A New Kind of Quinonic-Antibiotic Useful Against Multidrug-Resistant S. aureus and E. faecium Infections
Abstract
:1. Introduction
2. Results and Discussion
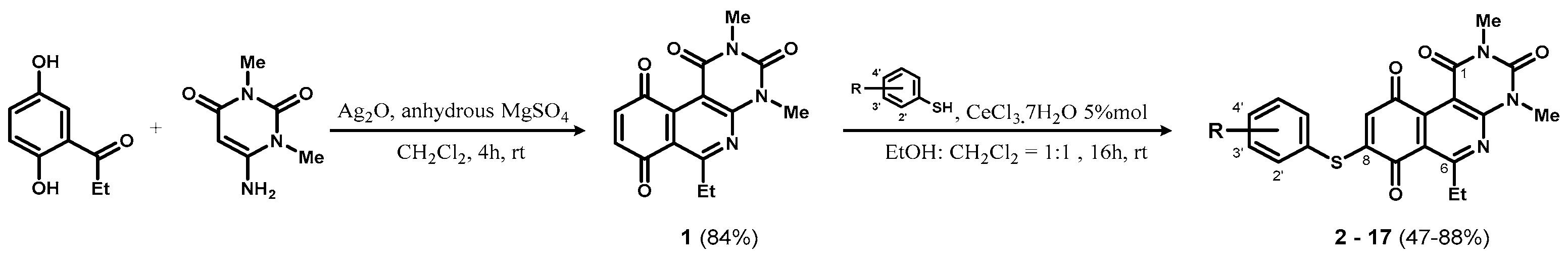
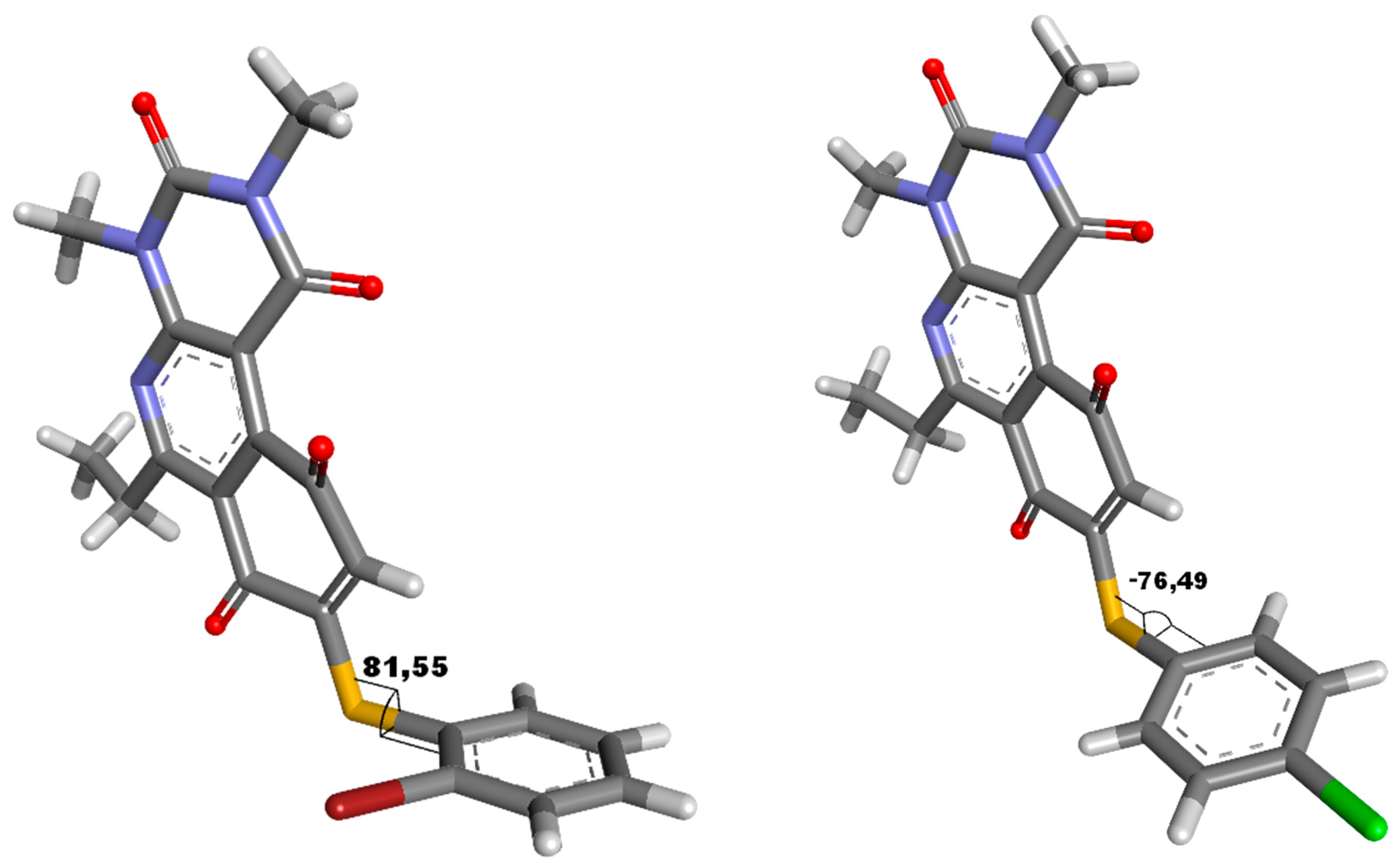
2.1. Synthesis and Determination of Physicochemical Parameters
2.2. Antibacterial Screening
2.3. Structure-Activity Relationship
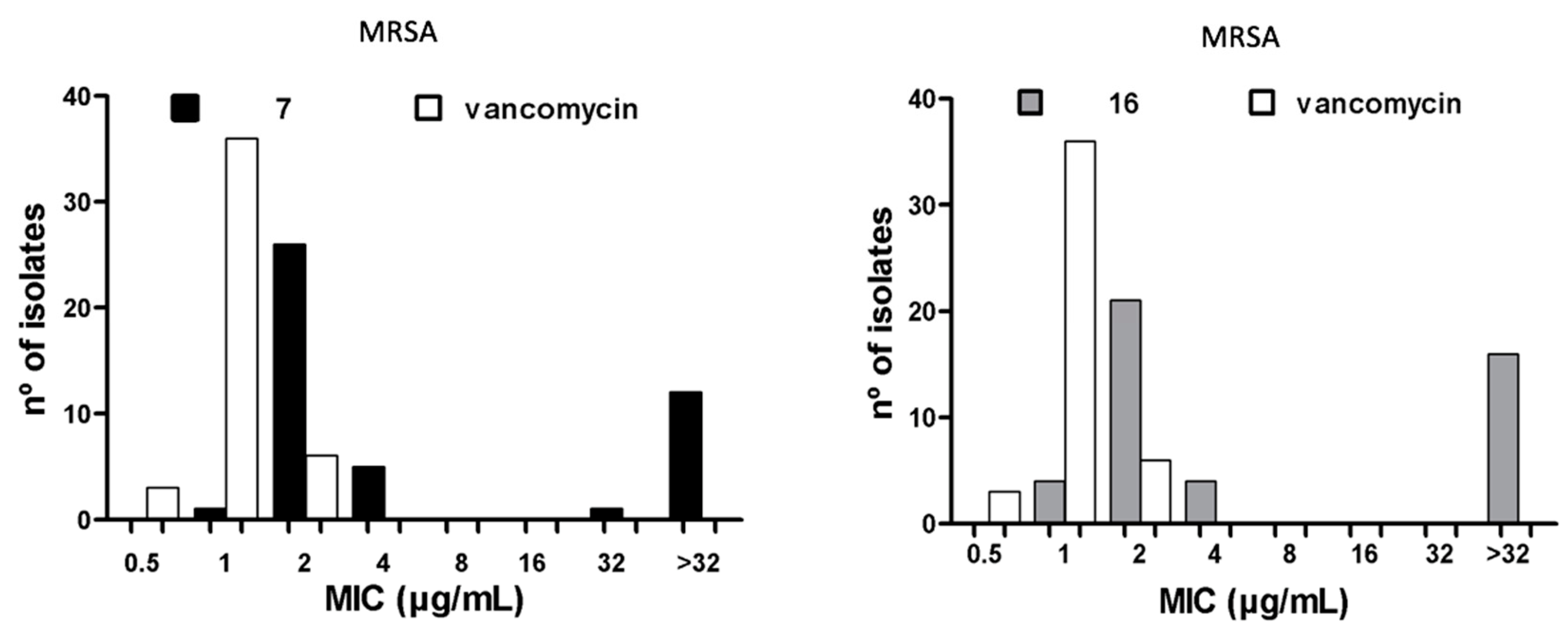
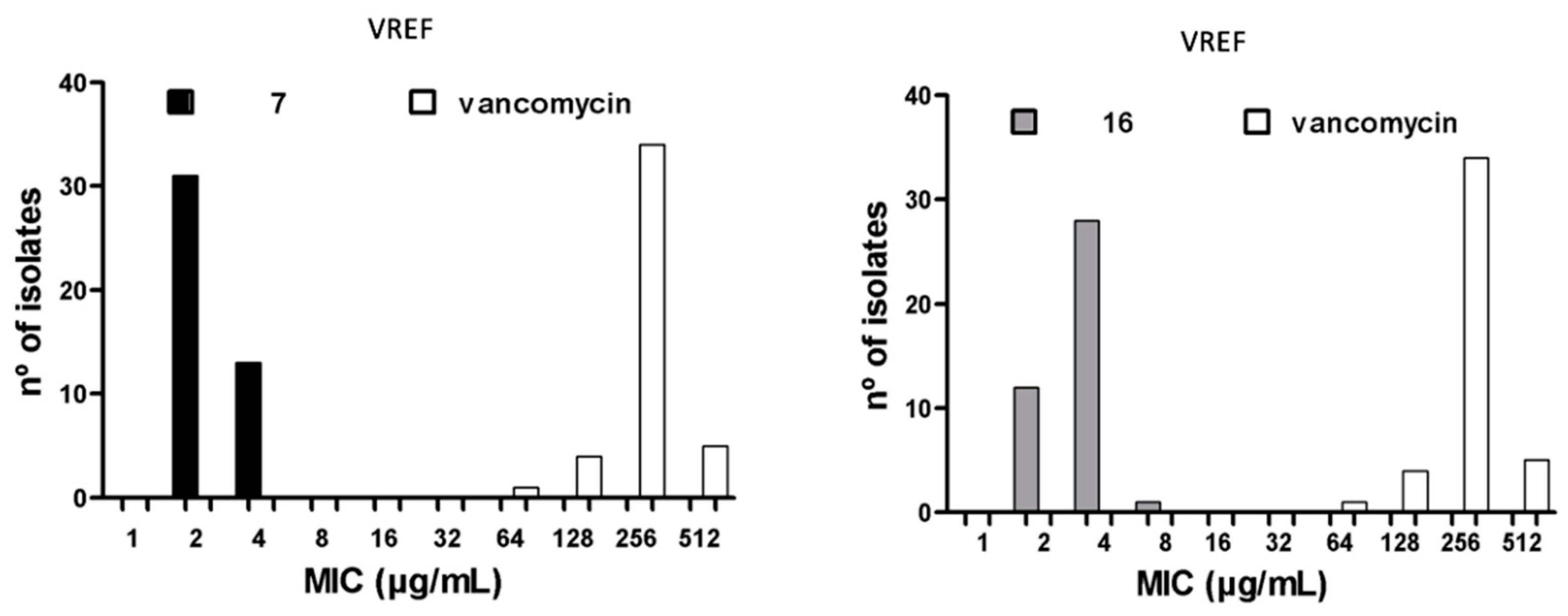
2.4. Antibacterial Activity of Compounds 7 and 16 Against Clinical MDR Isolates
2.5. Antibacterial Activity Against Heterogeneous Populations of Clinical Isolates
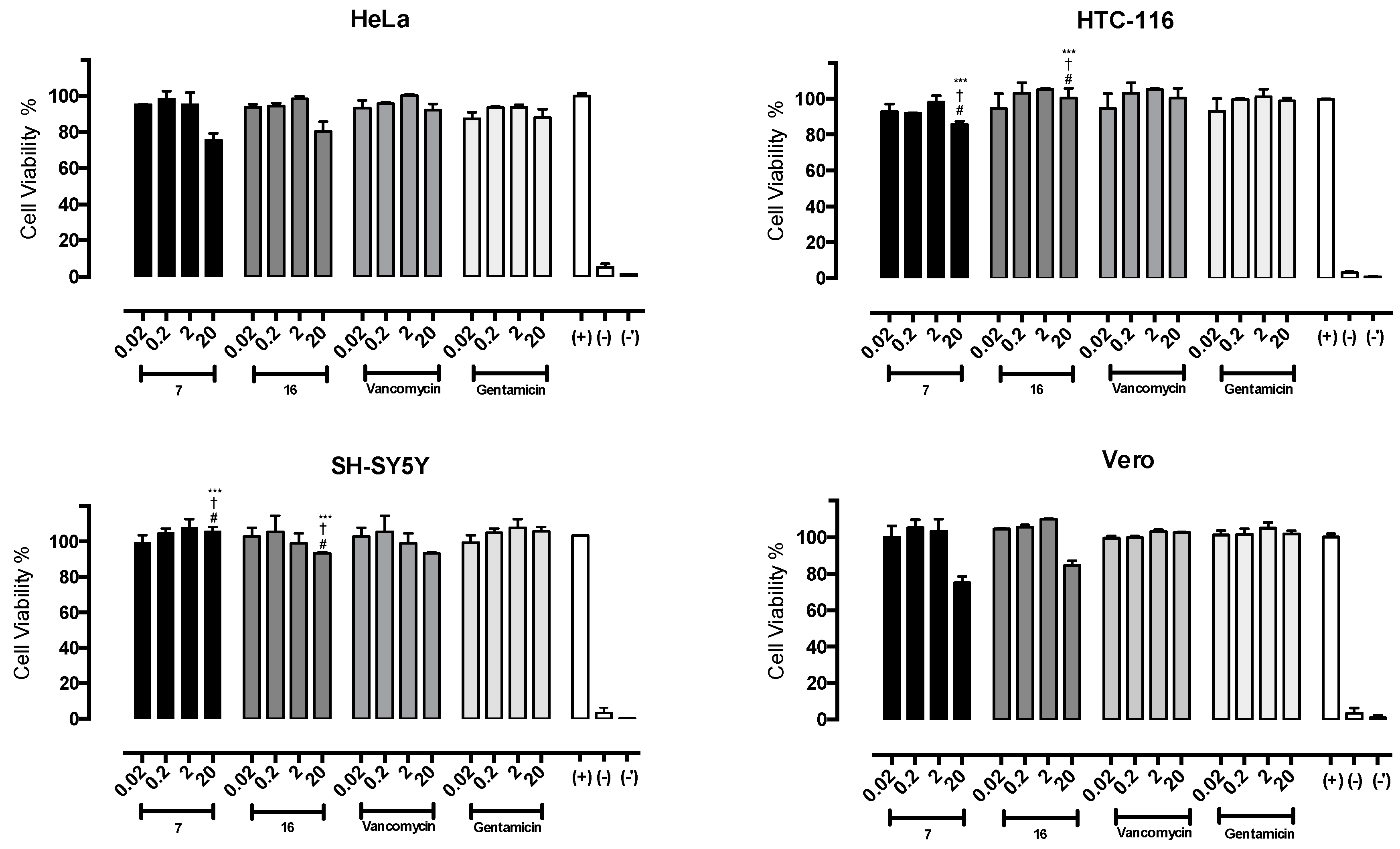
2.6. Cytotoxicity of Compounds 7 and 16
3. Materials and Methods
3.1. Materials
3.2. Chemical Synthesis
3.2.1. Procedure for the Synthesis of Compound 1
3.2.2. General Procedure for the Synthesis of Compounds 2–17
3.3. Crystalography
3.3.1. Preparation of Single Crystals
3.3.2. Single Crystal X-ray Diffraction
3.4. Evaluation of Antibacterial Activity and Cytotoxicity
3.4.1. Bacterial Strains
3.4.2. Evaluation of Antibacterial Activity
Minimal Inhibitory Concentration Determination
Minimal bactericidal concentration determination
3.4.3. Cell Cultures
3.4.4. Evaluation of Cellular Toxicity
3.5. Electrochemical Measuring
3.6. Determination of Theoretical Physicochemical Parameters
3.7. Statistical Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No eskape! An update from the infectious diseases society of america. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017; p. 7. [Google Scholar]
- CDC-Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/index.html (accessed on 10 July 2018).
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; WHO: Geneva, Switzerland, 2014; p. 232. [Google Scholar]
- Werner, G.; Coque, T.M.; Hammerum, A.M.; Hope, R.; Hryniewicz, W.; Johnson, A.; Klare, I.; Kristinsson, K.G.; Leclercq, R.; Lester, C.H.; et al. Emergence and spread of vancomycin resistance among enterococci in europe. Euro Surveill. 2008, 13, 5437–5453. [Google Scholar]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in us hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Moir, D.T.; Opperman, T.J.; Butler, M.M.; Bowlin, T.L. New classes of antibiotics. Curr. Opin. Pharmacol. 2012, 12, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Munoz-Crego, A. Looking for new targets: Simple coumarins as antibacterial agents. Med. Chem. 2012, 8, 1140–1145. [Google Scholar] [PubMed]
- Pucci, M.J. Novel genetic techniques and approaches in the microbial genomics era: Identification and/or validation of targets for the discovery of new antibacterial agents. Drugs R D 2007, 8, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M. Synthetic strategies to terpene quinones/hydroquinones. Mar. Drugs 2012, 10, 358–402. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W. The mechanism of action of quinone antibiotics. Mol. Cell. Biochem. 1983, 55, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Ray, R.; Hazra, B. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Amani, A.M. Synthesis, characterization and antibacterial and antifungal evaluation of some para-quinone derivatives. Drug Res. 2014, 64, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angewandte Chem. Int. Ed. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Kubo, A.; Nakahara, S.; Inaba, K.; Kitahara, Y. Synthesis of renierone, 7-methoxy-1,6-dimethyl-5,8-dihydroisoquinoline-5,8-dione and N-formyl-1,2-dihydrorenierone, antimicrobial metabolites from a marine sponge, reniera sp. Chem. Pharm. Bull. 1986, 34, 4056–4068. [Google Scholar] [CrossRef] [PubMed]
- Swapnaja, K.J.; Yennam, S.; Chavali, M.; Poornachandra, Y.; Kumar, C.G.; Muthusamy, K.; Jayaraman, V.B.; Arumugam, P.; Balasubramanian, S.; Sriram, K.K. Design, synthesis and biological evaluation of diaziridinyl quinone isoxazole hybrids. Eur. J. Med. Chem. 2016, 117, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Subramani, R.; Aalbersberg, W. Three bioactive sesquiterpene quinones from the fijian marine sponge of the genus hippospongia. Nat. Prod. Res. 2013, 27, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.K.; Yadav, D.B.; Singh, R.V.; Chaturvedi, A.K.; Shukla, P.K. Synthesis and biological evaluation of novel (l)-alpha-amino acid methyl ester, heteroalkyl, and aryl substituted 1,4-naphthoquinone derivatives as antifungal and antibacterial agents. Bioorg. Med. Chem. Lett. 2005, 15, 5324–5328. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.K.; Yadav, D.B.; Singh, R.V.; Vaish, M.; Chaturvedi, A.K.; Shukla, P.K. Synthesis and biological evaluation of novel 1,4-naphthoquinone derivatives as antibacterial and antiviral agents. Bioorg. Med. Chem. Lett. 2005, 15, 3463–3466. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [PubMed]
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Cockerill, F.R., III; Bradford, P.A.; Eliopoulos, G.M.; Hindler, J.A.; Jenkins, S.G.; Lewis, J.S., II; Limbago, B.; Nicolau, D.P.; Powell, M.; et al. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. Clin. Lab. Stand. Inst. 2015, 35, M100-S25. [Google Scholar]
- Verrax, J.; Beck, R.; Dejeans, N.; Glorieux, C.; Sid, B.; Pedrosa, R.C.; Benites, J.; Vasquez, D.; Valderrama, J.A.; Calderon, P.B. Redox-active quinones and ascorbate: An innovative cancer therapy that exploits the vulnerability of cancer cells to oxidative stress. Anti-Cancer Agents Med. Chem. 2011, 11, 213–221. [Google Scholar] [CrossRef]
- Brunmark, A.; Cadenas, E. Redox and addition chemistry of quinoid compounds and its biological implications. Free Radic. Biol. Med. 1989, 7, 435–477. [Google Scholar] [CrossRef]
- Hammett, L.P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Q.; Zhang, R.; He, W.; Ma, X.; Zhang, J.; Xia, F.; Zhao, F.; Cao, J.; Liu, Y.; et al. In vitro antimicrobial activity of the novel oxazolidinone tedizolid and comparator agents against staphylococcus aureus and linezolid-resistant Gram-positive pathogens: A multicentre study in china. Int. J. Antimicrob. Agents 2014, 44, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Prokocimer, P.; Bien, P.; Deanda, C.; Pillar, C.M.; Bartizal, K. In vitro activity and microbiological efficacy of tedizolid (TR-700) against Gram-positive clinical isolates from a phase 2 study of oral tedizolid phosphate (TR-701) in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 2012, 56, 4608–4613. [Google Scholar] [CrossRef] [PubMed]
- Sahm, D.F.; Deane, J.; Bien, P.A.; Locke, J.B.; Zuill, D.E.; Shaw, K.J.; Bartizal, K.F. Results of the surveillance of tedizolid activity and resistance program: In vitro susceptibility of Gram-positive pathogens collected in 2011 and 2012 from the united states and europe. Diagn. Microbiol. Infect. Dis. 2015, 81, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Schaadt, R.; Sweeney, D.; Shinabarger, D.; Zurenko, G. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob. Agents Chemother. 2009, 53, 3236–3239. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.S.; Goering, R.V. Activity of tedizolid (TR-700) against well-characterized methicillin-resistant staphylococcus aureus strains of diverse epidemiological origins. Antimicrob. Agents Chemother. 2013, 57, 2892–2895. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Baguneid, M.; Bouza, E.; Dryden, M.; Nathwani, D.; Wilcox, M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014, 20 (Suppl. 4), 3–18. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Poppe, S.; Schaadt, R.; Brown-Driver, V.; Finn, J.; Pillar, C.M.; Shinabarger, D.; Zurenko, G. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob. Agents Chemother. 2008, 52, 4442–4447. [Google Scholar] [CrossRef] [PubMed]
- WA, C. Post antibiotic effect. In Antibiotics in Laboratory Medicine, 2nd ed.; Victor, L., Ed.; Williams and Wilkins: Baltimore, MD, USA, 1996; pp. 296–329. [Google Scholar]
- Valderrama, J.A.; Gonzalez, M.F.; Colonelli, P.; Vasquez, D. Design and synthesis of angucyclinone 5-aza analogues. Synlett 2006, 2777–2780. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Colonelli, P.; Vasquez, D.; Gonzalez, M.F.; Rodriguez, J.A.; Theoduloz, C. Studies on quinones. Part 44: Novel angucyclinone n-heterocyclic analogues endowed with antitumoral activity. Bioorg. Med. Chem. 2008, 16, 10172–10181. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Velasquez, D.R. Diseño, Síntesis y Evaluación Antitumoral de Aza-Análogos de Anguciclinonas y Derivados de Aminopirimidoisoquinolinquinonas. Ph.D Thesis, Pontificia Universidad Católica de Chile, Santiago, Chile, 2009. [Google Scholar]
- Vasquez, D.; Rodriguez, J.A.; Theoduloz, C.; Calderon, P.B.; Valderrama, J.A. Studies on quinones. Part 46. Synthesis and in vitro antitumor evaluation of aminopyrimidoisoquinolinequinones. Eur. J. Med. Chem. 2010, 45, 5234–5242. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, J.A.; Ibacache, A.; Rodriguez, J.A.; Theoduloz, C.; Benites, J. Studies on quinones. Part 47. Synthesis of novel phenylaminophenanthridinequinones as potential antitumor agents. Eur. J. Med. Chem. 2011, 46, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Francart, T.; van Wieringen, A.; Wouters, J. Apex 3: A multi-purpose test platform for auditory psychophysical experiments. J. Neurosci. Methods 2008, 172, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS-2012/1. Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G. A short history of shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Sader, H.S.; Flamm, R.K.; Farrell, D.J.; Jones, R.N. Telavancin in vitro activity against a collection of methicillin-resistant staphylococcus aureus isolates, including resistant subsets, from the united states. Antimicrob. Agents Chemother. 2015, 59, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition. Clin. Lab. Stand. Inst. 2012, 32, M07-A9. [Google Scholar]
- Pearson, R.D.; Steigbigel, R.T.; Davis, H.T.; Chapman, S.W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 1980, 18, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Gudmundsson, S. Antibiotics in Laboratory Medicine, 4th ed.; Lorian, V., Ed.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1996; pp. 296–329. [Google Scholar]
- Taylor, P.C.; Schoenknecht, F.D.; Sherris, J.C.; Linner, E.C. Determination of minimum bactericidal concentrations of oxacillin for staphylococcus aureus: Influence and significance of technical factors. Antimicrob. Agents Chemother. 1983, 23, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | R | MR (cm3/mol) of R | LogP | E1/2 (mV) | Mol wt (g/mol) | MIC (µg/mL) | ||||
| MRSA | MSSA | E. faecalis | E. coli | P. aeruginosa | ||||||
| 1 | - | - | 0.55 | −0.539 | 299.28 | >32 | >32 | >32 | >32 | >32 |
| 2 | -H | 0.80 | 0.74 | −0.508 | 407.44 | 8 | 8 | 8 | >32 | >32 |
| 3 | 2′-Me | 6.08 | 2.41 | −0.549 | 421.47 | 32 | 32 | >32 | >32 | >32 |
| 4 | 2′-OMe | 7.24 | 1.80 | −0.568 | 437.47 | 2 | 4 | 4 | >32 | >32 |
| 5 | 2′-F | 1.64 | 2.09 | −0.564 | 425.43 | >32 | >32 | >32 | >32 | >32 |
| 6 | 2′-Cl | 5.93 | 2.49 | −0.623 | 441.89 | >32 | >32 | >32 | >32 | >32 |
| 7 | 2′-Br | 9.06 | 2.76 | −0.604 | 486.34 | 1 | 4 | 2 | >32 | >32 |
| 8 | 3′-Me | 6.08 | 2.41 | −0.510 | 421.47 | 4 | 4 | 4 | >32 | >32 |
| 9 | 3′-OMe | 7.24 | 1.80 | −0.551 | 437.47 | 4 | 8 | 4 | >32 | >32 |
| 10 | 3′-F | 1.64 | 2.09 | −0.427 | 425.43 | 4 | 4 | 8 | >32 | >32 |
| 11 | 3′-Cl | 5.93 | 2.49 | −0.374 | 441.89 | 2 | 32 | 4 | >32 | >32 |
| 12 | 3′-Br | 9.06 | 2.76 | −0.443 | 486.34 | 2 | 32 | 4 | >32 | >32 |
| 13 | 4′-Me | 6.08 | 2.41 | −0.519 | 421.47 | 4 | 4 | 16 | >32 | >32 |
| 14 | 4′-OMe | 7.24 | 1.80 | −0.520 | 437.47 | 16 | 16 | 16 | >32 | >32 |
| 15 | 4′-F | 1.64 | 2.09 | −0.484 | 425.43 | 8 | 8 | 8 | >32 | >32 |
| 16 | 4′-Cl | 5.93 | 2.49 | −0.494 | 441.89 | 4 | 4 | 4 | >32 | >32 |
| 17 | 4′-Br | 9.06 | 2.76 | −0.501 | 486.34 | 4 | 8 | 8 | >32 | >32 |
| VAN a | 1 | 1 | 1 | - | - | |||||
| GEN b | - | - | - | 0.5 | 1 | |||||
| Compound | Isolates | MIC [b] Range | MIC50 | MIC90 | GM [c] MIC | MBC [d] Range | MBC50 | MBC90 | GM [c] MBC | MBC50/MIC50 | MBC90/MIC90 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 32 [a] | 4–1 | 2 | 2 | 2.3 | 8–2 | 4 | 4 | 2.89 | 2 | 2 |
| 16 | 29 [a] | 4–1 | 2 | 4 | 2.11 | 4–1 | 2 | 4 | 3 | 1 | 1 |
| VAN | 45 | 2–1 | 1 | 1 | 1.13 | ND | ND | ND | ND | - | - |
| Compound | Isolates | MIC [b] Range | MIC50 | MIC90 | GM [c] MIC | MBC [d] Range | MBC50 | MBC90 | GM [c] MBC | MBC50/MIC50 | MBC90/MIC90 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 44 | 4–2 | 2 | 4 | 2.51 | 8–2 | 4 | 4 | 3.42 | 2 | 1 |
| 16 | 41 [a] | 8–2 | 4 | 4 | 3.13 | 8–2 | 4 | 8 | 4 | 1 | 2 |
| VAN | 44 | 512–128 | 256 | 512 | 256.3 | ND | ND | ND | ND | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanini-Salinas, J.; Andrades-Lagos, J.; Gonzalez Rocha, G.; Choquesillo-Lazarte, D.; Bollo Dragnic, S.; Faúndez, M.; Alarcón, P.; Silva, F.; Vidal, R.; Salas-Huenuleo, E.; et al. A New Kind of Quinonic-Antibiotic Useful Against Multidrug-Resistant S. aureus and E. faecium Infections. Molecules 2018, 23, 1776. https://doi.org/10.3390/molecules23071776
Campanini-Salinas J, Andrades-Lagos J, Gonzalez Rocha G, Choquesillo-Lazarte D, Bollo Dragnic S, Faúndez M, Alarcón P, Silva F, Vidal R, Salas-Huenuleo E, et al. A New Kind of Quinonic-Antibiotic Useful Against Multidrug-Resistant S. aureus and E. faecium Infections. Molecules. 2018; 23(7):1776. https://doi.org/10.3390/molecules23071776
Chicago/Turabian StyleCampanini-Salinas, Javier, Juan Andrades-Lagos, Gerardo Gonzalez Rocha, Duane Choquesillo-Lazarte, Soledad Bollo Dragnic, Mario Faúndez, Pedro Alarcón, Francisco Silva, Roberto Vidal, Edison Salas-Huenuleo, and et al. 2018. "A New Kind of Quinonic-Antibiotic Useful Against Multidrug-Resistant S. aureus and E. faecium Infections" Molecules 23, no. 7: 1776. https://doi.org/10.3390/molecules23071776










