 25.8 | 20.7 | 7.58 | 7.3 | 5.1 | 2.4 | 1.9 |
| A1 | + | + | ± | - | - | - | - | - |
| A2 | + | ± | ± | - | - | + | - | - |
| A3 | + | ± | ± | + | - | + | - | - |
| A4 | + | + | + | − | - | + | − | − |
| A5 | + | + | ± | + | − | + | − | − |
| B1 | − | ± | + | + | + | + | − | − |
| B2 | − | + | + | + | + | + | ± | − |
| B3 | − | + | + | + | + | + | − | − |
| B4 | − | ± | + | + | + | + | ± | − |
| B5 | − | - | + | + | + | + | + | − |
| C1 | − | − | + | − | − | − | − | − |
| C2 | − | − | + | + | + | + | ± | − |
| C3 | − | − | + | − | − | − | − | − |
| Sample | Staphylococcus Aureus | Escherichia coli | Chinese Cabbage Soft Rot Disease | |||
| Mean | SD | Mean | SD | Mean | SD | |
| A1 | 0.0 | 0.0 | 6.0 | 0.0 | 0.0 | 0.0 |
| A2 | 14.0 | 2.0 | 15.0 | 1.0 | 6.7 | 1.2 |
| A3 | 19.3 | 1.2 | 21.0 | 2.6 | 16.7 | 3.1 |
| A4 | 24.0 | 3.6 | 19.7 | 1.5 | 16.7 | 0.6 |
| A5 | 23.0 | 2.6 | 13.3 | 7.0 | 15.7 | 0.6 |
| B1 | 6.7 | 1.2 | 6.0 | 0.0 | 10.7 | 1.2 |
| B2 | 10.3 | 4.5 | 10.7 | 4.2 | 11.3 | 2.3 |
| B3 | 9.3 | 3.1 | 10.0 | 2.0 | 8.3 | 4.0 |
| B4 | 6.7 | 1.2 | 8.7 | 3.1 | 11.3 | 2.3 |
| B5 | 9.3 | 1.2 | 7.3 | 2.3 | 6.7 | 1.2 |
| 5% Methanol (c) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 10% Acetone (d) | 1.0 | 2.4 | 3.0 | 0.0 | 0.0 | 0.0 |
| Sample | Rice Bacterial Blight | Xanthomonas Axonopodispv. Citri | Ginger Bacterial Wilt | |||
| Mean | SD | Mean | SD | Mean | SD | |
| A1 | 6.0 | 0.0 | 0.0 | 0.0 | 2.0 | 1.2 |
| A2 | 6.0 | 0.0 | 11.7 | 1.5 | 6.0 | 0.0 |
| A3 | 6.0 | 0.0 | 14.0 | 2.6 | 12.0 | 1.0 |
| A4 | 12.3 | 5.5 | 16.3 | 1.2 | 14.0 | 0.0 |
| A5 | 17.7 | 3.2 | 16.7 | 3.1 | 16.3 | 0.6 |
| B1 | 6.0 | 0.0 | 6.0 | 0.0 | 6.0 | 0.0 |
| B2 | 7.3 | 2.3 | 8.0 | 3.5 | 7.3 | 2.3 |
| B3 | 6.0 | 0.0 | 8.0 | 3.5 | 7.3 | 2.3 |
| B4 | 7.3 | 2.3 | 9.3 | 3.1 | 10.0 | 3.5 |
| B5 | 6.0 | 0.0 | 6.0 | 0.0 | 6.0 | 0.0 |
| 5% Methanol (c) | 2.0 | 4.2 | 0.0 | 0.0 | 1.0 | 0.0 |
| 10% Acetone (d) | 3.0 | 4.2 | 1.0 | 0.0 | 2.0 | 0.0 |
| Samples | Escherichia coli | Staphylococcus Aureus | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| A1 | >250 | 0.0 | 125 | 5.3 |
| A2 | > 250 | 0.0 | 62.5 | 3.1 |
| A3 | >250 | 0.0 | 15.6 | 2.3 |
| A4 | >250 | 0.0 | 15.6 | 2.3 |
| A5 | >250 | 0.0 | 15.6 | 2.3 |
| B1 | 250 | 0.0 | >250 | 0.0 |
| B2 | 250 | 0.0 | >250 | 0.0 |
| B3 | 250 | 0.0 | >250 | 0.0 |
| B4 | 250 | 0.0 | 250 | 0.0 |
| B5 | 250 | 0.0 | 250 | 0.0 |
| Neomycin | 3.1 | 1.3 | 2.9 | 1.1 |
| Emodin | 16 | 2.0 | 20.0 | 3.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.H.; He, H.X.; Du, J.L.; He, Z.J.; Yao, S. Novel 3-Methyl-2-alkylthio Benzothiazolyl-Based Ionic Liquids: Synthesis, Characterization, and Antibiotic Activity. Molecules 2018, 23, 2011. https://doi.org/10.3390/molecules23082011
Zhang TH, He HX, Du JL, He ZJ, Yao S. Novel 3-Methyl-2-alkylthio Benzothiazolyl-Based Ionic Liquids: Synthesis, Characterization, and Antibiotic Activity. Molecules. 2018; 23(8):2011. https://doi.org/10.3390/molecules23082011
Chicago/Turabian StyleZhang, Teng He, Hao Xi He, Jun Liang Du, Zhi Jian He, and Shun Yao. 2018. "Novel 3-Methyl-2-alkylthio Benzothiazolyl-Based Ionic Liquids: Synthesis, Characterization, and Antibiotic Activity" Molecules 23, no. 8: 2011. https://doi.org/10.3390/molecules23082011





