Liquid Chromatography-Tandem Mass Spectrometry Simultaneous Determination and Pharmacokinetic Study of Fourteen Alkaloid Components in Dog Plasma after Oral Administration of Corydalis bungeana Turcz Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. UHPLC-MS/MS Optimization
2.2. Selection of Extraction Method
2.3. Selection of IS
2.4. Method Validation
2.4.1. Selectivity
2.4.2. Linearity and LLOQ
2.4.3. Accuracy and Precision
2.4.4. Extraction Recovery and Matrix Effect
2.4.5. Stability
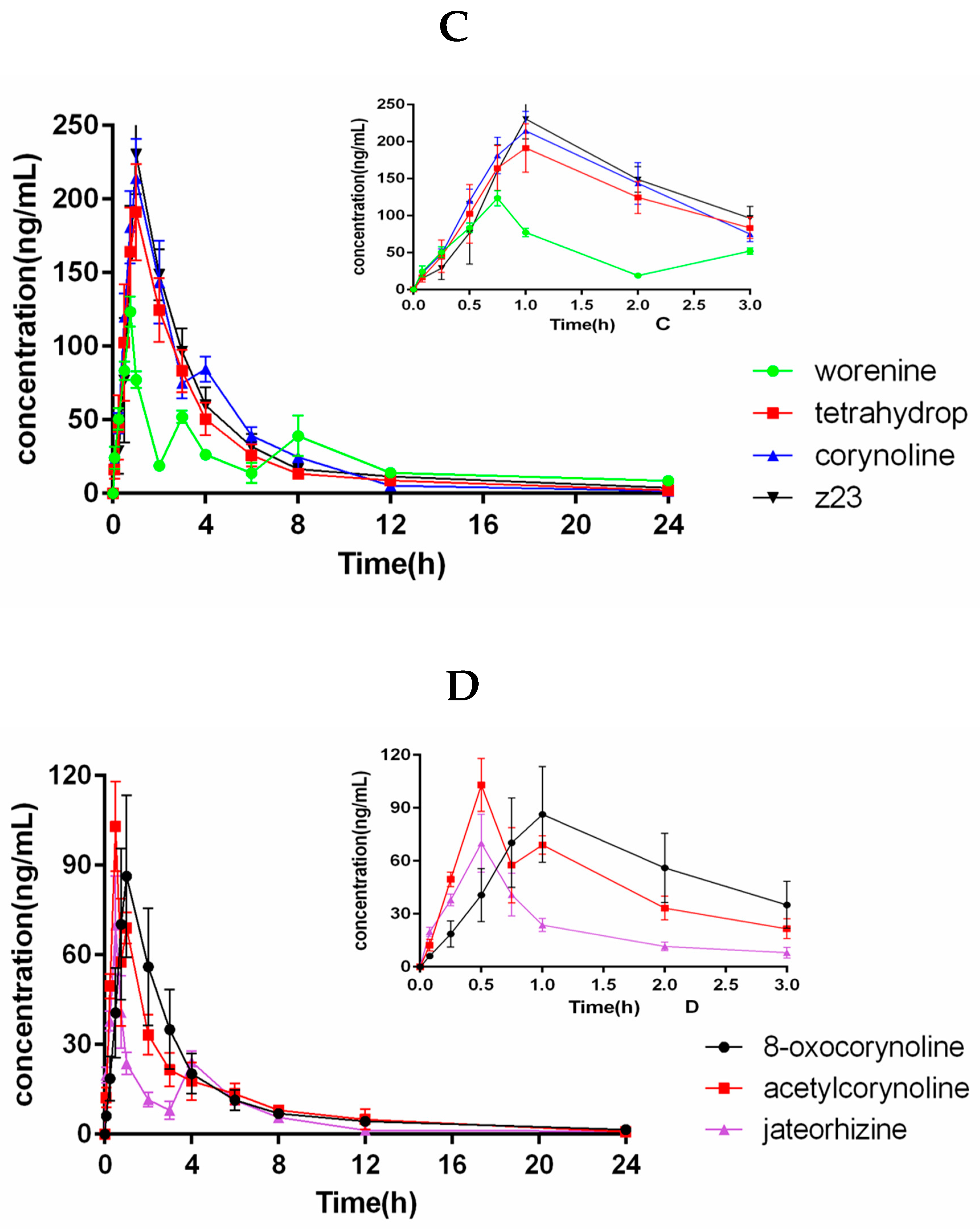
2.5. Pharmacokinetic Study
3. Materials and Methods
3.1. Material and Reagents
3.2. Instruments and Analytical Conditions
3.3. Preparation of Corydalis bungeana Turcz Extract
3.4. Preparation of Calibration Standards and Quality Control (QC) Samples
3.5. Animal Experiments
3.6. Preparation of Plasma Samples
3.7. Method Validation
3.7.1. Specificity
3.7.2. Linearity and Lower Limits of Quantification (LLOQ)
3.7.3. Precision and Accuracy
3.7.4. Extraction Recovery and Matrix Effect
3.7.5. Stability Experiments
3.8. Application to Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2015; p. 234. [Google Scholar]
- Zhai, X.T.; Chen, J.Q.; Jiang, C.H.; Song, J.; Li, D.Y.; Zhang, H.; Jia, X.B.; Tan, W.; Wang, S.X.; Yang, Y.; et al. Corydalis bungeana Turcz. attenuates LPS-induced inflammatory responses via the suppression of NF-κB signaling pathway in vitro and in vivo. J. Ethnopharmacol. 2016, 194, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, Y.; Wang, Z.; Wang, S.; Chen, L.; Wu, L.; Liu, G. UHPLC–ESI–MS/MS determination and pharmacokinetic study of two alkaloid components in rat plasma after oral administration of the extract of Corydalis bungeana Turcz. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 960, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, M.; Zhu, G.F.; Xi, X.; Li, K.; Wu, C.T.; Huang, L.X. Corynoline attenuates L PS-induced acute lung injury in mice by activating Nrf2. J. Int. Immunopharmacol. 2017, 48, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.L.; Liu, G.T. Protective action of corynoline, acetylcorynoline and protopine against experimental liver injury in mice. J. Acta Pharm. Sin. 1997, 32, 331–336. [Google Scholar]
- Fu, R.H.; Wang, Y.C.; Liu, S.P.; Chu, C.L.; Tsai, R.T.; Ho, Y.C.; Chang, W.L.; Chiu, S.C.; Harn, H.J.; Shyu, W.C.; et al. Acetylcorynoline Impairs the Maturation of Mouse Bone Marrow-Derived Dendritic Cells via Suppression of IκB Kinase and Mitogen-Activated Protein Kinase Activities. PLoS ONE 2013, 8, e58398. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Huang, K.L.; Zeng, J.G.; Li, S.; She, J.M.; Li, G.; Zhang, L. Optimization of microwave-assisted extraction of protopine and allocryptop-ine from stems of Macleaya cordata (Willd) R. Br. using response surface methodology. J. Sep. Sci. 2010, 33, 2160–2167. [Google Scholar] [PubMed]
- Song, L.S.; Ren, G.J.; Chen, Z.L.; Chen, Z.H.; Zhou, Z.N.; Cheng, H. Electrophysiological effects of protopine in cardiac myocytes: Inhibition of multiple cation channel currents. Columbia J. Pharmacol. 2000, 129, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Q.; Zhang, N.; Xue, C.; Leung, A.W.; Zhang, H.; Tang, Q.J.; Xu, C. Palmatine hydrochloride mediated photodynamic inactivation of breast cancer MCF-7 cells: effectiveness and mechanism of action. Photodiagn. Photodyn. Ther. 2016, 15, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Ye, X.L.; Cui, X.L.; He, K.; Jin, Y.N.; Chen, Z.; Li, X.G. Cytotoxicity and antihyperglycemic effect of minor constituents from Rhizoma Coptis in HepG2 cells. Fitoterapia 2012, 83, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.W.; Pan, C.S.; Huang, P.; Liu, Y.Y.; Wang, C.S.; Yan, L.; Hu, B.H.; Chang, X.; He, K.; Mu, H.N.; et al. Levo-tetrahydropalmatine attenuates mouse blood-brain barrier injury induced by focal cerebral ischemia and reperfusion: Involvement of Src kinase. Sci. Rep. 2015, 5, 11155–11162. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, W.; Tang, Y.; Bai, W.; Yang, F.; Xie, L.; Li, X.; Zhou, S.; Pan, S.; Chen, Q.; et al. L-tetrahydropalmatine, an active component of corydalis yanhusuo W.T. Wang, Protects against Myocardial Ischaemia-Reperfusion Injury in Rats. PLoS ONE 2012, 7, e38627. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.W.; Marshall, S.J.; Russell, P.F.; Anderson, M.M.; Phillipson, J.D.; Kirby, G.C.; Warhurst, D.C.; Schiff, P.L. In vitro antiplasmodial, antiamoebic, and cytotoxic activities of some monomeric Isoquinoline alkaloids. J. Nat. Prod. 2000, 63, 1638–1640. [Google Scholar] [CrossRef] [PubMed]
- Mišík, V.; Bezáková, L.; Máleková, Ľ.; Košťálová, D. Lipoxygenase inhibition and antioxidant properties of protoberberine and aporphine alkaloids isolated from Mahonia aquifolium. Planta Med. 1995, 61, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xiao, Y.; Yi, L.; Li, L.; Wang, M.; Tian, C.; Ma, H.; He, K.; Wang, Y.; Han, B.; et al. Coptisine from Rhizoma Coptidis suppresses HCT-116 Cells-related tumor growth in vitro and in vivo. Sci. Rep. 2017, 7, 38524. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.E.; Wickström, M.; Hassan, S.; Oberg, K.; Granberg, D. The cytotoxic agents NSC-95397, Brefeldin A, Bortezomib and Sanguinaryine induce apoptosis in neuroendocrine tumors in vitro. J. Anticancer Res. 2010, 30, 149–156. [Google Scholar]
- Du, X.H.; Huang, S.; Feng, H.R.; Chen, L.L.; Yang, S.; Lu, L.F. Inhibition of sanguinarine on S180 subcutaneously implanted tumors in mice. J. Chin. Med. Mater. 2014, 37, 1830–1833. [Google Scholar]
- Park, H.; Bergeron, E.; Senta, H.; Guillemette, K.; Beauvais, S.; Blouin, R.; Sirois, J.; Faucheux, N. Sanguinarine induces apoptosis of human osteosarcoma cells through the extrinsic and intrinsic pathways. Biochem. Biophys. Res. Commun. 2010, 399, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Cecen, E.; Altun, Z.; Ercetin, P.; Aktas, S.; Olgun, N. Promoting effects of sanguinarine on apoptotic gene expression in human neuroblastoma cells. Asian Pac. J. Cancer Prev. 2014, 15, 9445–9451. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Hu, B.; Yang, L.; Wang, P.; Wang, F.; Meng, X. Coptisine from Coptis Chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur. J. Pharmacol. 2016, 780, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.Q.; Wei, W.; Chen, L.M.; Liu, S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 2006, 108, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine improves glucose metabolism through induction of glycolysis. AJP Endocrinol. Metab. 2007, 294, E148–E156. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Lee, J.H.; Kim, S.H.; Kang, T.H.; Moon, J.S.; Kim, S.U. Synthesis of 13-(substituted benzyl) berberine and berberrubine derivatives as antifungal agents. Bioorg. Med. Chem. Lett. 2006, 16, 3913–3916. [Google Scholar] [CrossRef] [PubMed]
- Pongkittiphan, V.; Chavasiri, W.; Supabphol, R. Antioxidant effect of berberine and its phenolic derivatives against human fibrosarcoma cells. Asian Pac. J. Cancer Prev. 2015, 16, 5371–5376. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa, T.; Park, J.H. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, H.J.; Li, F.M. Preparation and simultaneous determination of corynoline and acetylcorynoline in the herb of Corydalis bungeana. China J. Chin. Mater. Med. 2003, 28, 346–352. [Google Scholar]
- Wen, C.; Cai, J.; Lin, C.; Ma, J.; Wang, X. Gradient elution liquid chromatography mass spectrometry determination of acetylcorynoline in rat plasma and its application to a pharmacokinetic study. Xenobiotica 2014, 44, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, P.; Wang, L.; Cheng, M.; Wu, Y.; Zheng, L.; Liu, Y.; Ding, L. Study on the pharmacokinetic profiles of corynoline and its potential interaction in traditional chinese medicine formula shuanghua baihe tablets in rats by LC–MS/MS. J. Pharm. Biomed. Anal. 2016, 117, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zheng, L.; Cheng, M.; Wu, Y.; Gu, P.; Liu, Y.; Ma, P.; Ding, L. Simultaneous determination of corynoline and acetylcorynoline in human urine by LC–MS/MS and its application to a urinary excretion study. J. Chromatogr. B 2016, 1014, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, X.; Wang, Z.; Sun, J.; Pan, R.; Yang, C.; Wu, L. Simultaneous determination and pharmacokinetics of five alkaloids in rat plasma by ultra high performance liquid chromatography with tandem mass spectrometry after the oral administration of Corydalis bungeana Turcz extract. J. Sep. Sci. 2015, 39, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Mooiman, K.D.; Maas-Bakker, R.F.; Beijnen, J.H.; Schellens, J.H.M.; Meijerman, I. Effect of chinese herbs on CYP3A4 activity and expression in vitro. J. Ethnopharmacol. 2013, 149, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liao, Q.; Li, S.; Bi, K.; Pan, B.; Xie, Z. Simultaneous determination of berberine, palmatine and jatrorrhizine by liquid chromatography–tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of coptis–evodia herb couple. J. Chromatogr. B 2008, 863, 195–205. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 7′-(3′,4′-dihydroxyphenyl)-N-[(4-me thoxyphenyl)-ethyl] propena-mide, coptisine, berberrubine, sanguinarine, worenine, berberine, jateorhizine, columbamine, palmatine, protopine, tetrahydropalmatine, corynoline, 8-oxocorynoline, acetylcorynoline are available from the authors. |
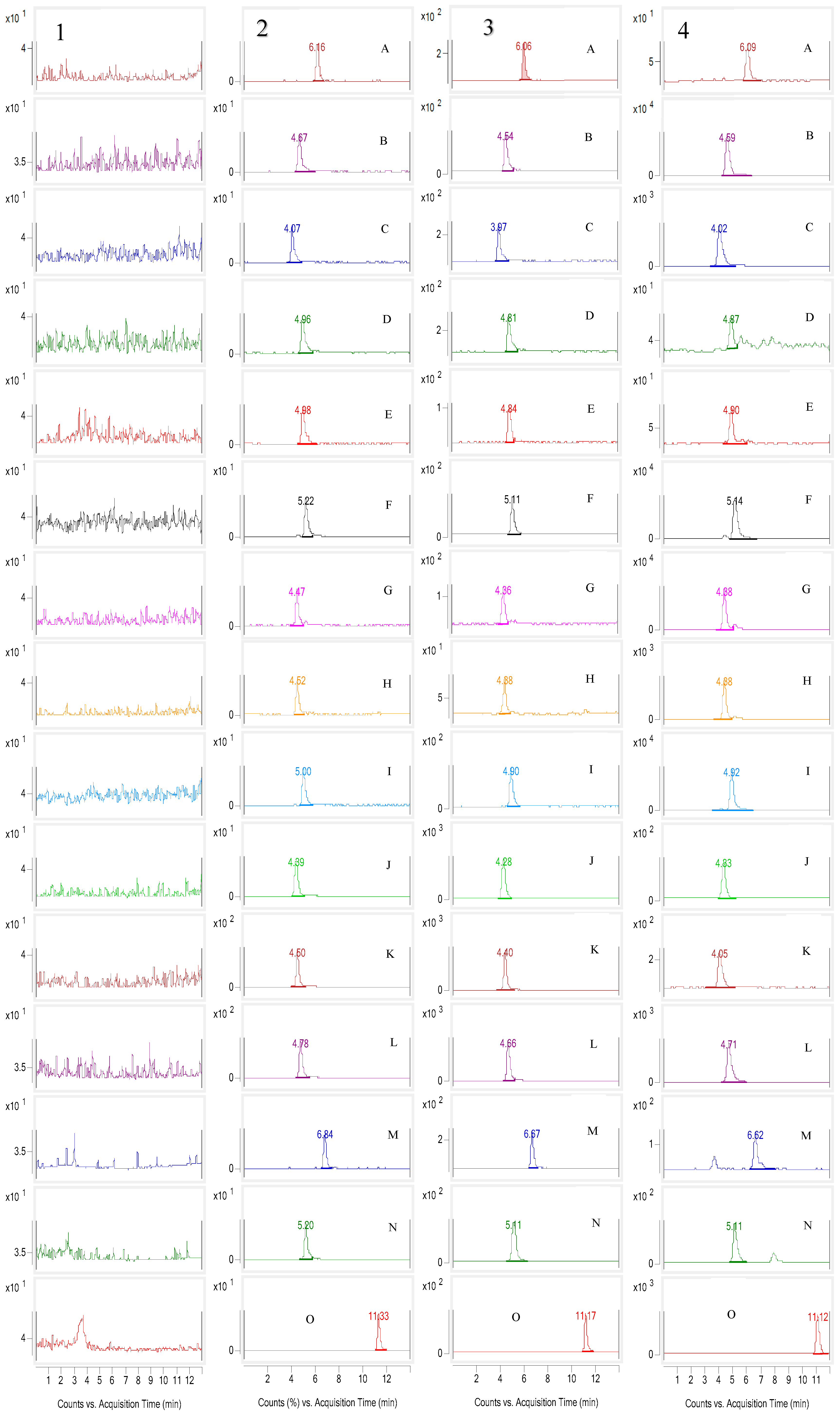



| Compounds | Linear Range (ng/mL) | Regression Equation | R2 | LLOQ (ng/mL) |
|---|---|---|---|---|
| Z23 | 5.34–2670 | Y = 6.19X + 5.50 × 10−3 | 0.9989 | 5.34 |
| Coptisine | 5.00–2500 | Y = 9.86X − 7.69 × 10−3 | 0.9985 | 5.00 |
| Berberrubine | 5.53–2763 | Y = 23.48X − 3.73 × 10−2 | 0.9978 | 5.53 |
| Sanguinarine | 5.00–2500 | Y = 15.22X − 2.64 × 10−2 | 0.9949 | 5.00 |
| Worenine | 5.55–2775 | Y = 0.776X + 3.39 × 10−4 | 0.9905 | 5.55 |
| Berberine | 5.40–2700 | Y = 9.75X + 6.15 × 10−2 | 0.9942 | 5.40 |
| Jateorhizine | 5.00–2500 | Y = 1.12X + 8.51 × 10−3 | 0.9910 | 5.00 |
| Columbamine | 5.58–2788 | Y = 0.323X + 6.27 × 10−3 | 0.9918 | 5.58 |
| Palmatine | 5.65–2825 | Y = 2.238X + 3.34 × 10−2 | 0.9985 | 5.65 |
| Protopine | 4.87–2435 | Y = 26.65X − 8.12 × 10−3 | 0.9909 | 4.87 |
| Tetrahydropalmatine | 5.07–2535 | Y = 58.74X − 4.69 × 10−2 | 0.9981 | 5.07 |
| Corynoline | 5.11–2555 | Y = 75.78X + 1.44 × 10−2 | 0.9904 | 5.11 |
| 8-oxocorynoline | 5.80–2900 | Y = 4.49X − 7.19 × 10−3 | 0.9922 | 5.80 |
| Acetylcorynoline | 6.50–3250 | Y = 15.10X − 2.09 × 10−3 | 0.9989 | 6.50 |
| Compounds | Spiked Cone. (ng/mL) | Measured Cone. (ng/mL) | Accuracy (%) | Intra-Day Precision (%) | Inter-Day Precision (%) |
|---|---|---|---|---|---|
| Z23 | 5.34 | 6.24 ± 0.75 | 13.83 | 12.15 | 11.23 |
| 10.86 | 12.09 ± 1.59 | 11.37 | 13.36 | 11.25 | |
| 133.5 | 152.6 ± 14.59 | 14.40 | 9.90 | 6.20 | |
| 2136 | 1934 ± 120.3 | −9.40 | 5.50 | 10.10 | |
| Coptisine | 5.00 | 4.47 ± 0.61 | −10.70 | 13.90 | 12.60 |
| 10.00 | 10.36 ± 1.31 | 3.58 | 12.58 | 12.86 | |
| 125.0 | 139.1 ± 9.12 | 11.24 | 6.22 | 8.68 | |
| 2000 | 1826 ± 109.0 | −8.70 | 6.00 | 5.70 | |
| Berberrubine | 5.53 | 6.26 ± 0.68 | 13.40 | 11.20 | 8.60 |
| 11.05 | 12.62 ± 1.42 | 14.21 | 11.75 | 6.65 | |
| 138.1 | 130.5 ± 13.62 | −5.50 | 10.80 | 6.70 | |
| 2210 | 2046 ± 201.5 | −7.40 | 10.30 | 6.00 | |
| Sanguinarine | 5.00 | 5.64 ± 0.79 | 12.80 | 14.70 | 6.50 |
| 10.00 | 11.32 ± 0.90 | 13.25 | 8.42 | 2.12 | |
| 125.0 | 112.4 ± 7.98 | −10.10 | 6.40 | 11.10 | |
| 2000 | 2117 ± 218.4 | 5.87 | 10.74 | 6.25 | |
| Worenine | 5.55 | 6.17 ± 0.61 | 11.10 | 10.30 | 6.00 |
| 11.10 | 12.09 ± 0.95 | 8.90 | 7.20 | 12.00 | |
| 138.8 | 128.6 ± 10.19 | −7.30 | 8.00 | 7.00 | |
| 2220 | 2443 ± 200.7 | 10.10 | 8.10 | 8.70 | |
| Berberine | 5.40 | 5.87 ± 0.68 | 8.70 | 12.10 | 6.80 |
| 10.80 | 11.55 ± 1.43 | 6.97 | 12.30 | 12.64 | |
| 135.0 | 151.3 ± 13.79 | 12.10 | 9.30 | 7.80 | |
| 2160 | 2275 ± 273.5 | 5.31 | 11.93 | 12.74 | |
| Jateorhizine | 5.00 | 5.69 ± 0.61 | 13.90 | 11.00 | 8.90 |
| 10.00 | 11.05 ± 0.88 | 10.48 | 7.95 | 8.15 | |
| 125.0 | 130.1 ± 9.93 | 4.10 | 7.96 | 4.36 | |
| 2000 | 2157 ± 212.1 | 7.82 | 9.86 | 9.66 | |
| Columbamine | 5.58 | 5.99 ± 0.73 | 7.50 | 12.20 | 11.90 |
| 11.15 | 12.48 ± 1.29 | 11.95 | 9.98 | 12.85 | |
| 139.4 | 124.4 ± 12.37 | −10.70 | 9.50 | 12.70 | |
| 2230 | 2292 ± 219.6 | 2.78 | 9.76 | 8.11 | |
| Palmatine | 5.65 | 6.40 ± 0.87 | 13.40 | 14.00 | 9.90 |
| 11.30 | 12.27 ± 1.01 | 8.56 | 8.61 | 3.90 | |
| 141.3 | 146.1 ± 10.79 | 3.42 | 7.73 | 3.90 | |
| 2260 | 2047 ± 235.1 | −9.40 | 11.50 | 11.00 | |
| Protopine | 4.87 | 5.53 ± 0.61 | 13.50 | 11.40 | 8.60 |
| 9.74 | 9.89 ± 0.72 | 1.50 | 6.40 | 11.93 | |
| 121.8 | 124.3 ± 5.19 | 2.11 | 3.78 | 6.38 | |
| 1948 | 2216 ± 154.3 | 13.77 | 7.36 | 2.55 | |
| Terahydro-palmatine | 5.07 | 5.71 ± 0.57 | 12.70 | 10.20 | 8.40 |
| 10.14 | 11.41 ± 1.27 | 12.50 | 11.44 | 8.79 | |
| 126.8 | 132.1 ± 8.62 | 4.19 | 6.36 | 7.66 | |
| 2028 | 2228 ± 236.0 | 9.87 | 11.15 | 4.65 | |
| Corynoline | 5.11 | 5.73 ± 0.61 | 12.10 | 11.10 | 6.70 |
| 10.22 | 11.11 ± 1.03 | 8.69 | 9.14 | 9.88 | |
| 127.8 | 139.5 ± 10.59 | 9.19 | 7.52 | 8.11 | |
| 2044 | 2172 ± 241.3 | 6.28 | 11.68 | 5.14 | |
| 8-oxocorynoline | 5.80 | 4.86 ± 0.67 | −12.60 | 14.00 | 10.70 |
| 11.60 | 12.09 ± 1.59 | 4.27 | 13.36 | 11.25 | |
| 145.0 | 125.1 ± 6.39 | −13.70 | 5.20 | 4.40 | |
| 2320 | 2109 ± 123.1 | −9.10 | 6.20 | 2.10 | |
| Acetylcorynoline | 6.50 | 5.79 ± 0.68 | −10.90 | 11.90 | 10.30 |
| 13.00 | 13.78 ± 1.36 | 6.02 | 10.12 | 7.97 | |
| 162.5 | 142.7 ± 15.79 | −12.20 | 11.40 | 8.20 | |
| 2600 | 2819 ± 208.7 | 8.40 | 7.60 | 5.30 |
| Compounds | Spiked Conc. (ng/L) | Matrix Effect (%) | RSD (%) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Z23 | 10.86 | 96.98 | 13.59 | 80.05 | 10.35 |
| 133.5 | 100.02 | 5.54 | 87.74 | 6.82 | |
| 2136 | 100.71 | 11.52 | 84.44 | 5.38 | |
| Coptisine | 10 | 99.68 | 10.66 | 78.35 | 10.55 |
| 125 | 98.35 | 8.45 | 86.10 | 13.60 | |
| 2000 | 99.42 | 11.29 | 79.21 | 6.19 | |
| Berberrubine | 11.05 | 95.07 | 8.25 | 85.44 | 8.39 |
| 138.1 | 102.76 | 7.14 | 79.63 | 9.79 | |
| 2210 | 100.33 | 5.55 | 83.76 | 6.94 | |
| Sanguinarine | 10 | 94.08 | 6.54 | 82.98 | 8.14 |
| 125 | 101.70 | 2.06 | 83.94 | 5.42 | |
| 2000 | 100.59 | 6.05 | 87.79 | 6.73 | |
| Worenine | 11.10 | 101.41 | 8.90 | 78.67 | 5.75 |
| 138.8 | 100.15 | 3.94 | 89.67 | 3.76 | |
| 2220 | 101.77 | 7.91 | 88.63 | 2.80 | |
| Berberine | 10.80 | 99.06 | 9.17 | 83.24 | 10.11 |
| 135 | 100.15 | 2.81 | 88.11 | 4.01 | |
| 2160 | 101.60 | 7.92 | 88.36 | 3.78 | |
| Jateorhizing | 10 | 101.01 | 11.80 | 82.65 | 7.11 |
| 125 | 99.92 | 4.74 | 86.10 | 4.97 | |
| 2000 | 99.66 | 5.48 | 87.46 | 4.28 | |
| Columbamine | 11.15 | 100.14 | 6.80 | 88.90 | 7.52 |
| 139.4 | 100.45 | 4.11 | 87.79 | 6.51 | |
| 2230 | 99.34 | 5.58 | 88.75 | 3.60 | |
| Palmatine | 11.30 | 101.91 | 7.90 | 82.17 | 6.47 |
| 141.3 | 101.49 | 7.40 | 86.68 | 5.99 | |
| 2260 | 99.51 | 4.11 | 88.70 | 4.69 | |
| Protopine | 9.74 | 99.78 | 8.37 | 78.40 | 10.49 |
| 121.8 | 100.86 | 4.78 | 89.59 | 2.22 | |
| 1948 | 99.08 | 9.96 | 82.63 | 7.96 | |
| Teahydropalmatine | 10.14 | 99.57 | 10.28 | 81.09 | 8.38 |
| 126.8 | 101.87 | 2.68 | 88.41 | 3.88 | |
| 2028 | 101.64 | 7.18 | 81.94 | 7.45 | |
| Corynoline | 10.22 | 99.31 | 5.29 | 84.75 | 5.77 |
| 127.8 | 101.63 | 7.06 | 84.52 | 6.18 | |
| 2044 | 101.41 | 8.70 | 83.65 | 10.00 | |
| 8-oxocorynoline | 11.60 | 100.57 | 8.74 | 78.03 | 9.25 |
| 145 | 100.92 | 7.17 | 87.53 | 11.00 | |
| 2320 | 99.80 | 2.36 | 87.12 | 5.51 | |
| Acetylcorynoline | 13 | 101.50 | 12.01 | 78.21 | 9.53 |
| 162.5 | 99.72 | 5.06 | 84.63 | 5.88 | |
| 2600 | 101.49 | 4.77 | 86.13 | 5.03 | |
| I.S. | 2000 | 99.12 | 11.88 | 84.71 | 8.97 |
| Compounds | Spiked Conc. (ng/mL) | Stability (% RE) | ||||
|---|---|---|---|---|---|---|
| Freeze-Thaw | Short-Term | Long-Term | Post-Preparative | Room Temperature for Stock-Solution | ||
| Z23 | 10.86 | 5.35 | 10.52 | −8.16 | 12.10 | 3.65 |
| 133.5 | 12.92 | 9.94 | 9.86 | 11.32 | 1.64 | |
| 2136 | 12.43 | 10.85 | 9.00 | −6.09 | 2.30 | |
| Coptisine | 10 | 10.21 | 10.66 | 5.48 | 7.64 | −4.12 |
| 125 | 8.93 | −3.05 | 6.73 | 8.40 | 1.35 | |
| 2000 | 12.83 | 7.57 | 13.69 | 10.58 | 3.70 | |
| Berberrubine | 11.05 | 12.88 | 11.76 | 11.85 | 5.99 | −2.05 |
| 138.1 | 5.15 | 5.82 | 3.75 | 7.97 | 1.25 | |
| 2210 | 10.06 | 10.78 | 11.01 | 5.08 | 3.65 | |
| Sanguinarine | 10 | 11.45 | 12.41 | 13.35 | 7.77 | 2.48 |
| 125 | 8.78 | −5.50 | 7.46 | −2.41 | 3.20 | |
| 2000 | 12.22 | 9.29 | 11.08 | 10.40 | −4.02 | |
| Worenine | 11.10 | 11.96 | −5.32 | −6.79 | 8.10 | 2.10 |
| 138.8 | 8.93 | 3.89 | 7.99 | 9.22 | −3.48 | |
| 2220 | 11.16 | 10.03 | 11.40 | 13.58 | 2.59 | |
| Berberine | 10.80 | 12.57 | 9.61 | 7.75 | 11.84 | 2.22 |
| 135 | 11.60 | 7.42 | 7.72 | 9.39 | 4.08 | |
| 2160 | 7.71 | 11.96 | 7.00 | 10.36 | −3.26 | |
| Jateorhizine | 10 | 10.65 | 7.31 | 12.90 | 6.63 | 1.16 |
| 125 | 8.68 | 3.05 | 10.30 | 8.51 | −3.58 | |
| 2000 | 6.24 | 6.64 | 8.26 | 8.41 | 1.32 | |
| Columbamine | 11.15 | 11.50 | 8.47 | 9.66 | 8.80 | 4.02 |
| 139.4 | 12.73 | 4.02 | 11.77 | 5.50 | 1.62 | |
| 2230 | 5.60 | 9.67 | 10.24 | 12.44 | 2.50 | |
| Palmatine | 11.30 | 10.26 | 9.42 | 7.79 | 6.92 | 2.77 |
| 141.3 | 8.08 | −6.30 | 10.77 | 9.22 | −4.66 | |
| 2260 | 8.37 | 6.73 | 11.04 | −7.08 | 1.30 | |
| Protopine | 9.74 | 3.72 | 5.52 | −5.39 | 1.65 | 2.17 |
| 121.8 | 4.17 | 8.75 | 7.93 | 11.35 | −3.87 | |
| 1948 | 10.26 | 12.18 | 13.11 | 10.57 | 2.02 | |
| Terahydropalmatine | 10.14 | 11.34 | 9.24 | −5.67 | 10.82 | 2.97 |
| 126.8 | 12.23 | 3.11 | 11.88 | 8.29 | 4.65 | |
| 2028 | 10.09 | 5.15 | 10.23 | 8.76 | 1.28 | |
| Corynoline | 10.22 | 6.11 | −2.63 | 13.95 | 7.14 | 4.42 |
| 127.8 | 6.78 | 9.25 | 6.81 | −2.98 | −3.74 | |
| 2044 | 7.95 | 6.81 | 9.74 | 5.40 | 1.99 | |
| 8-oxocorynoline | 11.60 | 1.18 | 1.62 | 3.08 | 3.71 | −4.08 |
| 145 | 9.69 | −3.92 | 5.35 | 10.01 | 3.77 | |
| 2320 | −3.58 | −2.89 | 3.13 | 10.60 | 2.30 | |
| Acetylcorynoline | 13 | 5.17 | 6.47 | 8.62 | 12.65 | −1.83 |
| 162.5 | −3.75 | 6.74 | 7.56 | −3.44 | 2.57 | |
| 2600 | −4.20 | −4.81 | 7.32 | 2.05 | 3.69 | |
| Analytes | Cmax (ng/mL) | tmax (h) | t1/2 (h) | AUC0→t (ng h/mL) | AUC0→∞ (ng h/mL) |
|---|---|---|---|---|---|
| Z23 | 237.0 ± 13.66 | 0.96 ± 0.10 | 6.34 ± 1.21 | 682.2 ± 74.81 | 746.7 ± 95.24 |
| Coptisine | 2256 ± 255.9 | 0.92 ± 0.13 | 2.48 ± 0.33 | 5957 ± 204.6 | 6032 ± 194.1 |
| Berberrubine | 450.1 ± 61.61 | 0.96 ± 0.10 | 4.88 ± 1.72 | 1156 ± 94.24 | 1204 ± 86.29 |
| Sanguinarine | 533.4 ± 29.12 | 0.96 ± 0.10 | 5.63 ± 1.36 | 1260 ± 107.4 | 1346 ± 109.4 |
| Worenine | 123.4 ± 10.34 | 0.71 ± 0.10 | 5.95 ± 0.84 | 396.9 ± 42.21 | 482.2 ± 91.51 |
| Berberine | 1369 ± 312.7 | 0.92 ± 0.13 | 2.49 ± 0.14 | 3691 ± 141.0 | 3767 ± 139.0 |
| Jateorhizine | 74.16 ± 8.71 | 0.54 ± 0.10 | 6.29 ± 1.09 | 151.1 ± 6.42 | 163.2 ± 11.04 |
| Columbamine | 2130 ± 195.7 | 0.92 ± 0.13 | 2.87 ± 0.42 | 6826 ± 170.0 | 7026 ± 289.6 |
| Palmatine | 1902 ± 84.86 | 0.96 ± 0.10 | 8.56 ± 1.29 | 8037 ± 459.3 | 9582 ± 596.2 |
| Protopine | 433.7 ± 73.92 | 0.88 ± 0.13 | 4.92 ± 1.49 | 1029 ± 106.2 | 1079 ± 135.6 |
| Terahydropalmatine | 205.0 ± 22.39 | 0.92 ± 0.13 | 5.58 ± 1.46 | 584.7 ± 59.85 | 622.5 ± 52.46 |
| Corynoline | 223.9 ± 15.29 | 0.96 ± 0.10 | 4.25 ± 0.81 | 733.8 ± 57.45 | 755.3 ± 58.92 |
| 8-oxocorynoine | 94.49 ± 22.77 | 0.92 ± 0.13 | 6.98 ± 1.76 | 260.5 ± 64.21 | 283.6 ± 62.41 |
| Acetylcorynoline | 106.7 ± 4.63 | 0.58 ± 0.13 | 5.52 ± 0.93 | 229.7 ± 18.60 | 244.4 ± 26.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Yan, G.; Wang, Z.; Wu, C.; Cui, B.; Ren, Y.; Yang, C. Liquid Chromatography-Tandem Mass Spectrometry Simultaneous Determination and Pharmacokinetic Study of Fourteen Alkaloid Components in Dog Plasma after Oral Administration of Corydalis bungeana Turcz Extract. Molecules 2018, 23, 1927. https://doi.org/10.3390/molecules23081927
Dong H, Yan G, Wang Z, Wu C, Cui B, Ren Y, Yang C. Liquid Chromatography-Tandem Mass Spectrometry Simultaneous Determination and Pharmacokinetic Study of Fourteen Alkaloid Components in Dog Plasma after Oral Administration of Corydalis bungeana Turcz Extract. Molecules. 2018; 23(8):1927. https://doi.org/10.3390/molecules23081927
Chicago/Turabian StyleDong, Hongrui, Guanyun Yan, Zhibin Wang, Chengcui Wu, Binbin Cui, Yixuan Ren, and Chunjuan Yang. 2018. "Liquid Chromatography-Tandem Mass Spectrometry Simultaneous Determination and Pharmacokinetic Study of Fourteen Alkaloid Components in Dog Plasma after Oral Administration of Corydalis bungeana Turcz Extract" Molecules 23, no. 8: 1927. https://doi.org/10.3390/molecules23081927




