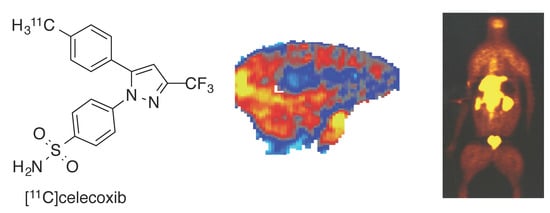
In Vivo Brain Imaging, Biodistribution, and Radiation Dosimetry Estimation of [11C]Celecoxib, a COX-2 PET Ligand, in Nonhuman Primates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Radiochemistry of [11C]Celecoxib and Cross Selectivity of Celecoxib to Brain Targets
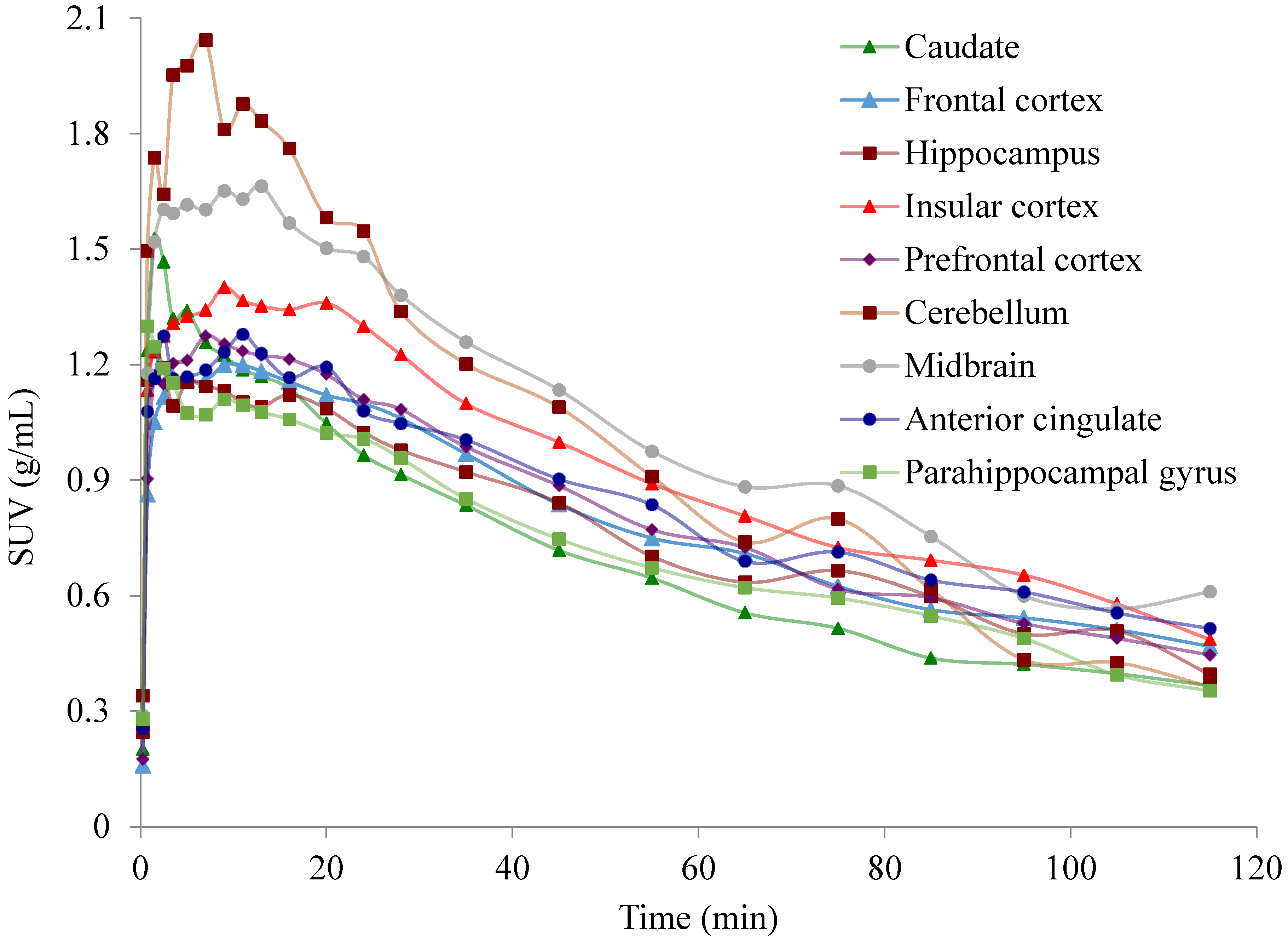
2.2. PET Imaging of [11C]Celecoxib in Baboon Brain
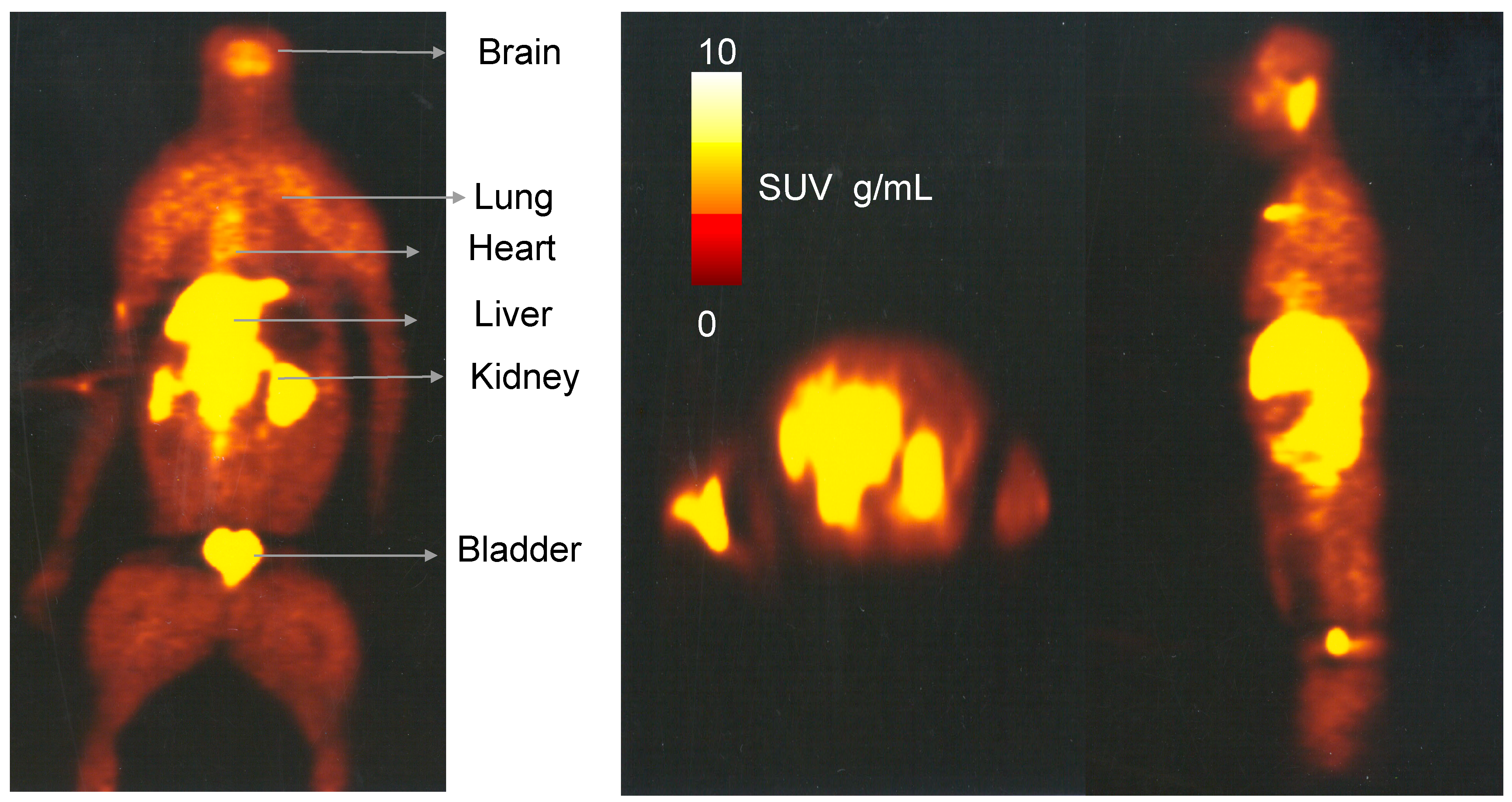
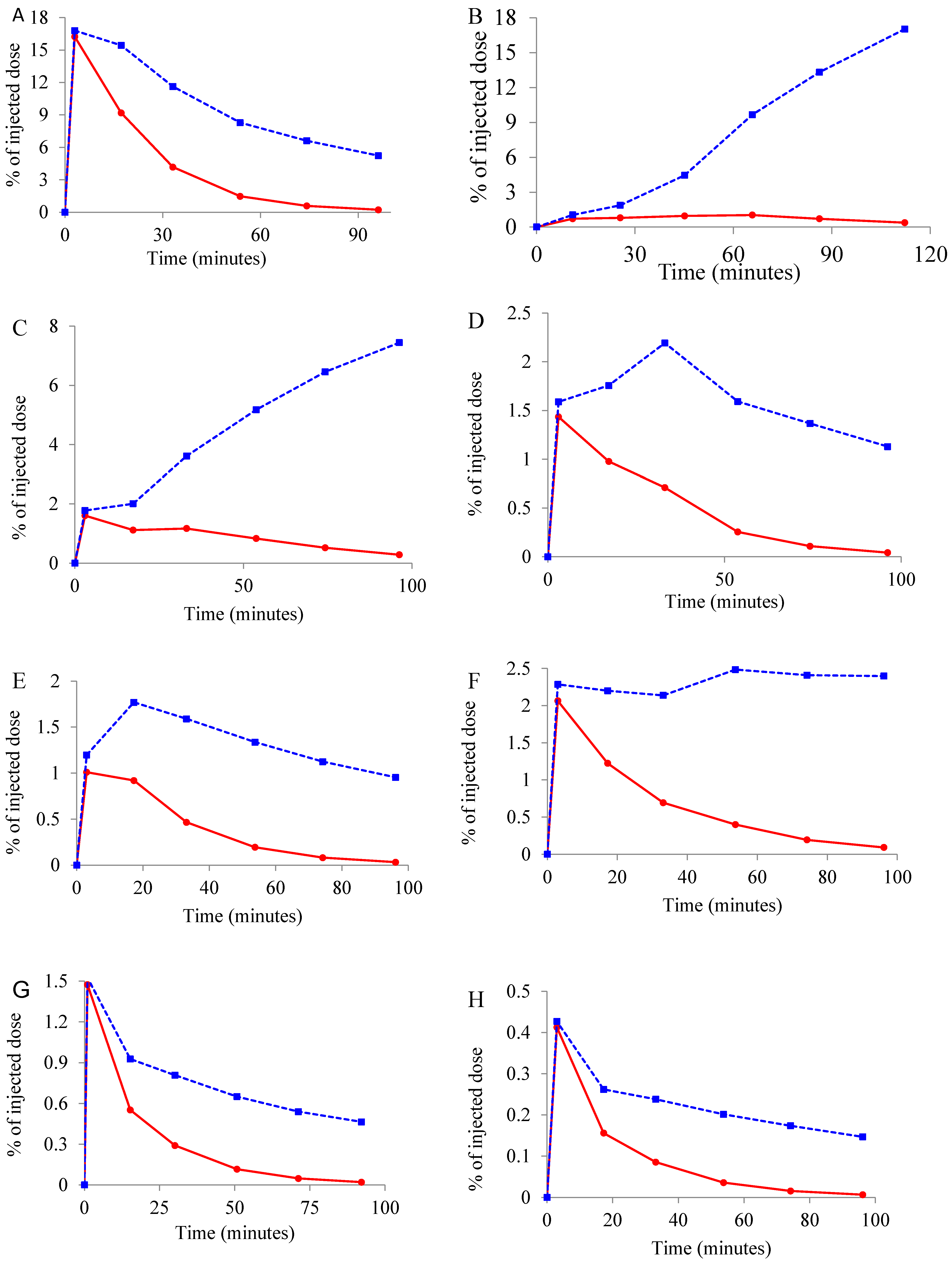
2.3. Whole Body Biodistribution and Radiation Dosimetry Estimation of [11C]Celecoxib in Baboons
3. Experimental
3.1. Brain PET Imaging and Quantification of [11C]Celecoxib in Baboons
3.2. Whole Body Biodistribution and Radiation Dosimetry Estimations of [11C]Celecoxib in Baboons
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzpatrick, W.D. Cyclooxygenase enzymes: Regulation and function. Curr. Pharm. Des. 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, J.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasojima, K.; Schwab, C.; McGeer, E.G.; McGeer, P.L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999, 830, 226–236. [Google Scholar] [CrossRef]
- Ho, L.; Pieroni, C.; Winger, D.; Purohit, D.P.; Aisen, P.S.; Pasinetti, G.M. Regional distribution of cyclooxygenase-2 in the hippocampal formation in Alzheimer’s disease. J. Neurosci. Res. 1999, 57, 295–303. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, N.; Zacharia, E.; Briasoulis, A.; Charakida, M.; Tousoulis, D. Celecoxib for the treatment of atherosclerosis. Expert Opin. Investig. Drugs 2016, 25, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, K.-P.; Tan, G.-S. Cyclooxygenase-2 inhibitors in lung cancer treatment: Bench to bed. Eur. J. Pharmacol. 2015, 769, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Venkat, A.; Chaitanya, P.; Neelima, T. Protective role of NSAIDs in the treatment of nonmelanoma skin cancer: A comprehensive review on animal and human trial studies. J. Pharma. Res. 2013, 2, 1–4. [Google Scholar]
- Marina, M.-A.; Alejandro, S.-L.; Fabian, S.-G.; Carmen, F.-L.; Helios, P.-G.; Nuria, G.; Alejandro, J. Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer’s disease: A systematic review and meta-Analysis of treatment effect. Drugs Aging 2015, 32, 139–147. [Google Scholar]
- Rayar, A.M.; Lagarde, N.; Ferroud, C.; Zagury, J.F.; Montes, M.; Sylla-Iyarret, V.M. Update on COX-2 selective inhibitors: Chemical classification, side effects and their use in cancers and neuronal diseases. Curr. Top. Med. Chem. 2017, 17, 2935–2956. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Zhang, Y.; Koboldt, C.M.; Muhammad, J.; Zweifel, B.S.; Shaffer, A.; Talley, J.J.; Masferrer, J.L.; Seibert, K.; Isakson, P.C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, J.L. Selective inhibitors of cyclooxygenase-2 (COX-2), celecoxib and parecoxib: A systematic review. Drugs Today (Barc.) 2010, 46 (Suppl. A), 1–25. [Google Scholar] [PubMed]
- Prabhakaran, J.; Underwood, M.D.; Majo, V.J.; Parsey, R.V.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J.; Kumar, J.S.D. Synthesis and in vivo evaluation of [18F]celecoxib: A potential PET probe for imaging COX-2 expression. Bioorg. Med. Chem. 2007, 15, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Paulson, S.K.; Zhang, J.Y.; Breau, A.P.; Hribar, J.D.; Liu, N.W.; Jessen, S.M.; Lawal, Y.M.; Cogburn, J.N.; Gresk, C.J.; Markos, C.S.; et al. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab. Dispos. 2000, 28, 514–521. [Google Scholar] [PubMed]
- Paulson, S.K.; Hribar, J.D.; Liu, N.W.; Hajdu, E.; Bible, R.H., Jr.; Piergies, A.; Karim, A. Metabolism and excretion of [14C]celecoxib in healthy male volunteers. Drug Metab. Dispos. 2000, 28, 308–314. [Google Scholar] [PubMed]
- Prabhakaran, J.; Majo, V.J.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J.; Kumar, J.S.D. Synthesis of [11C]celebrex: A PET probe for COX-2 expression. J. Label. Compd. Radiopham. 2005, 48, 887–895. [Google Scholar] [CrossRef]
- NIMH-PDSP. Available online: https://pdspdb.unc.edu/pdspWeb/ (accessed on 25 July 2018).
- Roth, B.L. NIMH-PDSP Assay Protocol Book. Available online: https://pdspdb.unc.edu/pdspWeb/content/UNC-CH%20Protocol%20Book.pdf (accessed on 25 July 2018).
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wolk, A.P.; Dewey, S.L.; Schlyer, D.J.; Macgregor, R.R.; Hitzmann, R.; Bendriem, B.; Gatley, S.J.; et al. Graphical analysis of reversible radioligandbinding from time-activity measurements applied to [N-11C-methyl-(2)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990, 10, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ogden, R.T. Estimation of kinetic parameters in graphical analysis of PET imaging data. Stat. Med. 2003, 22, 3557–3568. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.N.; Gunn, S.R.; Cunningham, V.J. Positron emission tomography compartmental models. J. Cereb. Blood Flow Metab. 2001, 21, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.S.D.; Majo, V.J.; Tamir, H.; Millak, M.S.; Hsing, S.-C.; Prabhakaran, J.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J.; Parsey, R.V. Synthesis and in vivo validation of [11C]MPT: A potential 5-HT1A receptor agonist PET ligand. J. Med. Chem. 2006, 49, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.N.; Sargent, P.A.; Bench, C.J.; Rabiner, E.A.; Osman, S.; Pike, V.W.; Hume, S.P.; Grasby, P.M.; Lammertsma, A.A. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage 1998, 8, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.J.; Simpson, N.R.; Wang, T.; Van Heertum, R.L.; Mann, J.J.; Parsey, R.V. Biodistribution and radiation dosimetry of [11C]DASB in baboons. Nucl. Med. Biol. 2004, 31, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Parsey, R.V.; Sokol, L.O.; Bélanger, M.J.; Kumar, J.S.D.; Simpson, N.R.; Wang, T.; Pratap, M.; Van Heertum, R.L.; Mann, J.J. Amyloid plaque imaging agent [C-11]-6-OH-BTA-1: Biodistribution and radiation dosimetry in baboon. Nucl. Med. Commun. 2005, 26, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Parsey, R.V.; Belanger, M.J.; Sullivan, G.M.; Simpson, N.R.; Stabin, M.G.; Van Heertum, R.L.; Mann, J.J. Biodistribution and Radiation Dosimetry of 11C-WAY100,635 in Humans. J. Nucl. Med. 2005, 46, 614–619. [Google Scholar] [PubMed]
- Murthy, R.; Erlandsson, K.; Kumar, J.S.D.; Van Heertum, R.L.; Mann, J.J.; Parsey, R.V. Biodistribution and radiation dosimetry of 11C-harmine in baboons. Nucl. Med. Commun. 2007, 28, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.S.D.; Bai, B.; Ng, H.H.; Mirsalis, J.C.; Erlandsson, K.; Milak, M.S.; Majo, V.J.; Prabhakaran, J.; Mann, J.J.; Parsey, R.V. Biodistribution, toxicology, and radiation dosimetry of 5-HT1A-receptor agonist positron emission tomography ligand [11C]CUMI-101. Int. J. Toxicol. 2011, 30, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; Postema, E.; Boerman, O.; Visschers, J.; Oyen, W.; Corstens, F. Software package for integrated data processing for internal dose assessment in nuclear medicine (SPRIND). Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 413–421. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Targets | Affinity (nM) | Targets | Affinity (nM) |
|---|---|---|---|
| COX-214 | 40 nM | COX-114 | 17,000 |
| 5-hydroxytryptamin 1-7 receptors | >10,000 | Adenosine receptors | >10,000 |
| Alpha1,2 receptors | >10,000 | AMPA receptors | >10,000 |
| Beta1-3 receptors | >10,000 | Calcium channel receptor | >10,000 |
| Benzodiazepine receptors | >10,000 | Dopamine 1-5 receptors | >10,000 |
| Cannabinoid 1,2 receptors | >10,000 | Dopamine transporter | 2487 |
| Delta opioid receptor | >10,000 | Prostanoid receptors | >10,000 |
| Prostaglandin receptors | >10,000 | Histamine 1-4 receptors | >10,000 |
| Gamma-aminobutyric acid receptors | >10,000 | Kainate receptors | >10,000 |
| Imidazoline receptor | >10,000 | Kappa Opioid receptor | >10,000 |
| Human Ether-à-go-go | >10,000 | Muscarinic 1-5 receptors | >10,000 |
| Norepinephrine transporter | >10,000 | mGluR receptors | >10,000 |
| Neurokinin receptors | >10,000 | Mu opioid receptor | >10,000 |
| Neurotensin receptors | >10,000 | Serotonin transporter | >10,000 |
| Sodium Channel | >10,000 | Smoothened receptor | >10,000 |
| Nociceptin opioid peptide receptor | >10,000 | Sigma 1, 2 receptors | >10,000 |
| NMDA receptors | >10,000 | VMAT receptors | >10,000 |
| Vanilloid receptor | >10,000 | NR2B receptor | >10,000 |
| Oxytocin receptors | >10,000 | Protein kinase C receptors | >10,000 |
| Peripheral benzodiazepine receptor | >10,000 | Vasopressin receptors | >10,000 |
| Organ | Residence Time |
|---|---|
| Gall Bladder | 0.015627 |
| Urinary Bladder | 0.01683 |
| Brain | 0.006497 |
| Heart | 0.00464 |
| Small Intestine | 0.010684 |
| Kidneys | 0.015125 |
| Liver | 0.063269 |
| Lungs | 0.002582 |
| Spleen | 0.001115 |
| Stomach wall | 0.003536 |
| Vertebra | 0.000769 |
| Remainder | 0.349441 |
| Organ | mGy/MBq | Mrad/mCi | Mrad/ID | % Limit |
|---|---|---|---|---|
| Adrenals | 3.45 × 10−3 | 1.28 × 101 | 3.83 × 102 | 7.7 |
| Brain | 1.82 × 10−3 | 6.73 | 2.02 × 102 | 4.0 |
| Breasts | 2.04 × 10−3 | 7.55 | 2.26 × 102 | 4.5 |
| Gallbladder Wall | 3.99 × 10−2 | 1.48 × 102 | 4.43 × 103 | 88.6 |
| LLI Wall | 2.95 × 10−3 | 1.09 × 101 | 3.27 × 102 | 6.5 |
| Small Intestine | 6.17 × 10−3 | 2.28 × 101 | 6.85 × 102 | 13.7 |
| Stomach Wall | 4.66 × 10−3 | 1.72 × 101 | 5.17 × 102 | 10.3 |
| ULI Wall | 3.55 × 10−3 | 1.31 × 101 | 3.94 × 102 | 7.9 |
| Heart Wall | 5.34 × 10−3 | 1.98 × 101 | 5.93 × 102 | 11.9 |
| Kidneys | 1.51 × 10−2 | 5.59 × 101 | 1.68 × 103 | 33.5 |
| Liver | 1.18 × 10−2 | 4.37 × 101 | 1.31 × 103 | 26.2 |
| Lungs | 1.97 × 10−3 | 7.29 | 2.19 × 102 | 4.4 |
| Muscle | 2.43 × 10−3 | 8.99 | 2.70 × 102 | 5.4 |
| Ovaries | ||||
| Pancreas | 3.65 × 10−3 | 1.35 × 101 | 4.05 × 102 | 8.1 |
| Red marrow | 2.23 × 10−3 | 8.25 | 2.48 × 102 | 8.3 |
| Osteogenic Cells | 3.28 × 10−3 | 1.21 × 101 | 3.64 × 102 | 7.3 |
| Skin | 1.90 × 10−3 | 7.03 | 2.11 × 102 | 4.2 |
| Spleen | 3.16 × 10−3 | 1.17 × 101 | 3.51 × 102 | 7.0 |
| Testes | 2.34 × 10−3 | 8.66 | 2.60 × 102 | 8.7 |
| Thymus | 2.40 × 10−3 | 8.88 | 2.66 × 102 | 5.3 |
| Thyroid | 2.25 × 10−3 | 8.33 | 2.50 × 102 | 5.0 |
| Urinary Bladder Wall | 1.38 × 10−2 | 5.11 × 101 | 1.53 × 103 | 30.6 |
| Uterus | ||||
| Total Body | 2.81 × 10−3 | 1.04 × 101 | 3.12 × 102 | |
| mSv/MBq | mrem/mCi | mrem/ID | ||
| Effective dose equivalent | 6.97 × 10−3 | 2.58 × 101 | 7.74 × 102 | |
| Effective dose | 4.04 × 10−3 | 1.49 × 101 | 4.48 × 102 | 14.9 |
| Organ | mGy/MBq | Mrad/mCi | Mrad/ID | % Limit |
|---|---|---|---|---|
| Adrenals | 4.37 × 10−3 | 1.62 × 101 | 4.85 × 102 | 9.7 |
| Brain | 2.18 × 10−3 | 8.07 | 2.42 × 102 | 4.8 |
| Breast | 2.60 × 10−3 | 9.62 | 2.89 × 102 | 5.8 |
| Gallbladder Wall | 4.47 × 10−2 | 1.65 × 102 | 4.96 × 103 | 99.2 |
| LLI Wall | 3.73 × 10−3 | 1.38 × 101 | 4.14 × 102 | 8.3 |
| Small Intestine | 7.22 × 10−3 | 2.67 × 101 | 8.01 × 102 | 16.0 |
| Stomach Wall | 5.65 × 10−3 | 2.09 × 101 | 6.27 × 102 | 12.5 |
| ULI Wall | 4.43 × 10−3 | 1.64 × 101 | 4.92 × 102 | 9.8 |
| Heart Wall | 6.94 × 10−3 | 2.57 × 101 | 7.70 × 102 | 15.4 |
| Kidneys | 1.66 × 10−2 | 6.14 × 101 | 1.84 × 103 | 36.9 |
| Liver | 1.56 × 10−2 | 5.77 × 101 | 1.73 × 103 | 34.6 |
| Lungs | 2.54 × 10−3 | 9.40 | 2.82 × 102 | 5.6 |
| Muscle | 3.03 × 10−3 | 1.12 × 101 | 3.36 × 102 | 6.7 |
| Ovaries | 3.90 × 10−3 | 1.44 × 101 | 4.33 × 102 | 14.4 |
| Pancreas | 4.53 × 10−3 | 1.68 × 101 | 5.03 × 102 | 10.1 |
| Red marrow | 2.72 × 10−3 | 1.01 × 101 | 3.02 × 102 | 10.1 |
| Osteogenic Cells | 4.39 × 10−3 | 1.62 × 101 | 4.87 × 102 | 9.7 |
| Skin | 2.38 × 10−3 | 8.81 | 2.64 × 102 | 5.3 |
| Spleen | 3.90 × 10−3 | 1.44 × 101 | 4.33 × 102 | 8.7 |
| Testes | ||||
| Thymus | 3.09 × 10−3 | 9.92 | 2.97 × 102 | 5.9 |
| Thyroid | 2.68 × 10−3 | 6.77 × 101 | 2.03 × 103 | 40.6 |
| Urinary Bladder Wall | 1.83 × 10−2 | 1.53 × 101 | 4.60 × 102 | 9.2 |
| Uterus | 4.14 × 10−3 | 0.00 × 10 | 0.00 | 0.0 |
| Total Body | 3.53 × 10−3 | 1.31 × 101 | 3.92 × 102 | |
| mSv/MBq | mrem/mCi | mrem/ID | ||
| Effective dose equivalent | 8.35 × 10−3 | 3.09 × 101 | 9.27 × 102 | |
| Effective dose | 4.83 × 10−3 | 1.79 × 101 | 5.36 × 102 | 17.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, J.S.D.; Bai, B.; Zanderigo, F.; DeLorenzo, C.; Prabhakaran, J.; Parsey, R.V.; Mann, J.J. In Vivo Brain Imaging, Biodistribution, and Radiation Dosimetry Estimation of [11C]Celecoxib, a COX-2 PET Ligand, in Nonhuman Primates. Molecules 2018, 23, 1929. https://doi.org/10.3390/molecules23081929
Kumar JSD, Bai B, Zanderigo F, DeLorenzo C, Prabhakaran J, Parsey RV, Mann JJ. In Vivo Brain Imaging, Biodistribution, and Radiation Dosimetry Estimation of [11C]Celecoxib, a COX-2 PET Ligand, in Nonhuman Primates. Molecules. 2018; 23(8):1929. https://doi.org/10.3390/molecules23081929
Chicago/Turabian StyleKumar, J. S. Dileep, Bing Bai, Francesca Zanderigo, Christine DeLorenzo, Jaya Prabhakaran, Ramin V. Parsey, and J. John Mann. 2018. "In Vivo Brain Imaging, Biodistribution, and Radiation Dosimetry Estimation of [11C]Celecoxib, a COX-2 PET Ligand, in Nonhuman Primates" Molecules 23, no. 8: 1929. https://doi.org/10.3390/molecules23081929