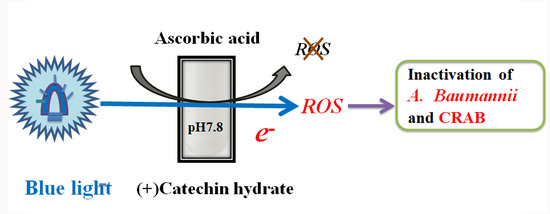
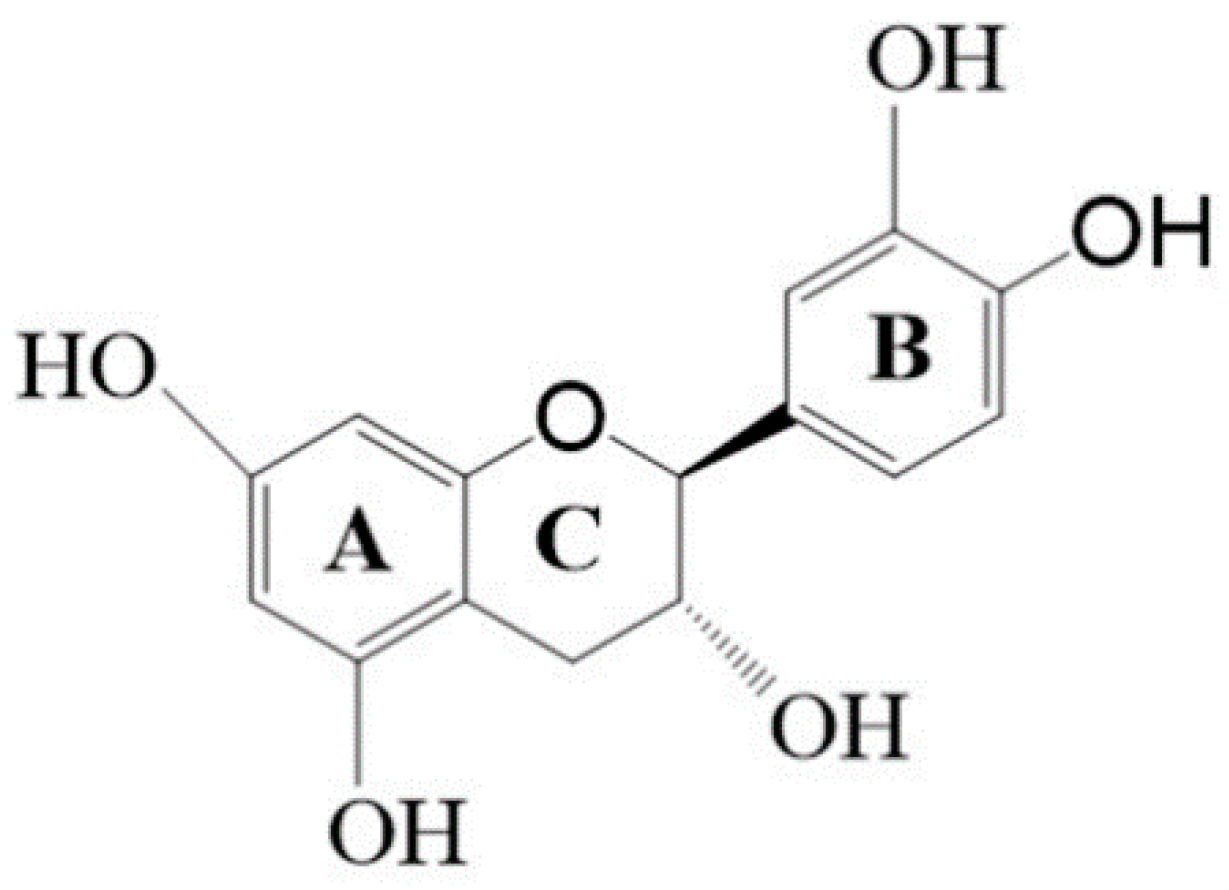
Effects of Blue-Light-Induced Free Radical Formation from Catechin Hydrate on the Inactivation of Acinetobacter baumannii, Including a Carbapenem-Resistant Strain
Abstract
:1. Introduction
2. Results
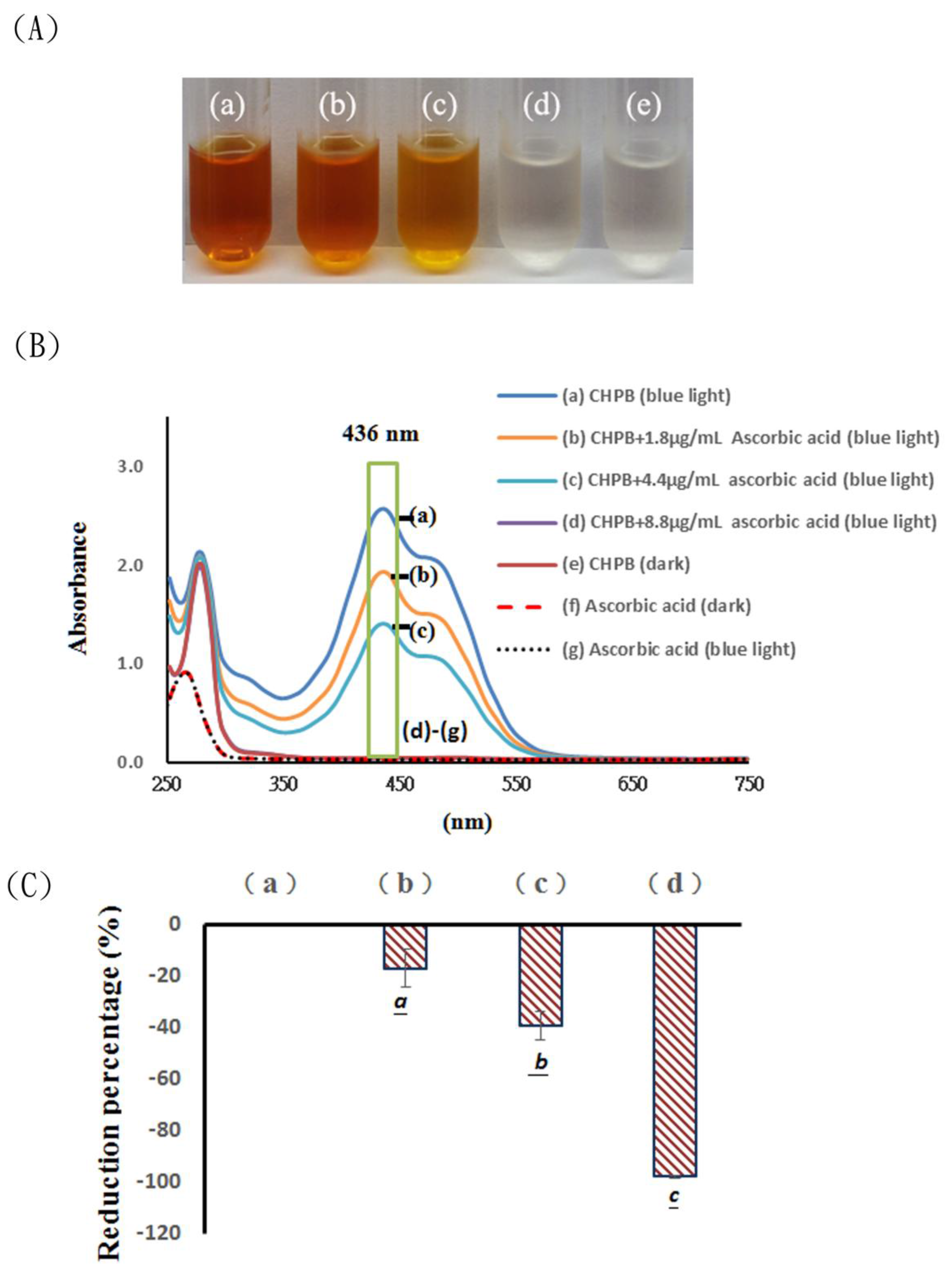
2.1. Effects of Blue Light Irradiation on Catechin Hydrate Treated with Ascorbic Acid
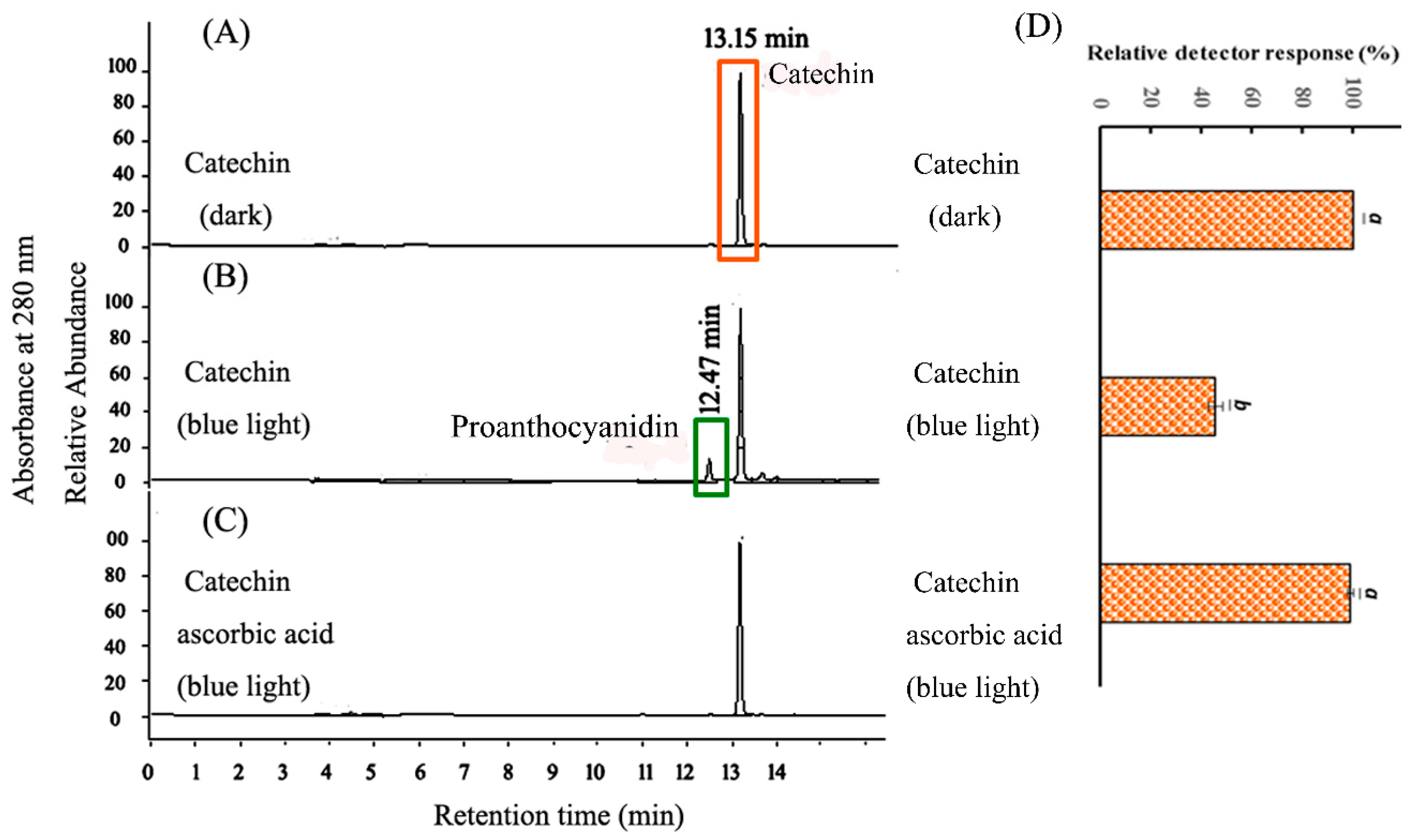
2.2. HPLC Analysis of CHPB Treated with Blue Light Irradiation and Ascorbic Acid
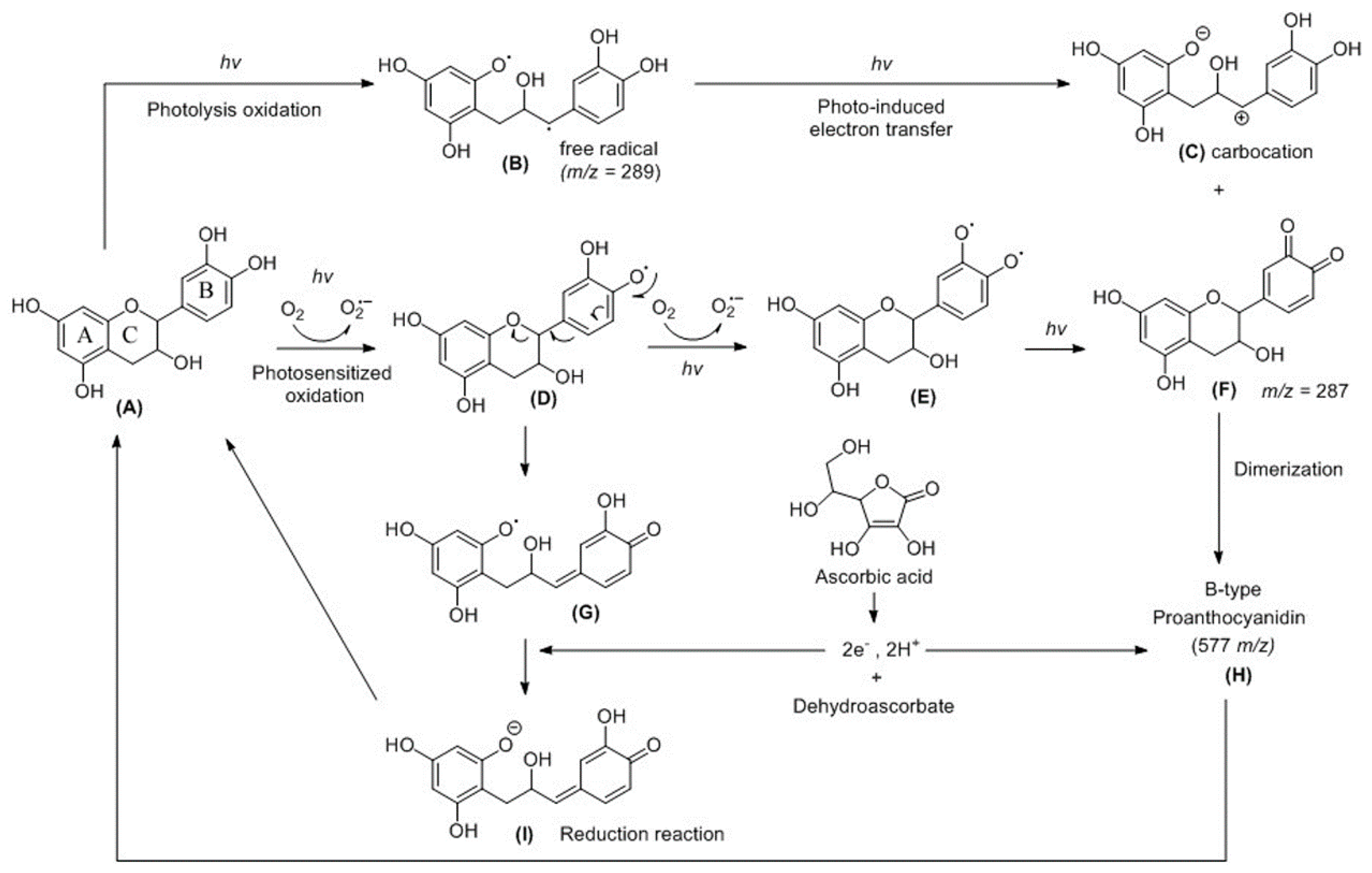
2.3. Probing of Radicals in the Photoreaction of CHPB
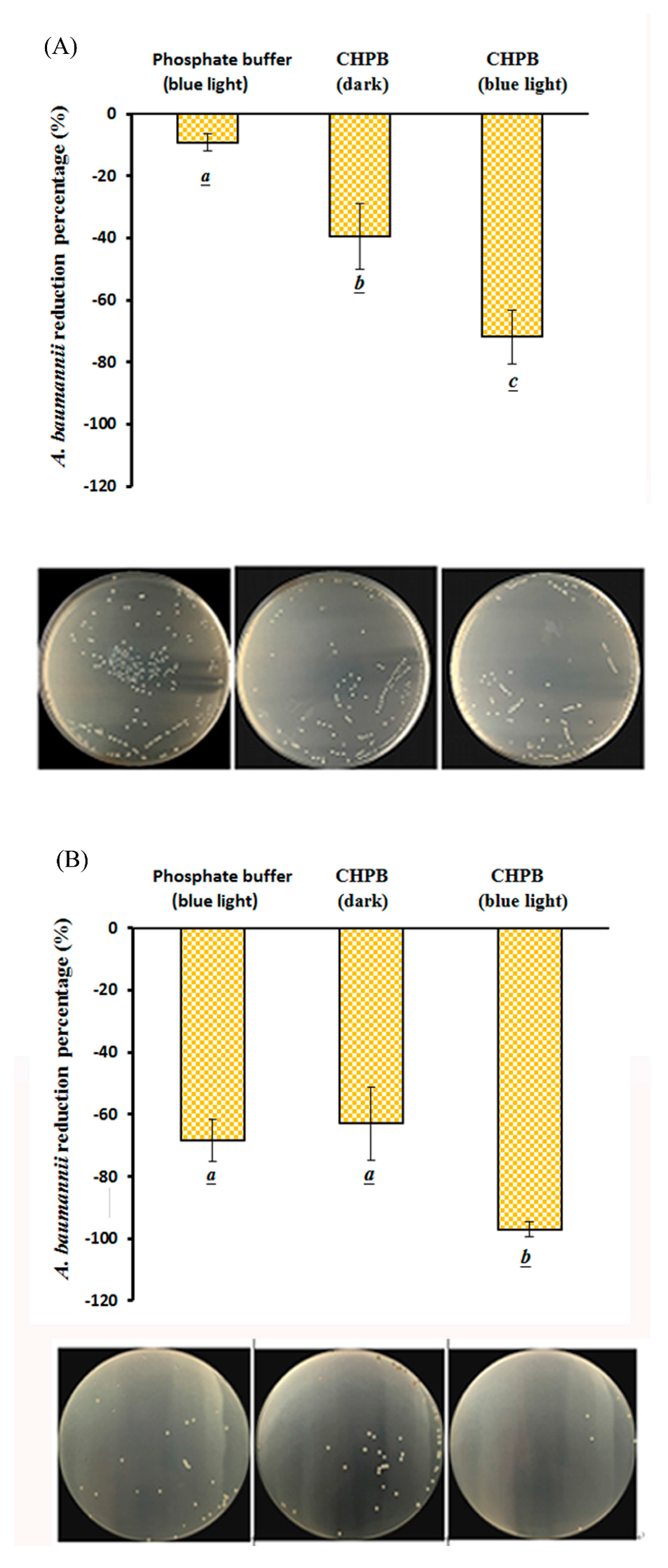
2.4. Viability of A. baumannii under CHPB Photoreaction
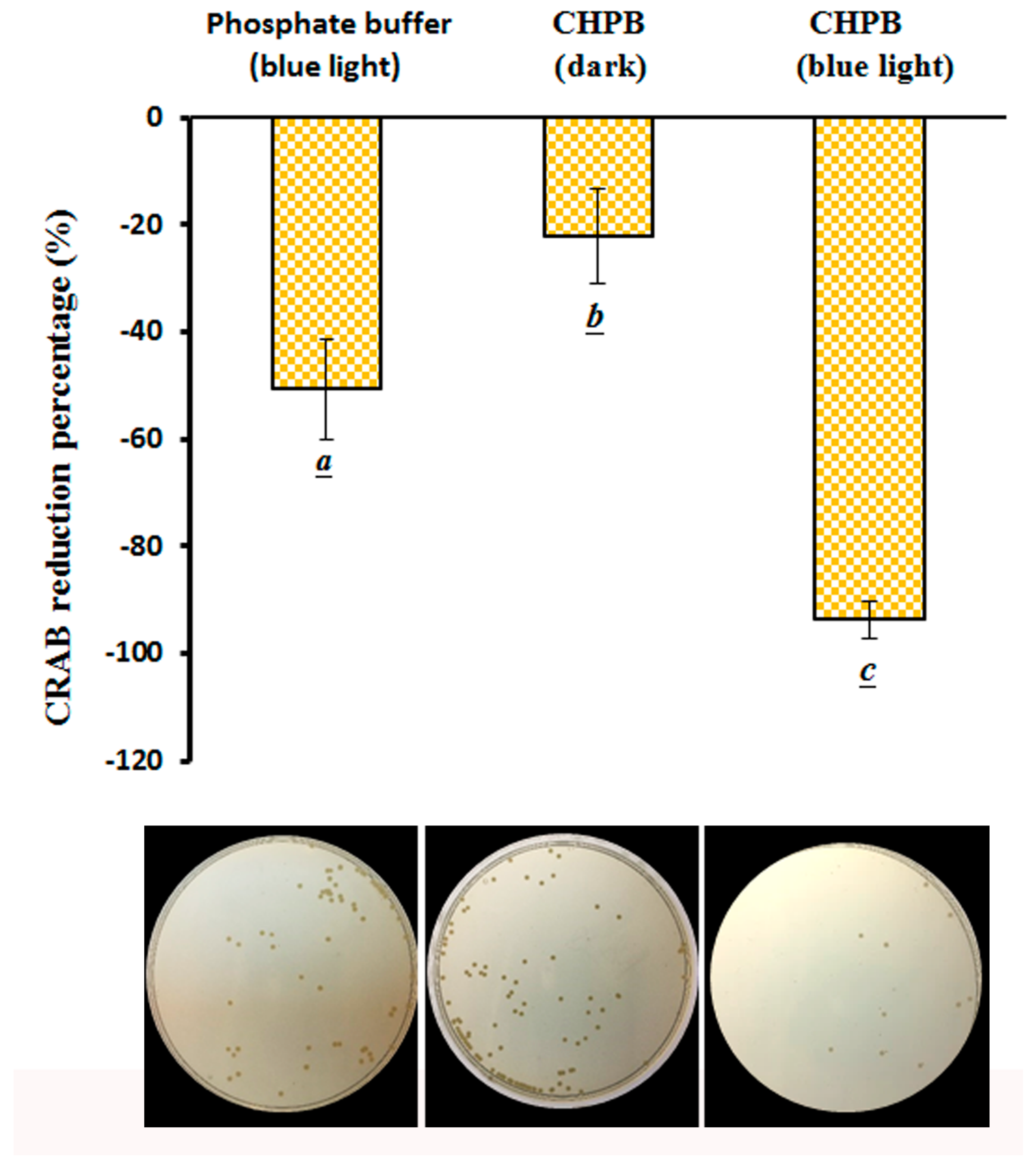
2.5. Viability of CRAB under the CHPB Photoreaction
3. Discussion
4. Materials and Methods
4.1. Chemicals
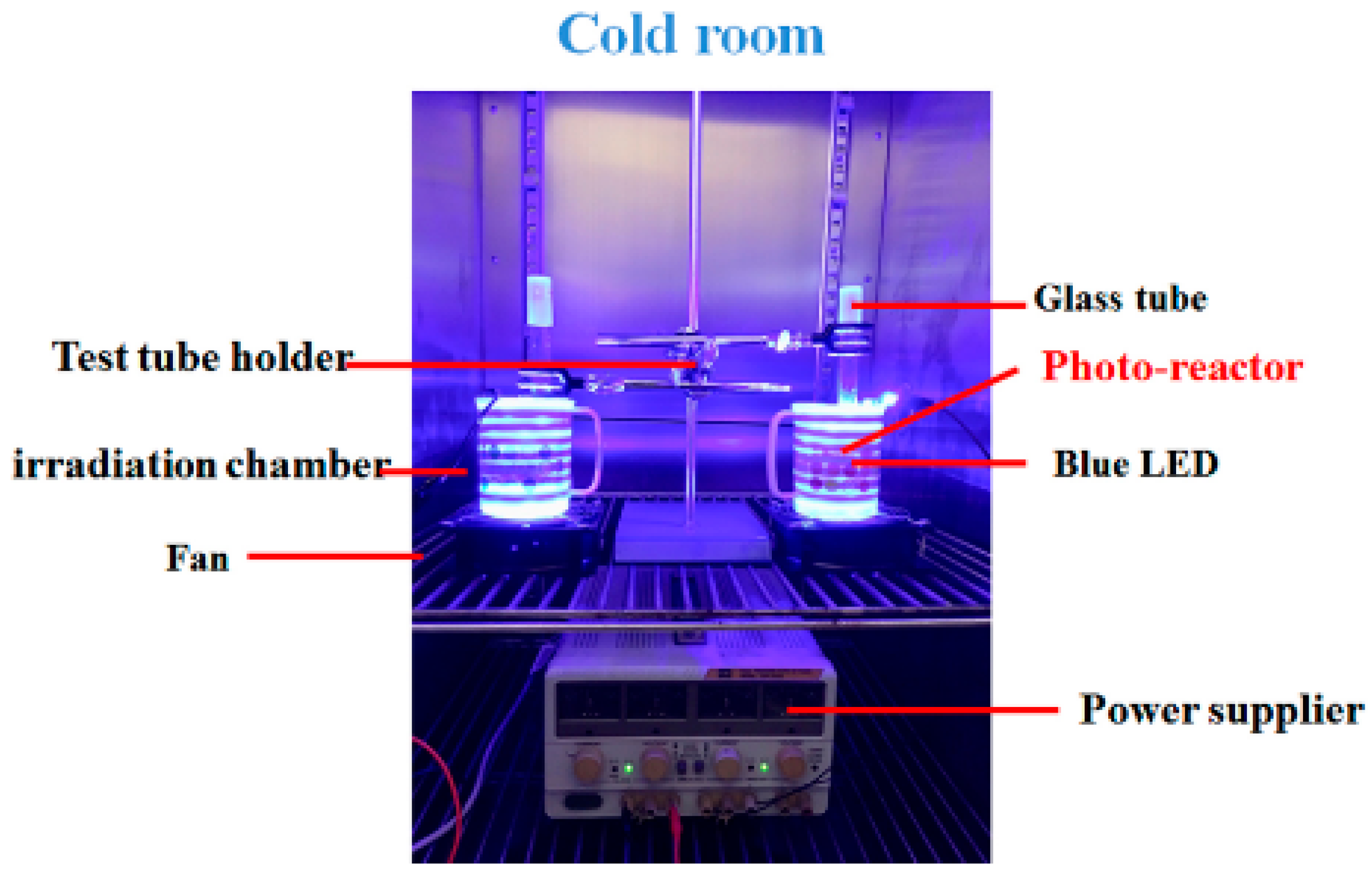
4.2. Setup of Irradiation Units
4.3. Effects of Ascorbic Acid on the CHPB Treated with Blue Light Irradiation
4.4. HPLC-DAD Analysis of CHPB Treated with Blue Light Irradiation
4.5. Detection of O2•− Using an NBT Reduction Assay
4.6. Survival of A. baumannii and CRAB after the Photoreaction Processes of CHPB
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mariod, A.; Matthäus, B.; Idris, Y.A.; Abdelwahab, S. Fatty acids, tocopherols, phenolics and the antimicrobial effect of Sclerocarya birrea kernels with different harvesting dates. J. Am. Oil Chem. Soc. 2010, 87, 377–384. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Shi, M.; Nie, Y.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R.; Ye, J.H. Ultraviolet B (UVB) photosensitivities of tea catechins and the relevant chemical conversions. Molecules 2016, 21, 1345. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chen, H.; Zhang, L.B.; Ye, J.H.; Lu, J.L.; Liang, Y.R. Effects of temperature, illumination, and sodium ascorbate on browning of green tea infusion. Food Sci. Biotechnol. 2009, 18, 932–938. [Google Scholar]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Chen, L.Y.; Wu, J.Y.; Liang, J.Y. Using chromatography and mass spectrometry to monitor isomerization of catechin in alkaline aqueous with thermal processing. J. Food Process. Preserv. 2018, 42, e13365. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cheng, C.W.; Liang, J.Y. Effect of esterification condensation on the Folin-Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm-Mouton, A.; Bonnet, S.L.; Ding, Y.; Li, X.C.; Ferreira, D.; van der Westhuizen, J.H. Photochemistry synthesis. Part 2: Enantiomerically pure polyhydroxy-1,1,3-triarylpropan-2-ols. J. Photochem. Photobiol. A Chem. 2012, 227, 18–24. [Google Scholar] [CrossRef]
- Liang, J.Y.; Wu, J.Y.; Yang, M.Y.; Hu, A.; Chen, L.Y. Photo-catalytic polymerization of catechin molecules in alkaline aqueous. J. Photochem. Photobiol. B Biol. 2016, 165, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Nakano, K.; Yanase, E. Investigation of color-deepening phenomenon in catechin-(4→8)-dimer as a proanthocyanidin model and structural determination of its derivatives by oxidation. Food Chem. 2018, 239, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Suematsu, S.; Hisanobu, Y.; Saigo, H.; Matsuda, R.; Hara, K. Effects of pH and temperature on reaction kinetics of catechins in green tea infusion. Biosci. Biotechnol. Biochem. 1993, 57, 907–910. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. A colorimeteric method for the determination of phenol (and derivatives) in urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Brett, A.M.O. Catechin electrochemical oxidation mechanisms. Anal. Chim. Acta 2004, 518, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Yuann, J.M.P.; Wang, J.S.; Jian, H.L.; Lin, C.C.; Liang, J.Y. Effects of Clinacanthus nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. MC-Trans. Biotechnol. 2012, 4, 45–59. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Juen, J.W.; Jian, H.L.; Liang, J.Y. The effect of illuminance on light induced reduction of nitro blue tetrazolium. MC-Trans. Biotechnol. 2010, 2, e2. [Google Scholar]
- Donnelly, J.K.; McLellan, K.M.; Walker, J.L.; Robinson, D.S. Superoxide dismutases in foods. A review. Food Chem. 1989, 33, 243–270. [Google Scholar] [CrossRef]
- Cheng, C.W.; Chen, L.Y.; Chou, C.W.; Liang, J.Y. Investigations of riboflavin photolysis via coloured light in the nitro blue tetrazolium assay for superoxide dismutase activity. J. Photochem. Photobiol. B Biol. 2015, 148, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Vanderslice, J.T. Comprehensive review of vitamin B2 analytical methodology. J. Micronutr. Anal. 1990, 8, 257–310. [Google Scholar]
- Lin, Y.; Eitenmiller, R.R.; Landen, W.O. Riboflavin. In Vitamin Analysis for the Health and Food Sciences, 2nd ed.; CRC Press: Florida, FL, USA, 2008; pp. 329–360. [Google Scholar]
- Liang, J.Y.; Yuann, J.P.; Hsie, Z.J.; Huang, S.T.; Chen, C.C. Blue light induced free radicals from riboflavin in degradation of crystal violet by microbial viability evaluation. J. Photochem. Photobiol. B Biol. 2017, 174, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Yuann, J.M.; Cheng, C.W.; Jian, H.L.; Lin, C.C.; Chen, L.Y. Blue light induced free radicals from riboflavin on E. coli DNA damage. J. Photochem. Photobiol. B Biol. 2013, 119, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Cheng, C.W.; Yu, C.H.; Chen, L.Y. Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. J. Photochem. Photobiol. B Biol. 2015, 143, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Cheng, C.W.; Hsieh, Z.J.; Liang, J.Y. Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5’-phosphate photolysis. J. Photochem. Photobiol. B Biol. 2017, 173, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Osterburg, A.; Gardner, J.; Hyon, S.H.; Neely, A.; Babcock, G. Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG). Clin. Microbiol. Infect. 2009, 15, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Seifert, H.; Snelling, A.M.; Heritage, J.; Hawkey, P.M. Survival of Acinetobacter baumannii on dry surfaces: Comparison of outbreak and sporadic isolates. J. Clin. Microbiol. 1998, 36, 1938–1941. [Google Scholar] [PubMed]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Viehman, J.A.; Nguyen, M.H.; Doi, Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 2014, 74, 1315–1333. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J. Photochem. Photobiol. B Biol. 2015, 150, 2–10. [Google Scholar]
- Rineh, A.; Bremner, J.B.; Hamblin, M.R.; Ball, A.R.; Tegos, G.P.; Kelso, M.J. Attaching NorA efflux pump inhibitors to methylene blue enhances antimicrobial photodynamic inactivation of Escherichia coli and Acinetobacter baumannii in vitro and in vivo. Bioorg. Med. Chem. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Tegos, G.P.; Lu, Z.; Huang, L.; Zhiyentayev, T.; Franklin, M.J.; Baer, D.G.; Hamblin, M.R. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob. Agents Chemother. 2009, 53, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Ashkenazi, H. Photoinactivation of Acinetobacter baumannii and Escherichia coli B by a cationic hydrophilic porphyrin at various light wavelengths. Curr. Microbiol. 2001, 42, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Tsai, Y.H.; Hu, A.; Liou, J.W.; Chang, K.C.; Chang, H.H. Altered susceptibility to the bactericidal effect of photocatalytic oxidation by TiO2 is related to colistin resistance development in Acinetobacter baumannii. Appl. Microbiol. Biotechnol. 2016, 100, 8549–8561. [Google Scholar] [CrossRef] [PubMed]
- Boluki, E.; Kazemian, H.; Peeridogaheh, H.; Alikhani, M.Y.; Shahabi, S.; Beytollahi, L.; Ghorbanzadeh, R. Antimicrobial activity of photodynamic therapy in combination with colistin against a pan-drug resistant Acinetobacter baumannii isolated from burn patient. Photodiagnosis Photodyn. Ther. 2017, 18, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Chang, K.C.; Chen, L.Y.; Wang, P.C.; Chou, C.C.; Wu, Z.B.; Hu, A. Blue light irradiation triggers the antimicrobial potential of ZnO nanoparticles on drug-resistant Acinetobacter baumannii. J. Photochem. Photobiol. B Biol. 2018, 180, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fourie, T.G.; Ferreira, D.; Roux, D.G. Flavonoid synthesis based on photolysis of flavan-3-ols, 3-hydroxyflavanones, and 2-benzylbenzofuranones. J. Chem. Soc. Perkin Trans. 1977, 1, 125–133. [Google Scholar] [CrossRef]
- Faria, J.L.; Steenken, S. Photoionization (.lambda. = 248 or 308 nm) of triphenylmethyl radical in aqueous solution. Formation of triphenylmethyl carbocation. J. Am. Chem. Soc. 1990, 112, 1277–1279. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Y.; Wu, Y.; Tan, H.; Meng, F.; Wang, Y.S.; Li, M.; Zhao, L.; Liu, L.; Qian, Y.; et al. Analysis of accumulation patterns and preliminary study on the condensation mechanism of proanthocyanidins in the tea plant [Camellia sinensis]. Sci. Rep. 2015, 5, 8742. [Google Scholar] [CrossRef] [PubMed]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Oxidative stress: Adaptation, damage, repair and death. In Free Radicals in Biology and Medicine; Oxford University Press Inc.: New York, NY, USA, 2003; pp. 246–350. [Google Scholar]
- Miklasinska, M.; Kepa, M.; Wojtyczka, R.D.; Idzik, D.; Dziedzic, A.; Wasik, T.J. Catechin Hydrate Augments the Antibacterial Action of Selected Antibiotics against Staphylococcus aureus Clinical Strains. Molecules 2016, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Loes, A.N.; Ruyle, L.; Arvizu, M.; Gresko, K.E.; Wilson, A.L.; Deutch, C.E. Inhibition of urease activity in the urinary tract pathogen Staphylococcus saprophyticus. Lett. Appl. Microbiol. 2014, 58, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rajaonson, S.; Diallo, B.; Mol, A.; El Jaziri, M.; Baucher, M. Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2010, 76, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Harrington, O.D.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Dai, T. In vivo investigation of antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii burn infections using bioluminescence imaging. J. Vis. Exp. 2017, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.A.L. Tea flavanols: An overview. In Tea in Health and Disease Prevention; Victor, R.P., Ed.; Academic Press: Massachusetts, MA, USA, 2013; pp. 73–78. [Google Scholar]
Sample Availability: Samples of the compounds (catechin hydrate) are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.-J.; Hung, Y.-A.; Wong, T.-W.; Lee, N.-Y.; Yuann, J.-M.P.; Huang, S.-T.; Wu, C.-Y.; Chen, I.-Z.; Liang, J.-Y. Effects of Blue-Light-Induced Free Radical Formation from Catechin Hydrate on the Inactivation of Acinetobacter baumannii, Including a Carbapenem-Resistant Strain. Molecules 2018, 23, 1631. https://doi.org/10.3390/molecules23071631
Yang M-J, Hung Y-A, Wong T-W, Lee N-Y, Yuann J-MP, Huang S-T, Wu C-Y, Chen I-Z, Liang J-Y. Effects of Blue-Light-Induced Free Radical Formation from Catechin Hydrate on the Inactivation of Acinetobacter baumannii, Including a Carbapenem-Resistant Strain. Molecules. 2018; 23(7):1631. https://doi.org/10.3390/molecules23071631
Chicago/Turabian StyleYang, Meei-Ju, Yi-An Hung, Tak-Wah Wong, Nan-Yao Lee, Jeu-Ming P. Yuann, Shiuh-Tsuen Huang, Chun-Yi Wu, Iou-Zen Chen, and Ji-Yuan Liang. 2018. "Effects of Blue-Light-Induced Free Radical Formation from Catechin Hydrate on the Inactivation of Acinetobacter baumannii, Including a Carbapenem-Resistant Strain" Molecules 23, no. 7: 1631. https://doi.org/10.3390/molecules23071631