Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization
Abstract
:1. Introduction
2. Results
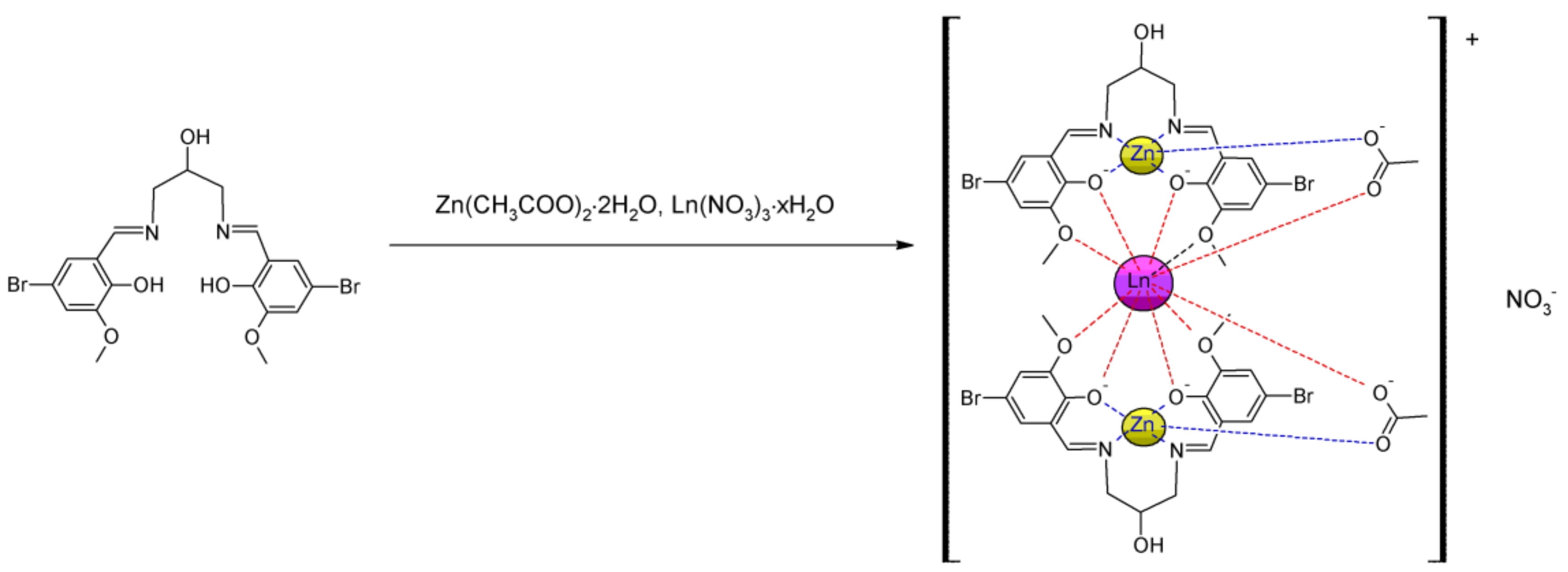
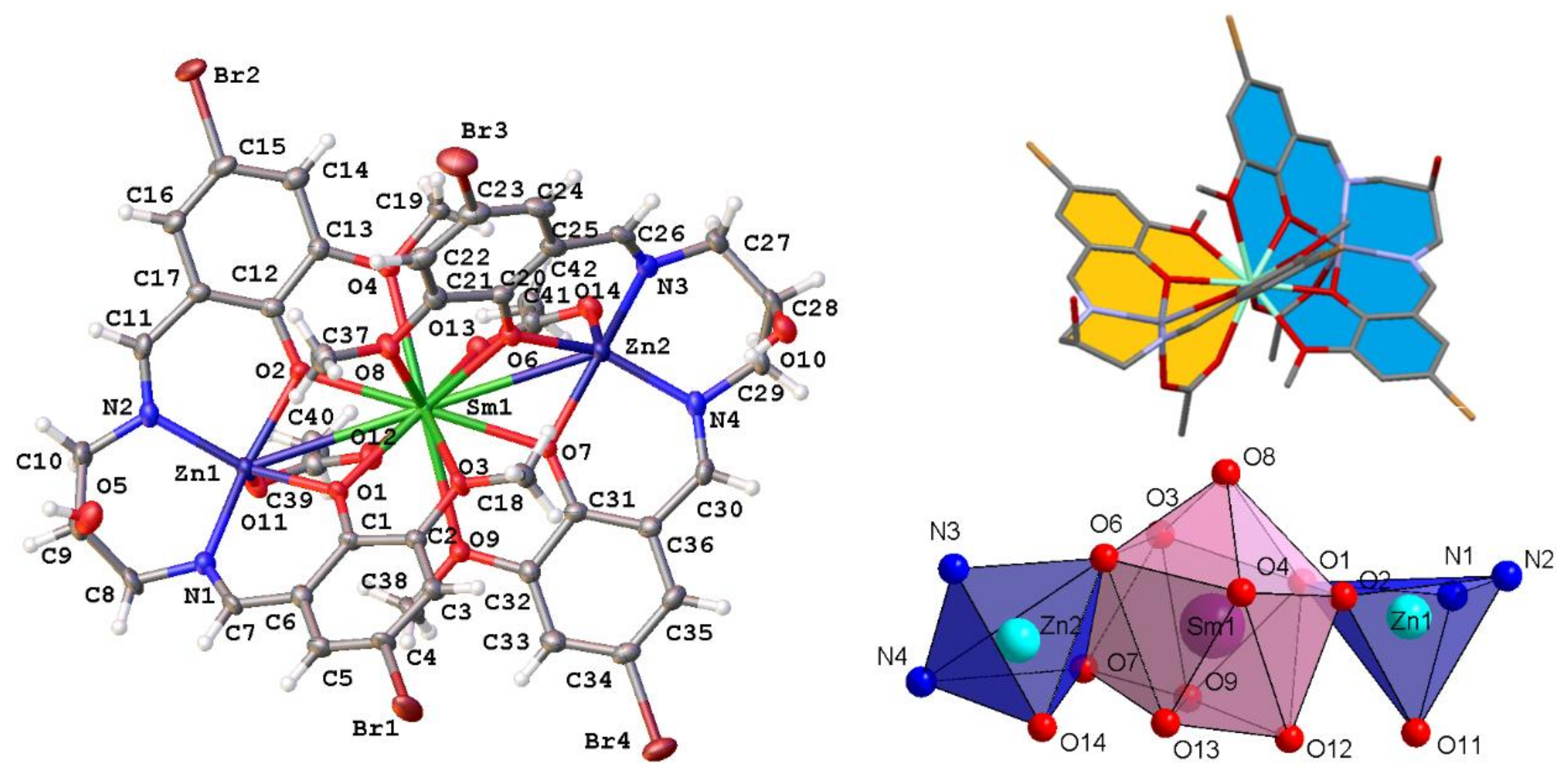
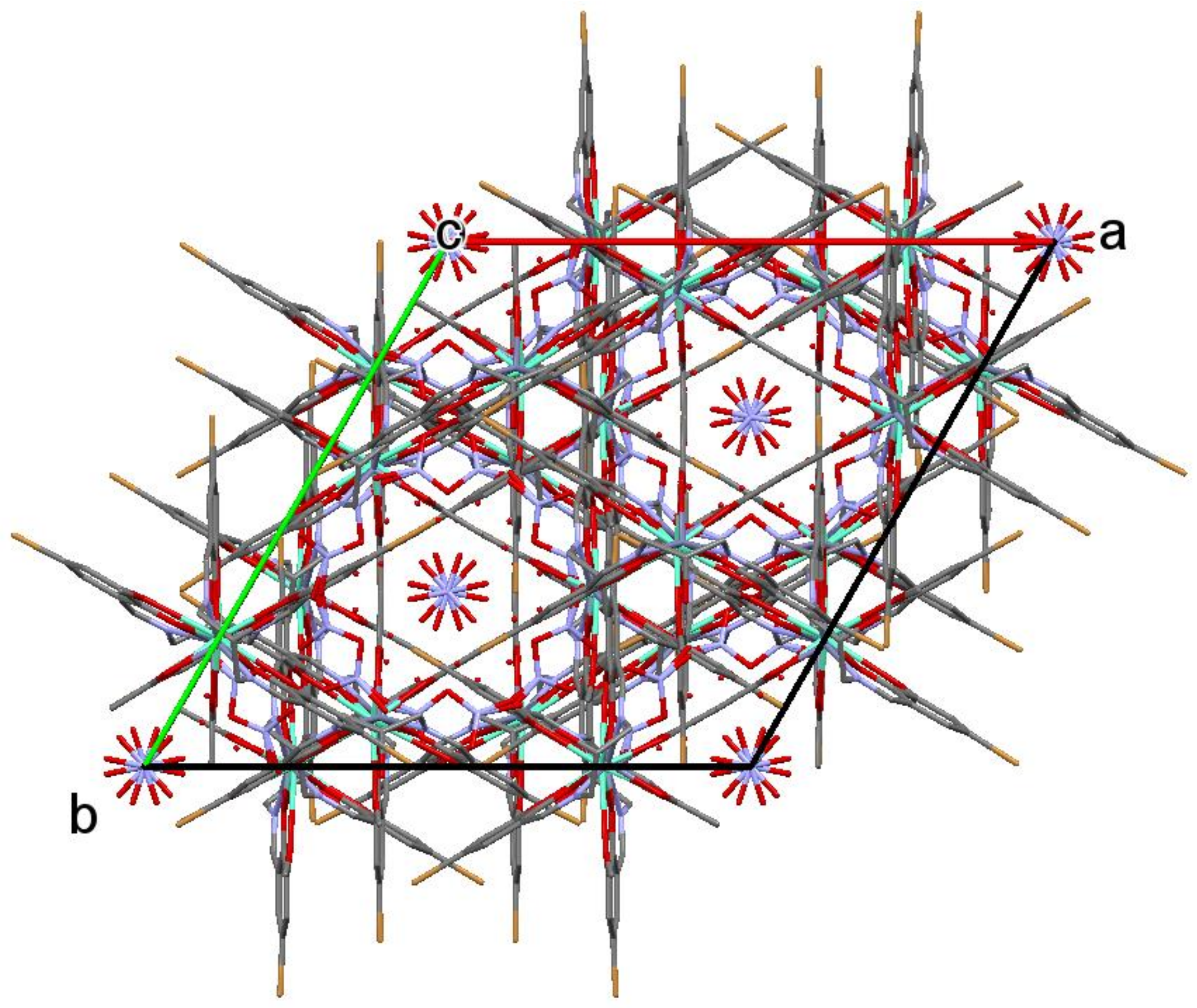
2.1. Crystal Structure
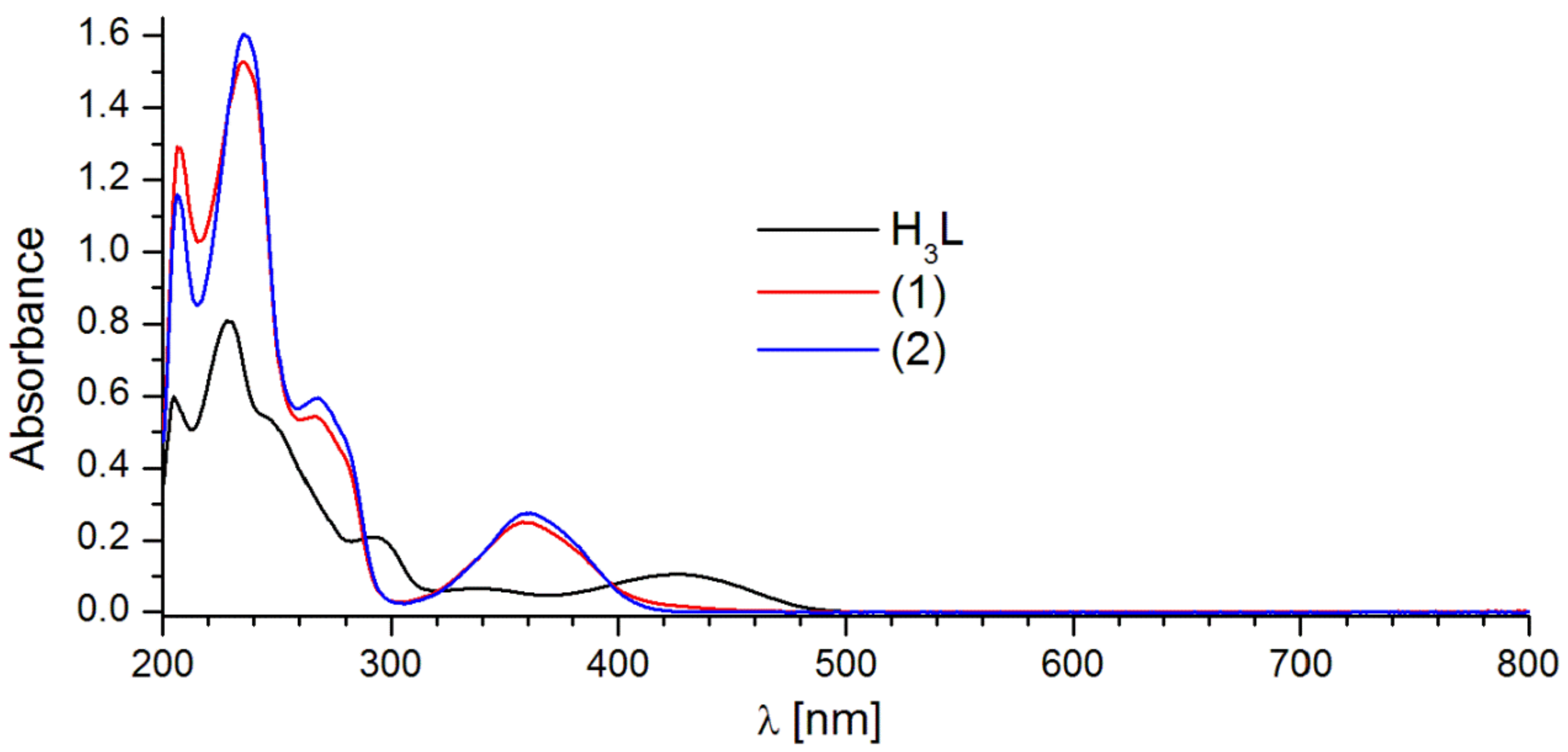
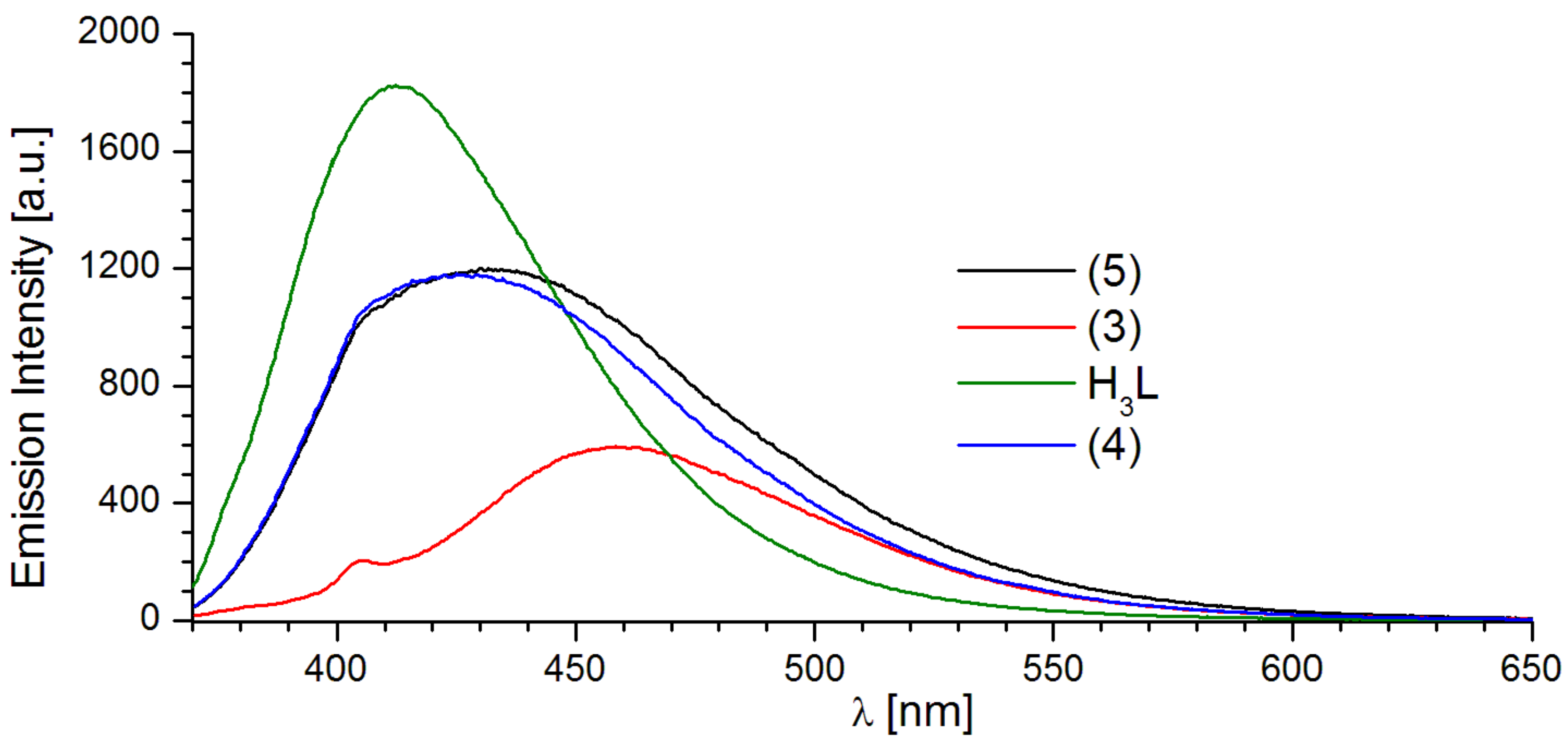
2.2. Luminescent Properties
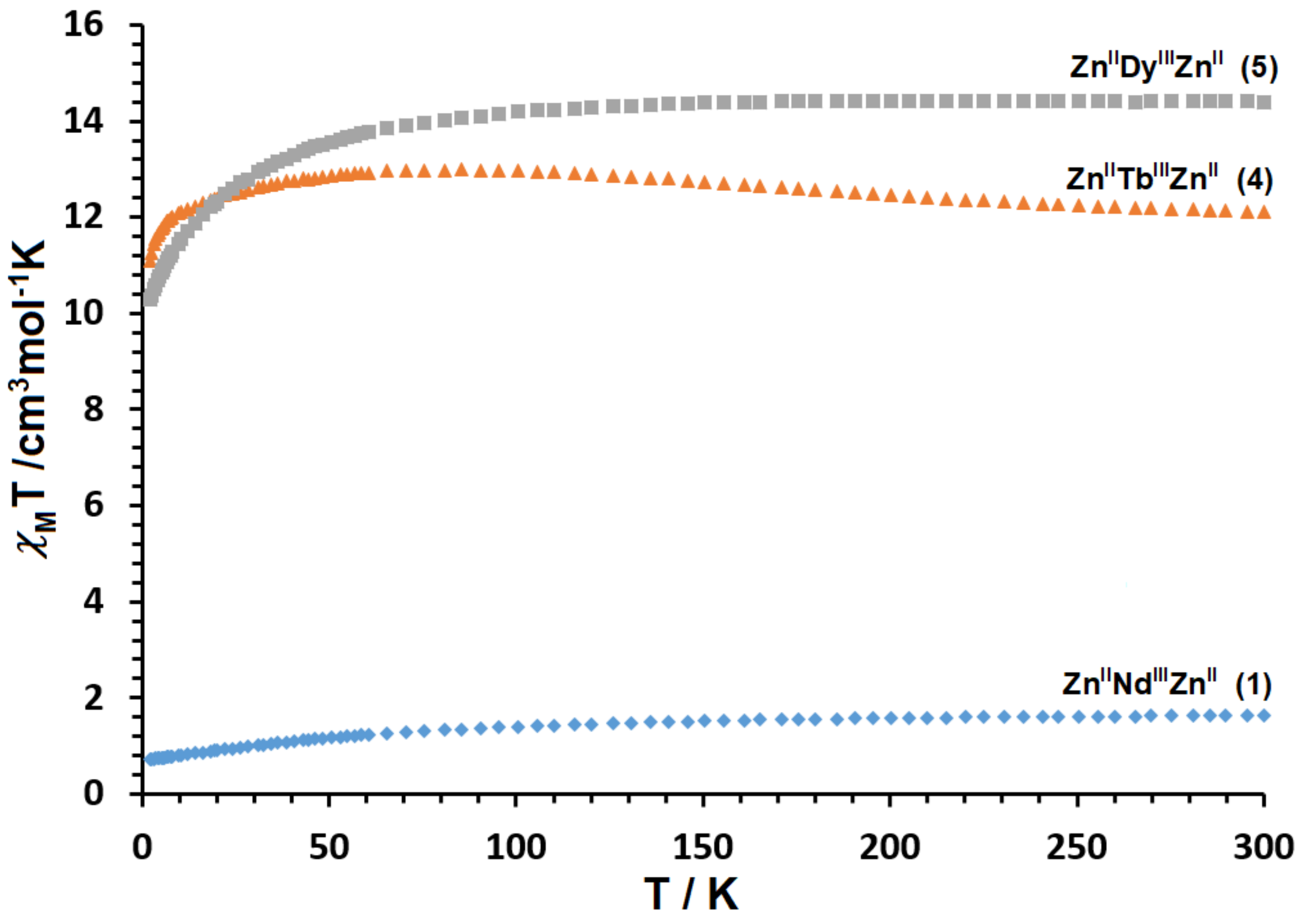
2.3. Magnetic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the H3L
3.3. Synthesis of the ZnII–LnIII–ZnII Complexes
3.3.1. [Zn2Nd(ac)2(HL)2]NO3·3H2O (1)
3.3.2. [Zn2Sm(ac)2(HL)2]NO3·3CH3OH·0.3H2O (2)
3.3.3. [Zn2Eu(ac)2(HL)2]NO3·5.33H2O (3)
3.3.4. [Zn2Tb(ac)2(HL)2]NO3·5.33H2O (4)
3.3.5. [Zn2Dy(ac)2(HL)2]NO3·5.33H2O (5)
3.4. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schmitz, S.; van Leusen, J.; Izarova, N.V.; Lan, Y.; Wernsdorfer, W.; Kögerler, P.; Monakhov, K.Y. Supramolecular 3d-4f Single-Molecule Magnet Architectures. Dalton Trans. 2016, 45, 16148–16152. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Tsipis, A.C.; Kumar, P.; Townrow, O.P.E.; Abdul-Sada, A.; Akien, G.R.; Baldansuren, A.; Spivey, A.C.; Kostakis, G.E. 3d/4f Coordination Clusters as Cooperative Catalysts for Highly Diastereoselective Michael Addition Reactions. Inorg. Chem. 2017, 56, 9563–9573. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.-Z.; Chen, Q.-S.; Zhang, C.-J.; Li, P.-X.; Wang, M.-S.; Guo, G.-C. Photochromism and Photomagnetism of a 3d-4f Hexacyanoferrate at Room Temperature. J. Am. Chem. Soc. 2015, 137, 10882–10885. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Li, H.-F.; Chen, P.; Gao, T.; Sun, W.-B.; Li, G.-M.; Yan, P.-F. Synthesis, Structure, and Tunable White Light Emission of Heteronuclear Zn2Ln2 Arrays Using a Zinc Complex as Ligand. CrystEngComm 2016, 18, 917–923. [Google Scholar] [CrossRef]
- Wong, W.-K.; Liang, H.; Wong, W.-Y.; Cai, Z.; Li, K.-F.; Cheah, K.-W. Synthesis and near-Infrared Luminescence of 3d-4f Bi-Metallic Schiff Base Complexes. New J. Chem. 2002, 26, 275–278. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, W.; Huang, X.; Cheng, Y.; Shen, P. Selective Fluorescent Probe Based on Schiff Base Derived from Hydroxymethyl Coumarin and Aminated Sudan I Dye for Mg2+ Detection. Arab. J. Chem. 2017, 10, S2729–S2735. [Google Scholar] [CrossRef]
- Jiang, X.-J.; Li, M.; Lu, H.-L.; Xu, L.-H.; Xu, H.; Zang, S.-Q.; Tang, M.-S.; Hou, H.-W.; Mak, T.C.W. A Highly Sensitive C3-Symmetric Schiff-Base Fluorescent Probe for Cd2+. Inorg. Chem. 2014, 53, 12665–12667. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Yang, Z.; Hao, L.; Wang, Z.; Luo, T.; Obst, M.; Liu, P.; Shen, Y.; Zhang, S.; Li, J. A Novel Approach to Study the Structure-Property Relationships and Applications in Living Systems of Modular Cu2+ Fluorescent Probes. Sci. Rep. 2016, 6, 28972. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Majeed, A.; Al-Sammarrae, K.; Salih, N.; Salimon, J.; Abdullah, B. Metal Complexes of Schiff Base: Preparation, Characterization and Antibacterial Activity. Arab. J. Chem. 2017, 10, S1639–S1644. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Zhang, D.-Y.; Suo, J.-J.; Gu, W.; Tian, J.-L.; Liu, X.; Yan, S.-P. Synthesis, Magnetism and Spectral Studies of Six Defective Dicubane Tetranuclear {M4O6} (M = NiII, CoII, ZnII) and Three Trinuclear CdII Complexes with Polydentate Schiff Base Ligands. Dalton Trans. 2016, 45, 10233–10248. [Google Scholar] [CrossRef] [PubMed]
- Sivchik, V.V.; Solomatina, A.I.; Chen, Y.-T.; Karttunen, A.J.; Tunik, S.P.; Chou, P.-T.; Koshevoy, I.O. Halogen Bonding to Amplify Luminescence: A Case Study Using a Platinum Cyclometalated Complex. Angew. Chem. Int. Ed. 2015, 54, 14057–14060. [Google Scholar] [CrossRef] [PubMed]
- Sanetra, J.; Armatys, P.; Chrzaszcz, R.; Pielichowski, J.; Barta, P.; Niziol, S.; Saliraoui, B. Synthesis and Luminescent Properties of Br-Substituted Poiy(n-Vinylcarbazoles). Synth. Met. 1999, 101, 82–83. [Google Scholar] [CrossRef]
- Van Deun, R.; Fias, P.; Driesen, K.; Binnemans, K.; Görller-Walrand, C. Halogen Substitution as an Efficient Tool to Increase the near-Infrared Photoluminescence Intensity of Erbium(III) Quinolinates in Non-Deuterated DMSO. Phys. Chem. Chem. Phys. 2003, 5, 2754–2757. [Google Scholar] [CrossRef]
- Albrecht, M.; Osetska, O.; Klankermayer, J.; Fröhlich, R.; Gumy, F.; Bünzli, J.-C.G. Enhancement of near-IR Emission by Bromine Substitution in Lanthanide Complexes with 2-Carboxamide-8-Hydroxyquinoline. Chem. Commun. 2007, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi-Anarjan, P.; Bikas, R.; Hosseini-Monfared, H.; Aleshkevych, P.; Mayer, P. Synthesis, Characterization, EPR Spectroscopy and Catalytic Activity of a New oxidovanadium(IV) Complex with N2O2-Donor Ligand. J. Mol. Struct. 2017, 1131, 258–265. [Google Scholar] [CrossRef]
- Datta, A.; Das, K.; Massera, C.; Clegg, J.K.; Sinha, C.; Huang, J.-H.; Garribba, E. A Mixed Valent Heterometallic CuII/NaI Coordination Polymer with Sodium–phenyl Bonds. Dalton Trans. 2014, 43, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, Y.; Liu, X.; Tian, J.; Yan, S. Three Series of Heterometallic NiII–LnIII Schiff Base Complexes: Synthesis, Crystal Structures and Magnetic Characterization. Dalton Trans. 2017, 46, 12558–12573. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Mayans, J.; Shipman, M.A.; Tizzard, G.J.; Coles, S.J.; Blight, B.A.; Escuer, A.; Kostakis, G.E. Four New Families of Polynuclear Zn-Ln Coordination Clusters. Synthetic, Topological, Magnetic, and Luminescent Aspects. Cryst. Growth Des. 2017, 17, 1524–1538. [Google Scholar] [CrossRef] [Green Version]
- Tsuchimoto, M.; Ishii, T.; Imaoka, T.; Yamamoto, K.; Yoshioka, N.; Sunatsuki, Y. Synthesis and Structures of Vanadium–Cerium Trinuclear Complexes with Schiff-Base Ligands. Bull. Chem. Soc. Jpn. 2006, 79, 1393–1397. [Google Scholar] [CrossRef]
- Tsuchimoto, M.; Ishii, T.; Imaoka, T.; Yamamoto, K. Synthesis and Electrochemical Properties of Oxovanadium Complexes with a Pentadentate Schiff Base Ligand. Bull. Chem. Soc. Jpn. 2004, 77, 1849–1854. [Google Scholar] [CrossRef]
- Lan, Y.; Novitchi, G.; Clérac, R.; Tang, J.-K.; Madhu, N.T.; Hewitt, I.J.; Anson, C.E.; Brooker, S.; Powell, A.K. Di-, Tetra- and Hexanuclear iron(III), manganese(II/III) and copper(II) Complexes of Schiff-Base Ligands Derived from 6-Substituted-2-Formylphenols. Dalton Trans. 2009, 10, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Chakrabarty, P.P.; Saha, S.; Jana, A.D.; Schollmeyer, D.; García-Granda, S. Ligand Mediated Structural Diversity and Role of Different Weak Interactions in Molecular Self-Assembly of a Series of copper(II)–sodium(I) Schiff-Base Heterometallic Complexes. Inorg. Chim. Acta 2013, 408, 172–180. [Google Scholar] [CrossRef]
- Elmali, A.; Zeyrek, C.T.; Elerman, Y. Crystal Structure, Magnetic Properties and Molecular Orbital Calculations of a Binuclear copper(II) Complex Bridged by an Alkoxo-Oxygen Atom and an Acetate Ion. J. Mol. Struct. 2004, 693, 225–234. [Google Scholar] [CrossRef]
- Mitra, M.; Maji, A.K.; Ghosh, B.K.; Raghavaiah, P.; Ribas, J.; Ghosh, R. Catecholase Activity of a Structurally Characterized Dinuclear iron(III) Complex [FeIII2(L)2] [H3L = N,N′-bis(3-Methoxysalicylaldimine)-1,3-Diaminopropan-2-Ol]. Polyhedron 2014, 67, 19–26. [Google Scholar] [CrossRef]
- Smith, K.I.; Borer, L.L.; Olmstead, M.M. Vanadium(IV) and Vanadium(V) Complexes of Salicyladimine Ligands. Inorg. Chem. 2003, 42, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- Dolai, M.; Ali, M.; Titiš, J.; Boča, R. Cu(II)–Dy(III) and Co(III)–Dy(III) Based Single Molecule Magnets with Multiple Slow Magnetic Relaxation Processes in the Cu(II)–Dy(III) Complex. Dalton Trans. 2015, 44, 13242–13249. [Google Scholar] [CrossRef] [PubMed]
- Chiboub Fellah, F.Z.; Boulefred, S.; Chiboub Fellah, A.; El Rez, B.; Duhayon, C.; Sutter, J.-P. Binuclear CuLn Complexes (LnIII = Gd, Tb, Dy) of Alcohol-Functionalized Bicompartmental Schiff-Base Ligand. Hydrogen Bonding and Magnetic Behaviors. Inorg. Chim. Acta 2016, 439, 24–29. [Google Scholar] [CrossRef]
- Datta, A.; Clegg, J.K.; Huang, J.-H.; Pevec, A.; Garribba, E.; Fondo, M. Hydroxo-Bridged 1-D Coordination Polymer of Cu(II) Incorporating with Salicyladimine Precursor: Spectral and Temperature Dependent Magneto Structural Correlation. Inorg. Chem. Commun. 2012, 24, 216–220. [Google Scholar] [CrossRef]
- Liao, S.; Yang, X.; Jones, R.A. Self-Assembly of Luminescent Hexanuclear Lanthanide Salen Complexes. Cryst. Growth Des. 2012, 12, 970–974. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Dey, A.; Das, S.; Rouzières, M.; Clérac, R. Syntheses, Structures, and Magnetic Properties of a Family of Heterometallic Heptanuclear [Cu5Ln2] (Ln = Y(III), Lu(III), Dy(III), Ho(III), Er(III), and Yb(III)) Complexes: Observation of SMM Behavior for the Dy(III) and Ho(III) Analogues. Inorg. Chem. 2013, 52, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Maeda, M.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. SMM Behavior Observed in Ce(III)Zn(II)2 Linear Trinuclear Complex. Chem. Lett. 2013, 42, 1276–1278. [Google Scholar] [CrossRef]
- Maeda, M.; Hino, S.; Yamashita, K.; Kataoka, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. Correlation between Slow Magnetic Relaxation and the Coordination Structures of a Family of Linear Trinuclear Zn(II)–Ln(III)–Zn(II) Complexes (Ln = Tb, Dy, Ho, Er, Tm and Yb). Dalton Trans. 2012, 41, 13640–13648. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Morita, Y.; Utsuno, F.; Nabeshima, T. Multiple Folding Structures Mediated by Metal Coordination of Acyclic Multidentate Ligand. Inorg. Chem. 2009, 48, 10670–10678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, W.; Liu, H.; Zhang, Z.; Lü, X.; Song, J.; Fan, D.; Wong, W.-K.; Jones, R.A. Photo-Luminescent Hetero-Trinuclear Zn2Ln (Ln = Nd, Yb, Er or Gd) Complexes Based on the Binuclear Zn2L Precursor. Inorg. Chem. Commun. 2012, 24, 148–152. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Ma, J.-C.; Zhu, L.-C.; Dong, W.-K.; Zhang, Y. Four 3d-4f Heteromultinuclear zinc(II)–lanthanide(III) Complexes Constructed from a Distinct Hexadentate N2O2-Type Ligand: Syntheses, Structures and Luminescence Properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Tian, Y.-M.; Li, H.-F.; Han, B.-L.; Zhang, Q.; Sun, W.-B. A Salen-Type Trinuclear Zn2Gd Complex. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, m1500–m1501. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.; Yang, X.; Stanley, J.M.; Jones, R.A.; Holliday, B.J. Synthesis and Crystal Structure of a New Heterotrinuclear Schiff-Base Zn–Gd Complex. J. Chem. Crystallogr. 2010, 40, 1060–1064. [Google Scholar] [CrossRef]
- Yang, X.; Jones, R.A.; Lynch, V.; Oye, M.M.; Holmes, A.L. Synthesis and near Infrared Luminescence of a Tetrametallic Zn2Yb2 Architecture from a Trinuclear Zn3L2 Schiff Base Complex. Dalton Trans. 2005, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Schipper, D.; Liao, A.; Stanley, J.M.; Jones, R.A.; Holliday, B.J. Anion Dependent Self-Assembly of Luminescent Zn–Ln (Eu and Tb) Salen Complexes. Polyhedron 2013, 52, 165–169. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Miroslaw, B. Synthesis, Crystal Structures and Magnetic Behavior of NiII–4f–NiII Compounds. Polyhedron 2012, 43, 47–54. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Pełka, R.; Miroslaw, B.; Hnatejko, Z. Heterometallic Trinuclear 3d-4f-3d Compounds Based on a Hexadentate Schiff Base Ligand. Polyhedron 2014, 68, 180–190. [Google Scholar] [CrossRef]
- Lo, W.K.; Wong, W.K.; Wong, W.Y.; Guo, J.; Yeung, K.T.; Cheng, Y.K.; Yang, X.; Jones, R.A. Heterobimetallic Zn(II)-Ln(III) Phenylene-Bridged Schiff Base Complexes, Computational Studies, and Evidence for Singlet Energy Transfer as the Main Pathway in the Sensitization of near-Infrared Nd3+ Luminescence. Inorg. Chem. 2006, 45, 9315–9325. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Thakurta, S.; Sadhukhan, D.; Pilet, G.; Rosair, G.M.; Nonat, A.; Charbonnière, L.J.; Mitra, S. Thermally Stable Luminescent zinc–Schiff Base Complexes: A Thiocyanato Bridged 1D Coordination Polymer and a Supramolecular 1D Polymer. Polyhedron 2013, 65, 6–15. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, B.; Li, Y. Synthesis and Luminescence Properties of Polymer-Rare Earth Complexes Containing Salicylaldehyde-Type Bidentate Schiff Base Ligand. Luminescence 2017, 32, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Panja, S.K.; Verma, A.; Takaya, T.; Iwata, K.; Sunkari, S.S.; Saha, S. NIR Luminescent Heterodinuclear [ZnIILnIII] Complexes: Synthesis, Crystal Structures and Photophysical Properties. J. Lumin. 2017, 192, 156–165. [Google Scholar] [CrossRef]
- Feng, X.; Feng, Y.-Q.; Chen, J.J.; Ng, S.-W.; Wang, L.-Y.; Guo, J.-Z. Reticular Three-Dimensional 3d-4f Frameworks Constructed through Substituted Imidazole-Dicarboxylate: Syntheses, Luminescence and Magnetic Properties Study. Dalton Trans. 2015, 44, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-S.; Dong, W.-K.; Zhang, Y.; Chen, L.; Ding, Y.-J. Four Salamo-Type 3d-4f Hetero-Bimetallic [ZnIILnIII] Complexes: Syntheses, Crystal Structures, and Luminescent and Magnetic Properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Pasatoiu, T.D.; Madalan, A.M.; Kumke, M.U.; Tiseanu, C.; Andruh, M. Temperature Switch of LMCT Role: From Quenching to Sensitization of Europium Emission in a ZnII−EuIII Binuclear Complex. Inorg. Chem. 2010, 49, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Görrler-Warland, C.; Binnemans, K.B. Spectral Intensities of F-F Transitions. In Handbook on the Physics and Chemistry of Rare Earths; Geschneidner, K.A., Eyring, L., Lander, G.H., Eds.; Elsevier: New York, NY, USA, 1998; pp. 101–264. [Google Scholar]
- Tanner, P.A. Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Hänninen, P., Härmä, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Tao, C.-H.; Ma, J.-C.; Zhu, L.-C.; Zhang, Y.; Dong, W.-K. Heterobimetallic 3d-4f Zn(II)–Ln(III) (Ln = Sm, Eu, Tb and Dy) Complexes with a N2O4 Bisoxime Chelate Ligand and a Simple Auxiliary Ligand Py: Syntheses, Structures and Luminescence Properties. Polyhedron 2017, 128, 38–45. [Google Scholar] [CrossRef]
- Cristóvão, B.; Hnatejko, Z. Lanthanide(III) Compounds with the N2O4-Donor Schiff Base—Synthesis, Spectral, Thermal, Magnetic and Luminescence Properties. J. Mol. Struct. 2015, 1088, 50–55. [Google Scholar] [CrossRef]
- Craze, A.R.; Huang, X.-D.; Etchells, I.; Zheng, L.-M.; Bhadbhade, M.M.; Marjo, C.E.; Clegg, J.K.; Moore, E.G.; Avdeev, M.; Lindoy, L.F.; et al. Synthesis and Characterisation of New Tripodal Lanthanide Complexes and Investigation of Their Optical and Magnetic Properties. Dalton Trans. 2017, 46, 12177–12184. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; VCH Publishers, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.P.; Titos-Padilla, S.; Oyarzabal, I.; Gupta, T.; Duhayon, C.; Rajaraman, G.; Colacio, E. Analysis of the Role of Peripheral Ligands Coordinated to ZnII in Enhancing the Energy Barrier in Luminescent Linear Trinuclear Zn-Dy-Zn Single-Molecule Magnets. Chemistry 2015, 21, 15785–15796. [Google Scholar] [CrossRef] [PubMed]
- Dolai, M.; Mistri, T.; Panja, A.; Ali, M. Diversity in Supramolecular Self-Assembly through Hydrogen-Bonding Interactions of Non-Coordinated Aliphatic –OH Group in a Series of Heterodinuclear CuIIM (M = NaI, ZnII, HgII, SmIII, BiIII, PbII and CdII). Inorg. Chim. Acta 2013, 399, 95–104. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. Crysalis-Pro Software System ver. 1.171.38.46; Rigaku Corporation: Oxford, UK, 2016. [Google Scholar]
- Clark, R.C.; Reid, J.S. The Analytical Calculation of Absorption in Multifaceted Crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, 51, 887–897. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |


| Parameter. | 1 | 2 | Parameter | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Ln1–Zn1 | 3.513(1) | 3.5268(7) | Ln1–Zn1 | 3.458(1) | 3.4242(9) | 3.414(1) |
| Ln1–Zn2 | 3.556(1) | 3.4915(7) | Ln1–O1 | 2.354(5) | 2.330(5) | 2.367(4) |
| Ln1–O1 | 2.472(5) | 2.414(3) | Ln1–O2 | 2.409(5) | 2.386(5) | 2.303(4) |
| Ln1–O2 | 2.442(5) | 2.414(3) | Ln1–O3 | 2.637(6) | 2.649(5) | 2.879(4) |
| Ln1–O3 | 2.825(6) | 2.650(4) | Ln1–O4 | 2.856(5) | 2.905(5) | 2.612(5) |
| Ln1–O4 | 2.729(6) | 2.704(3) | Ln1–O7 | 2.375(5) | 2.345(5) | 2.324(4) |
| Ln1–O6 | 2.442(5) | 2.412(3) | Zn1–O1 | 2.056(6) | 2.064(5) | 2.094(4) |
| Ln1–O7 | 2.457(5) | 2.440(3) | Zn1–O2 | 2.082(5) | 2.089(5) | 2.054(5) |
| Ln1–O8 | 2.671(5) | 2.689(4) | Zn1–O6 | 1.997(6) | 2.001(5) | 1.988(5) |
| Ln1–O9 | 2.730(6) | 2.803(4) | Zn1–N1 | 2.082(7) | 2.083(7) | 2.034(6) |
| Ln1–O12 | 2.437(6) | 2.369(4) | Zn1–N2 | 2.035(7) | 2.049(7) | 2.092(6) |
| Ln1–O13 | 2.384(6) | 2.407(4) | Zn1–O1–Ln1 | 103.0(1) | 102.2(2) | 103.0(2) |
| Zn1–O1 | 2.067(5) | 2.063(3) | Zn1–O2–Ln1 | 100.4(1) | 99.6(2) | 99.7(2) |
| Zn1–O2 | 2.044(5) | 2.077(3) | Δ b (Zn1) | 0.48 | 0.49 | 0.48 |
| Zn1–O11 | 1.996(6) | 2.005(4) | σc (Zn1) | 35.5 | 36.0 | 34.9 |
| Zn1–N1 | 2.022(8) | 2.075(4) | ωd | 62.4(4) | 61.6(3) | 62.2(2) |
| Zn1–N2 | 2.084(7) | 2.069(4) | εe | 62.0(3) | 61.3(4) | 62.0(3) |
| Zn2–O6 | 2.064(5) | 2.059(4) | φf | 173.3(2) | 172.8(1) | 172.9(1) |
| Zn2–O7 | 2.068(5) | 2.077(3) | ||||
| Zn2–O14 | 2.009(6) | 1.993(4) | ||||
| Zn2–N3 | 2.052(8) | 2.079(5) | ||||
| Zn2–N4 | 2.074(6) | 2.050(4) | ||||
| Zn1–O1–Ln1 | 101.1(2) | 103.7(1) | ||||
| Zn1–O2–Ln1 | 102.7(2) | 103.3(1) | ||||
| Zn2–O6–Ln1 | 103.9(2) | 102.4(1) | ||||
| Zn2–O7–Ln1 | 103.3(2) | 100.9(1) | ||||
| Δ b (Zn1; Zn2) | 0.46; 0.50 | 0.51; 0.49 | ||||
| σc (Zn1; Zn2) | 34.7; 34.8 | 34.5; 35.7 | ||||
| ωd | 36.2(4) | 34.6 (3) | ||||
| εe | 42.6(4) | 42.1(3) | ||||
| φf | 178.8(1) | 178.8(3) |
| Compound | λabs a (nm) | λex/λem a (nm) | λex/λem b (nm) |
|---|---|---|---|
| H3L | 229.0; 292.5; 341.5; 425.5 | 337/414.0 | 363/541.0 |
| 1 | 235.5; 267.0; 358.5 | 359/421.0 | 357/457.0 |
| 2 | 235.5; 268.0; 359.5 | 359/458.0; 561.0; 598.0; 644.0 | 357/460.0; 561.0; 598.0; 644.0 |
| 3 | 234.5; 267.5. 359.0 | 359/458.0 | 357/461.0 |
| 4 | 236.0. 267.0; 359.0 | 359/424.0 | 357/507.0 |
| 5 | 234.0; 267.5; 359.5 | 359/430.0 | 357/519.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroslaw, B.; Cristóvão, B.; Hnatejko, Z. Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules 2018, 23, 1761. https://doi.org/10.3390/molecules23071761
Miroslaw B, Cristóvão B, Hnatejko Z. Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules. 2018; 23(7):1761. https://doi.org/10.3390/molecules23071761
Chicago/Turabian StyleMiroslaw, Barbara, Beata Cristóvão, and Zbigniew Hnatejko. 2018. "Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization" Molecules 23, no. 7: 1761. https://doi.org/10.3390/molecules23071761
APA StyleMiroslaw, B., Cristóvão, B., & Hnatejko, Z. (2018). Heterometallic ZnII–LnIII–ZnII Schiff Base Complexes with Linear or Bent Conformation—Synthesis, Crystal Structures, Luminescent and Magnetic Characterization. Molecules, 23(7), 1761. https://doi.org/10.3390/molecules23071761








