Development of Dermal Films Containing Miconazole Nitrate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Products Obtained in Form of Polymeric Matrix Films
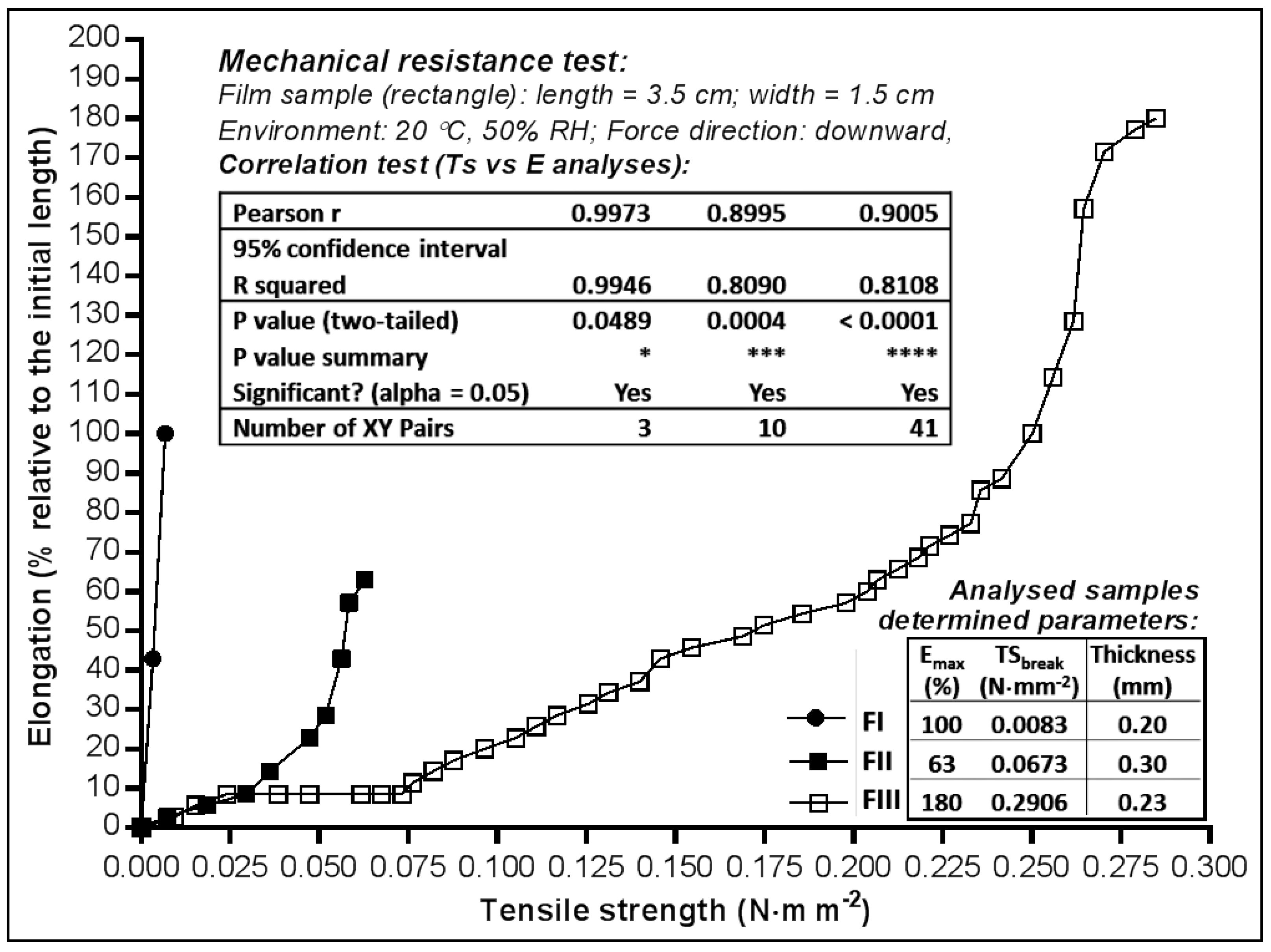
2.2. Mechanical Properties of MN Dermal Films
2.2.1. Tensile Strength and Elongation Capacity
2.2.2. Adhesive Capacity and Behaviour towards Vapour Moisture
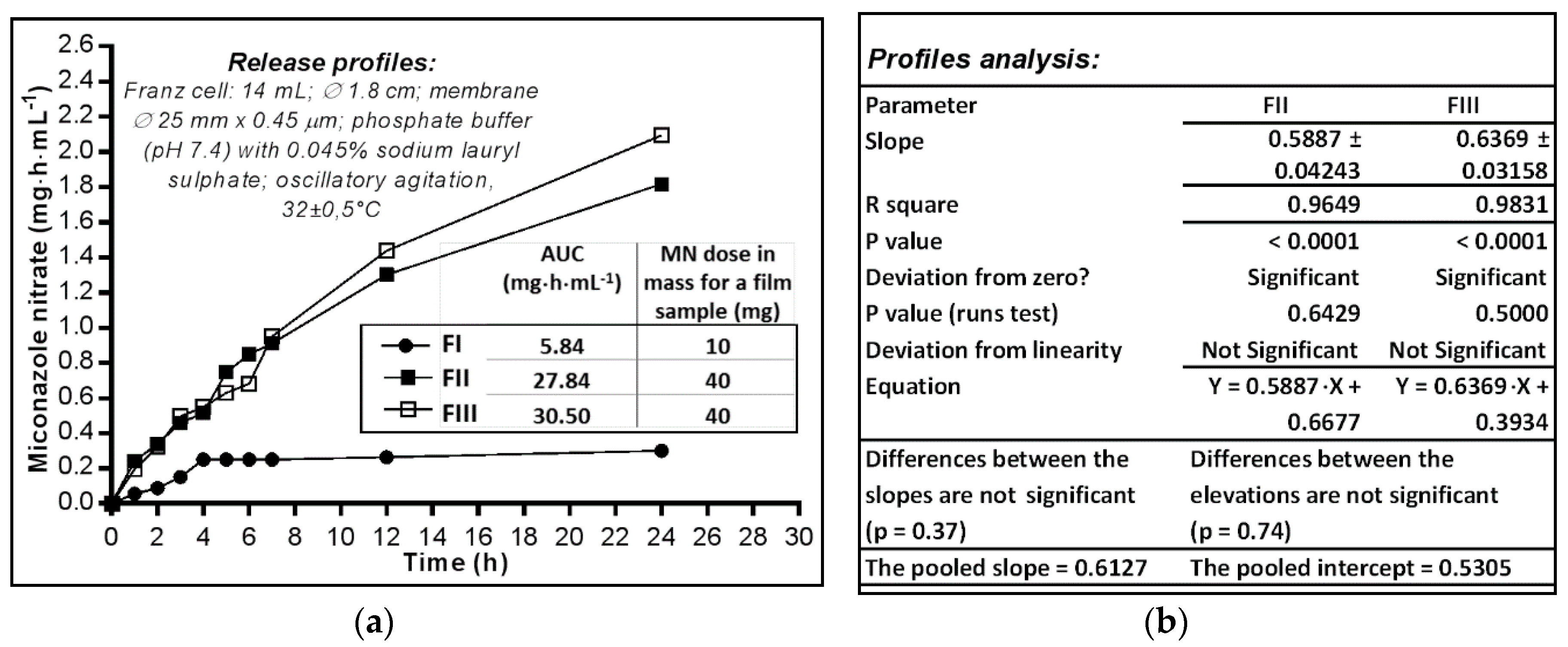
2.3. In Vitro Availability of Miconazole Nitrate Dermal Films
2.3.1. Analysis of In Vitro Release Profiles
2.3.2. Analysis of In Vitro Release Profiles
3. Materials and Methods
3.1. Preparation of MN-Dermal Films
3.1.1. Materials
3.1.2. Films Preparation Technique
3.2. Evaluation of Physicochemical and Mechanical Parameters
3.2.1. Measurement of Physical Parameters
3.2.2. Mechanical Resistance Test
3.2.3. Stickiness (Adhesion) Test (St)
3.2.4. Water Vapour Absorption Test (Aw)
3.2.5. Water Vapour Loss by Desiccation Test (Lw)
3.2.6. Water Vapour Permeability Test (Pw)
3.3. Evaluation of Dermal films In Vitro Availability
3.3.1. Determination of In Vitro Release Profiles
3.3.2. The Release Profile
3.3.3. Kinetic of Release Modelling
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hofsäss, M.; Souza, J.; Silva-Barcellos, N.M.; Bellavinha, K.R.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Parr, A.; Langguth, P.; Polli, J.E.; et al. Biowaiver. Monographs for immediate-release solid oral dosage forms: Folic acid. J. Pharm. Sci. 2017, 106, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Kenechukwu, F.C.; Attama, A.; Ibezim, E.C.; Nnamani, P.O.; Umeyor, C.E.; Uronnachi, E.M.; Gugu, T.H.; Momoh, M.A.; Ofokansi, K.C.; Akpa, P.A. Surface-modified mucoadhesive microgels as a controlled release system for miconazole nitrateto improve localized treatment of vulvovaginal candidiasis. Eur. J. Pharm. Sci. 2018, 111, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Kenechukwu, F.C.; Attama, A.A.; Ibezim, E.C. Novel solidified reverse micellar solution-based mucoadhesive nano lipid gels encapsulating miconazole nitrate-loaded nanoparticles for improved treatment of oropharyngeal candidiasis. J. Microencapsul. 2017, 34, 592–609. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Formulation and in-vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, X.; Liu, Y.; Han, Y.; Lin, M.; Wang, W.; Guan, X.; Zhu, S.; Zhang, H.; Wang, Q.; et al. The efficacy and safety of miconazole nitrate mucoadhesive tablets versus itraconazole capsules in the treatment of oral candidiasis: An open-label, randomized, multicenter trial. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Scutariu, M.; Matei, M.; Macovei, G.A.; Surdu, A. Clinical-statistical study of oral mucosa pathology induced by the acrylic resins from removable dentures in older patients. Mat. Plast. 2015, 52, 402–407. [Google Scholar]
- Gupta, A.; Kar, H.K. Antimycotic studies of miconazole nanovesicles formulation vs candida strain. J. Drugs Dermatol. 2016, 15, 734–737. [Google Scholar] [PubMed]
- Ozeki, C.; Moro, O. A study of the suppression of body odour in elderly subjects by anti-fungal agents. Int. J. Cosmet. Sci. 2016, 38, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mago, N.; Khuller, G.K. Effect of supplementation of ergosterol on miconazole action in vivo and in vitro in Candida albicans. Indian J. Exp. Biol. 1991, 29, 841–844. [Google Scholar] [PubMed]
- Firooz, A.; Namdar, R.; Nafisi, S.; Maibach, H.I. Nano-sized technologies for miconazole skin delivery. Curr. Pharm. Biotechnol. 2016, 17, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Donato, R.K.; Dalla Lana, D.F.; Donato, K.J.; Ortega, G.G.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium salts as antifungal agents: Strong antibiofilm activity against multidrug-resistant Candida tropicalis isolates. Lett. Appl. Microbiol. 2015, 60, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bossche, H.; Dromer, F.; Improvisi, I.; Lozano-Chiu, M.; Rex, J.H.; Sanglards, D. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 1998, 36, 119–128. [Google Scholar] [PubMed]
- Marichal, P.; Vanden Bossche, H. Mechanisms of resistance to azole antifungals. Acta Biochim. Pol. 1995, 42, 509–516. [Google Scholar] [PubMed]
- De Luca, L. Naturally occurring and synthetic imidazoles: Their chemistry and their biological activities. Curr. Med. Chem. 2006, 13, 1–23. [Google Scholar] [PubMed]
- Ahmed, T.A.; El-Say, K.M.; Mahmoud, M.F.; Samy, A.M.; Badawi, A.A. Miconazole nitrate oral disintegrating tablets: In vivo performance and stability study. AAPS PharmSciTech 2012, 13, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Heeres, J.; Meerpoel, L.; Lewi, P. Conazoles. Molecules 2010, 15, 4129–4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agut, J.; Palacín, C.; Sacristán, A.; Ortiz, J.A. Inhibition of ergosterol synthesis by sertaconazole in Candida albicans. Arzneimittelforschung 1992, 42, 718–720. [Google Scholar] [PubMed]
- Abruzzo, A.; Nicoletta, FP.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. Int. J. Pharm. 2017, 531, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; Brayden, D.J. Buccal delivery of small molecules and biologics: Of mucoadhesive polymers, films, and nanoparticles. Curr. Opin. Pharmacol. 2017, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.; Browne, L.; Capp, A.; Fox, C.; Graham, J.; Hollis, J.; Nasser, E. Randomized, paired comparison of No-Sting Barrier Film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 241–246. [Google Scholar] [CrossRef]
- Täuber, A.; Müller-Goymann, C.C. In vitro permeation and penetration of ciclopirox olamine from poloxamer 407-based formulations—Omparison of isolated human stratum corneum, bovine hoof plates and keratin films. Int. J. Pharm. 2015, 489, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Zeyohannes, S.S.; Rasad, M.S.B.A.; Yusuff, M.M. A facile synthesis method of hydroxyethyl cellulose-silver nanoparticle scaffolds for skin tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musazzi, U.M.; Santini, B.; Selmin, F.; Marini, V.; Corsi, F.; Allevi, R.; Ferretti, A.M.; Prosperi, D.; Cilurzo, F.; Colombo, M.; et al. Impact of semi-solid formulations on skin penetration of iron oxide nanoparticles. J. Nanobiotechnol. 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Bellesi, F.A.; Ruiz-Henestrosa, V.M.P.; Maldonado-Valderrama, J.; Del Castillo Santaella, T.; Pilosof, A.M.R. Comparative interfacial in vitro digestion of protein and polysaccharide oil/water films. Colloids Surf. B Biointerfaces 2018, 161, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B.; Özdemir, S.; Barla Demirkoz, A.; Üner, M. Optimization of piribedil mucoadhesive tablets for efficient therapy of Parkinson’s disease: Physical characterization and ex vivo drug permeation through buccal mucosa. Drug Dev. Ind. Pharm. 2017, 43, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, S.; Bulitta, J.B.; Youn, Y.S.; Yoo, S.D.; Shin, B.S. Development of a physiologically relevant population pharmacokinetic in vitro-in vivo correlation approach for designing extended-release oral dosage formulation. Mol. Pharm. 2017, 14, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, S.A. Comparison of the solubilization properties of polysorbate 80 and isopropanol/water solvent systems for organic compounds extracted from three pharmaceutical packaging configurations. Eur. J. Pharm. Sci. 2016, 93, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.D.; Kim, Y.M.; Kim, B.I.; Je, J.Y. Preparation and antibacterial activities of chitosan-gallic acid/polyvinyl alcohol blend film by LED-UV irradiation. J. Photochem. Photobiol. B 2017, 176, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, R.; Aliahmadi, A.; Rafati, H. Antibacterial hydroxypropyl methyl cellulose edible films containing nanoemulsions of Thymus daenensis essential oil for food packaging. Carbohydr. Polym. 2017, 175, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.R.; Choi, Y.W.; Cui, J.H.; Lee, B.J. Effect of solvents on physical properties and release characteristics of monolithic hydroxypropylmethylcellulose matrix granules and tablets. Arch. Pharm. Res. 2005, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C. Sol-gel behavior of hydroxypropylmethylcellulose (HPMC) in ionic media including drug release. Materials (Basel) 2011, 4, 1861–1905. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Grulke, E.A.; Delassus, P.T.; Smith, P.B.; Kocher, C.W.; Landes, B.G. A model for antiplasticization in polystyrene. Macromolecules 1995, 28, 2944–2954. [Google Scholar] [CrossRef]
- Chamarthy, S.P.; Pinal, R. Moisture induced antiplasticization in microcrystalline cellulose compacts. Tablets Capsul. 2007, 5, 22–33. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; Santos, L.O.D.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Thomazine, M.; Carvalho, R.A.; Sobral, P.J.A. Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J. Food Sci. 2005, 70, E172–E176. [Google Scholar] [CrossRef]
- Miconazole Nitrate. U.S. National Library of Medicine: National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Miconazolenitrate#section=Experimental-Properties (accessed on 8 August 2016).
- Saudagar, R.B.; Gangurde, P.A. Formulation, development and evaluation of film-forming gel for prolonged dermal delivery of miconazole nitrate. Int. J. Chem. Tech Res. 2017, 10, 282–299. [Google Scholar]
- Ofokansi, K.C.; Kenechukwu, F.C.; Ogwu, N.N. Design of novel miconazole nitrate transdermal films based on Eudragit RS100 and HPMC hybrids: Preparation, physical characterization, in vitro and ex vivo studies. Drug Deliv. 2015, 22, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2011, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Antonoaea, P.; Todoran, N.; Rédai, E.; Ciurba, A.; Bogdan, B.; Moldovan, M.; Muntean, D.L. Evaluation of mechanical properties of nonsteroidal anti-inflammatory matrix type transdermal therapeutic systems. Acta Medica Marisiensis. 2017, 63, 56–61. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.; Healy, A.M.; Cabral, L.; Pereira de Sousa, V. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Jung, S.; Shin, H.; Kim, B. Permeation characteristics of hazardous substances in tattoo dye using franz diffusion cells. J. Environ. Health Sci. 2016, 42, 61–70. [Google Scholar] [CrossRef]
- Shah, V.; Raval, S.; Peer, S.; Upadhyay, U.M. A comparative evaluation of different membranes for their diffusion efficiency: An in vitro study. Pharma Sci. Monit. 2010, 1, 41–49. [Google Scholar]
- Motulsky, H.J.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press Inc.: New York, NY, USA, 2004; pp. 134–160. ISBN 0190291397, 9780190291396. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Ingredient | Code | Formula/Quantity (% m/m) | Function | ||
|---|---|---|---|---|---|
| FI | FII | FIII | |||
| Miconazole nitrate | MN | 1.25 | 5.00 | 5.00 | Active pharmaceutical ingredient |
| Hydroxyethyl cellulose 250 M | HEC | - | 3.00 | 2.00 | Film forming agent, non-ionic, water highly viscous soluble polymer |
| Polyethylene glycol 400 | PEG400 | - | 1.00 | 1.00 | Permeation enhancer, solubilizer, plasticizer |
| Hydroxypropyl methylcellulose 15,000 | HPMC | 1.00 | - | - | Film forming agent, non-ionic, water highly viscous soluble polymer, water retention agent |
| Propylene glycol | PG | 10.00 | - | - | Humectant agent, solubilizer, plasticizer |
| Polysorbate 20 | PSB20 | 1.00 | - | - | Surfactant, non-ionic, oil in water emulsifier, permeation enhancer, solubilizer |
| Ethanol | - | 30.00 | 10.00 | 10.00 | Co-solvent |
| Ultrapure water | - | 56.75 | 81.00 | 82.00 | Solvent |
| Total | 100 | 100 | 100 | ||
| Parameter | Dermal Film Formula | ||
|---|---|---|---|
| FI | FII | FIII | |
| Appearance | Translucent film | Opaque film | Opaque film |
| Surface | Slightly rough | Shiny smooth | Shiny smooth |
| Thickness (mm ± SD 1) | 0.20 ± 0.009 | 0.30 ± 0.004 | 0.23 ± 0.007 |
| MN theoretical content | |||
| mg in 63.585 cm2 film: | 250 | 1000 | 1000 |
| mg in 2.54 cm2 film: | 10 | 40 | 40 |
| mg·cm−2: | 3.931 | 15.726 | 15.726 |
| Model | Kinetic Function/Equation 1 [41] | Parameter 2 | FII | FIII |
|---|---|---|---|---|
| 1 | First-order | 0.0071 | 0.0072 | |
| 0.9361 | 0.9750 | |||
| 2 | First-order with | 0.0516 | 0.0543 | |
| −0.3832 | −0.0765 | |||
| 0.9848 | 0.9774 | |||
| 3 | First-order with | 0.0903 | 0.0374 | |
| 10.8379 | 21.6643 | |||
| 0.9872 | 0.9794 | |||
| 4 | First-order with and | 0.0799 | 0.0200 | |
| −0.1667 | −0.3614 | |||
| 11.5726 | 35.9946 | |||
| 0.9864 | 0.9795 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bîrsan, M.; Apostu, M.; Todoran, N.; Antonoaea, P.; Rusu, A.; Ciurba, A. Development of Dermal Films Containing Miconazole Nitrate. Molecules 2018, 23, 1640. https://doi.org/10.3390/molecules23071640
Bîrsan M, Apostu M, Todoran N, Antonoaea P, Rusu A, Ciurba A. Development of Dermal Films Containing Miconazole Nitrate. Molecules. 2018; 23(7):1640. https://doi.org/10.3390/molecules23071640
Chicago/Turabian StyleBîrsan, Magdalena, Mihai Apostu, Nicoleta Todoran, Paula Antonoaea, Aura Rusu, and Adriana Ciurba. 2018. "Development of Dermal Films Containing Miconazole Nitrate" Molecules 23, no. 7: 1640. https://doi.org/10.3390/molecules23071640






