Synthesis and Evaluation of New Potential Benzo[a]phenoxazinium Photosensitizers for Anticancer Photodynamic Therapy
Abstract
:1. Introduction
2. Results and Discussion
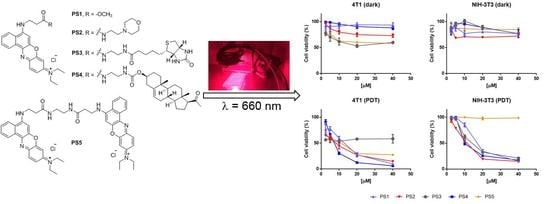
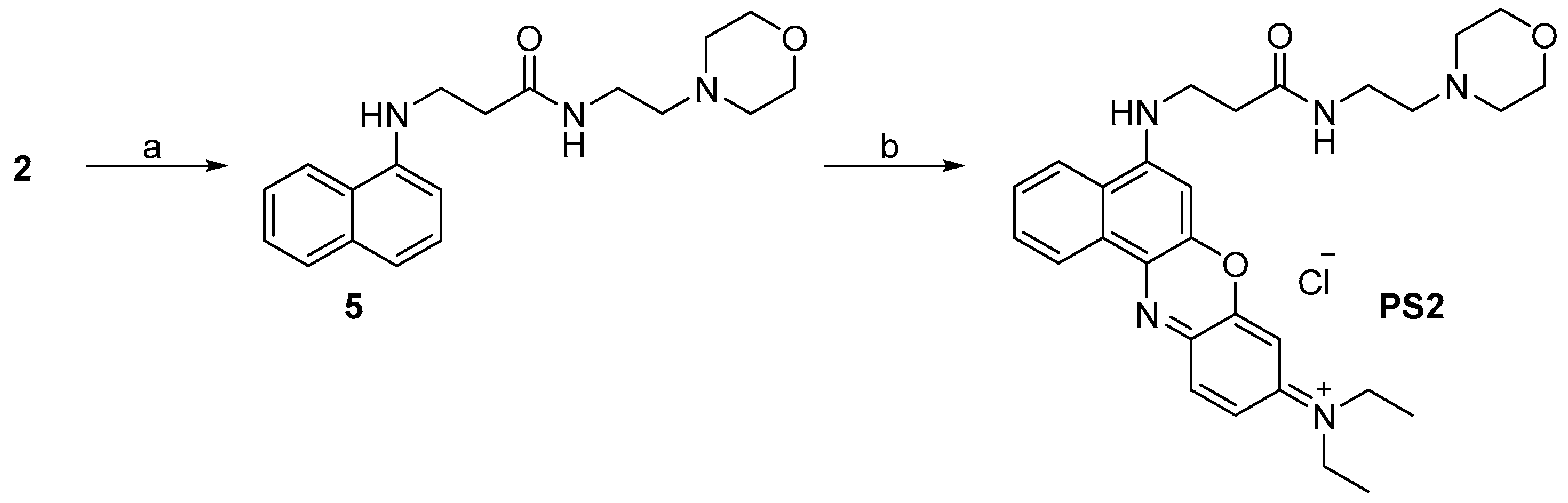
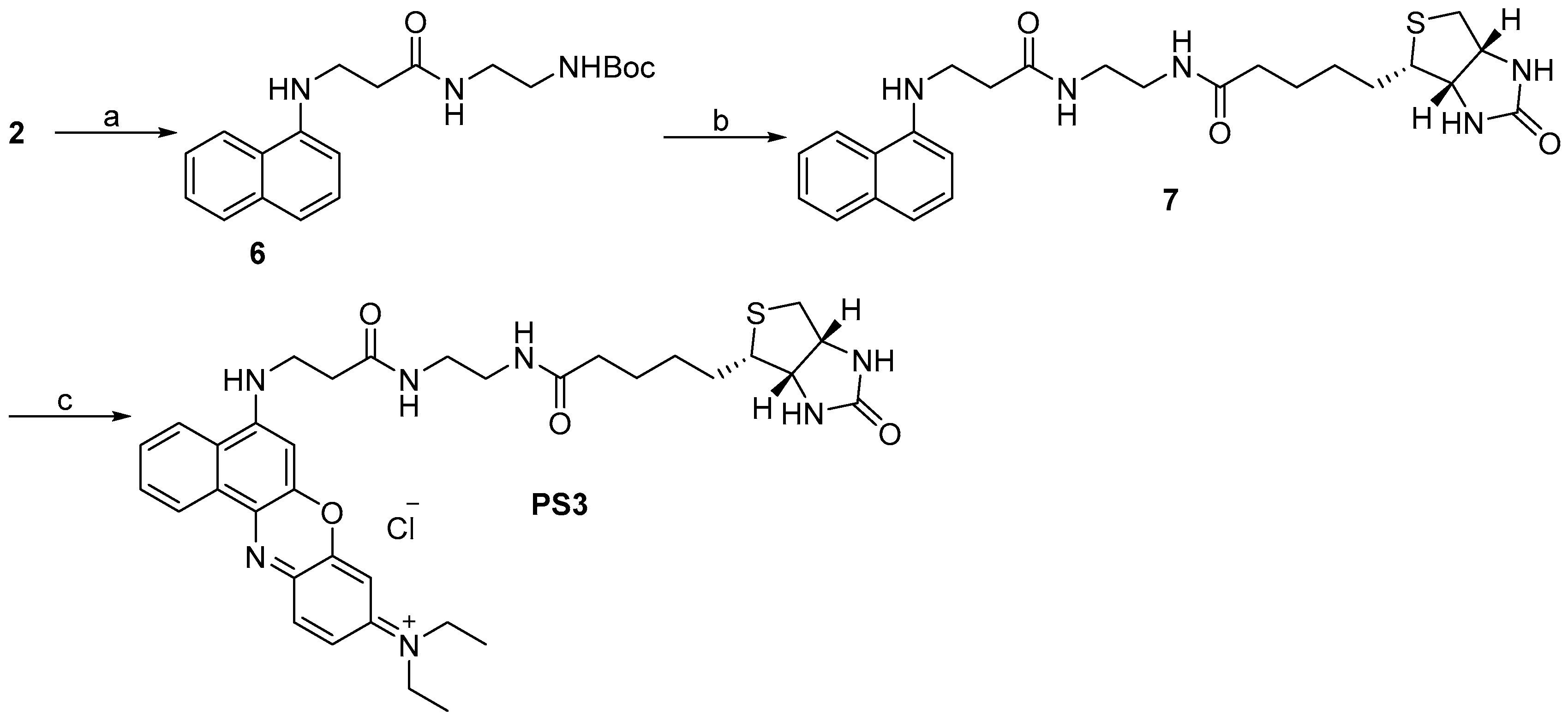
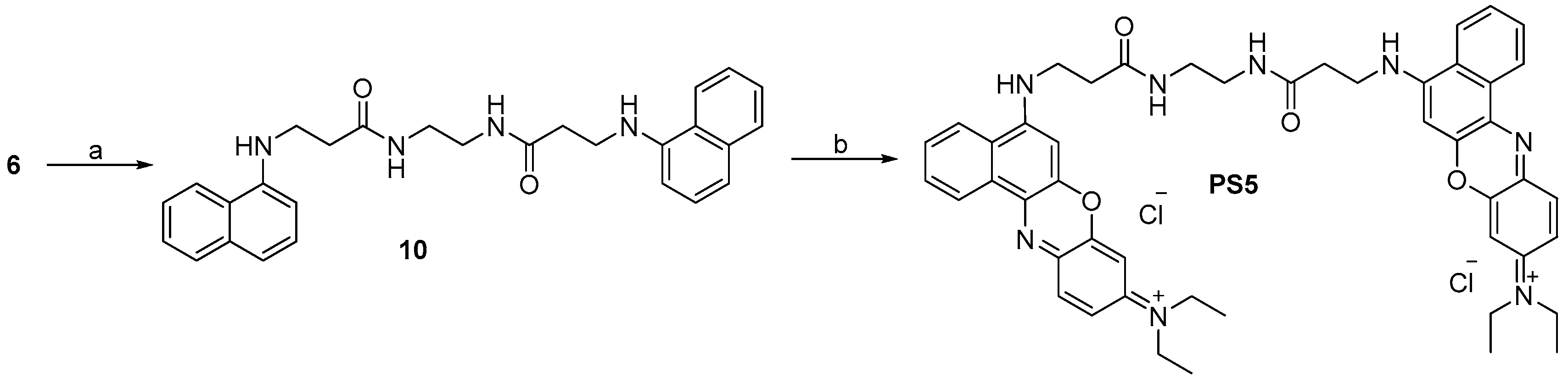
2.1. Design and Synthesis
2.2. Absorption and Emission Studies
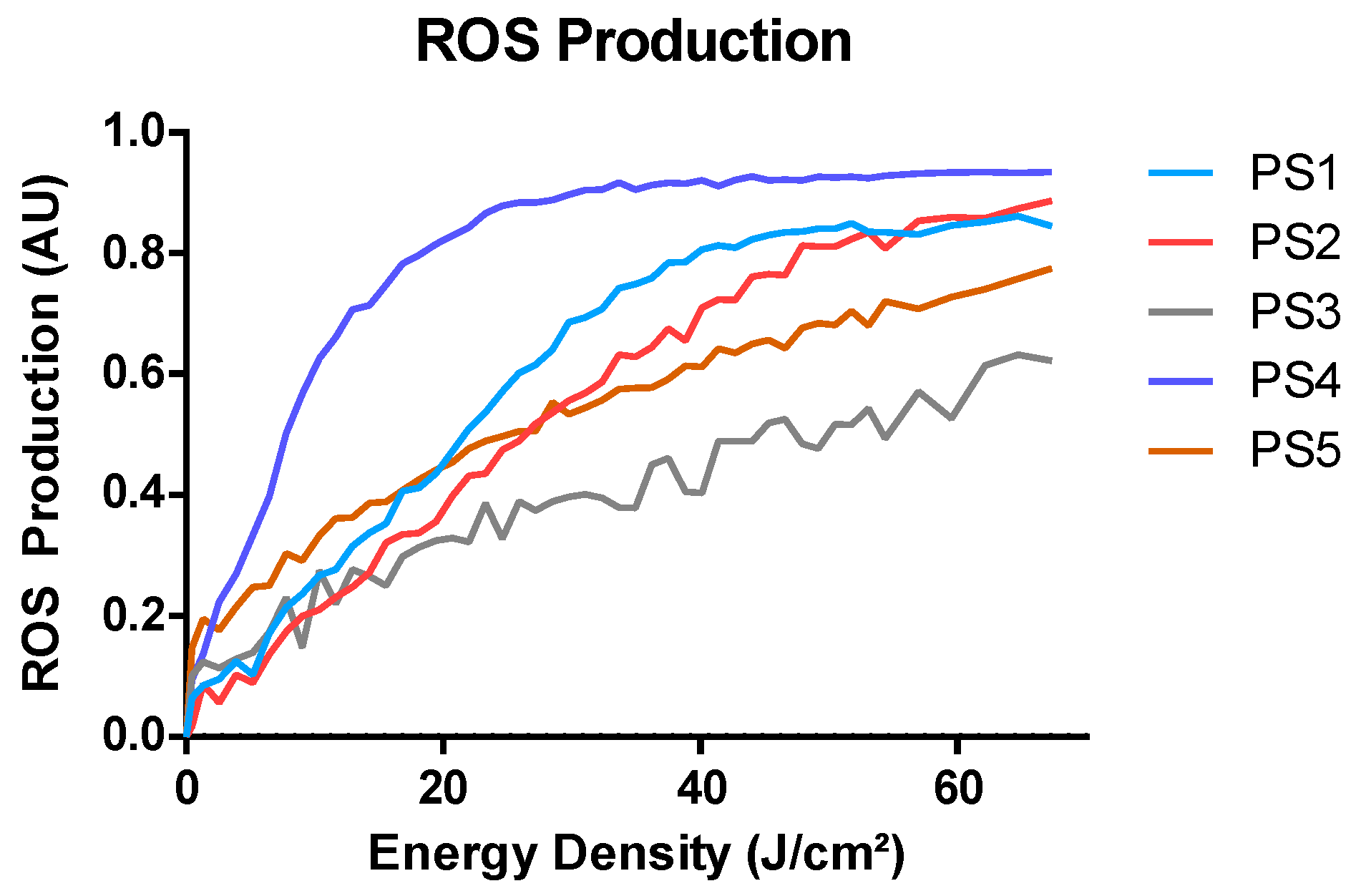
2.3. ROS Production
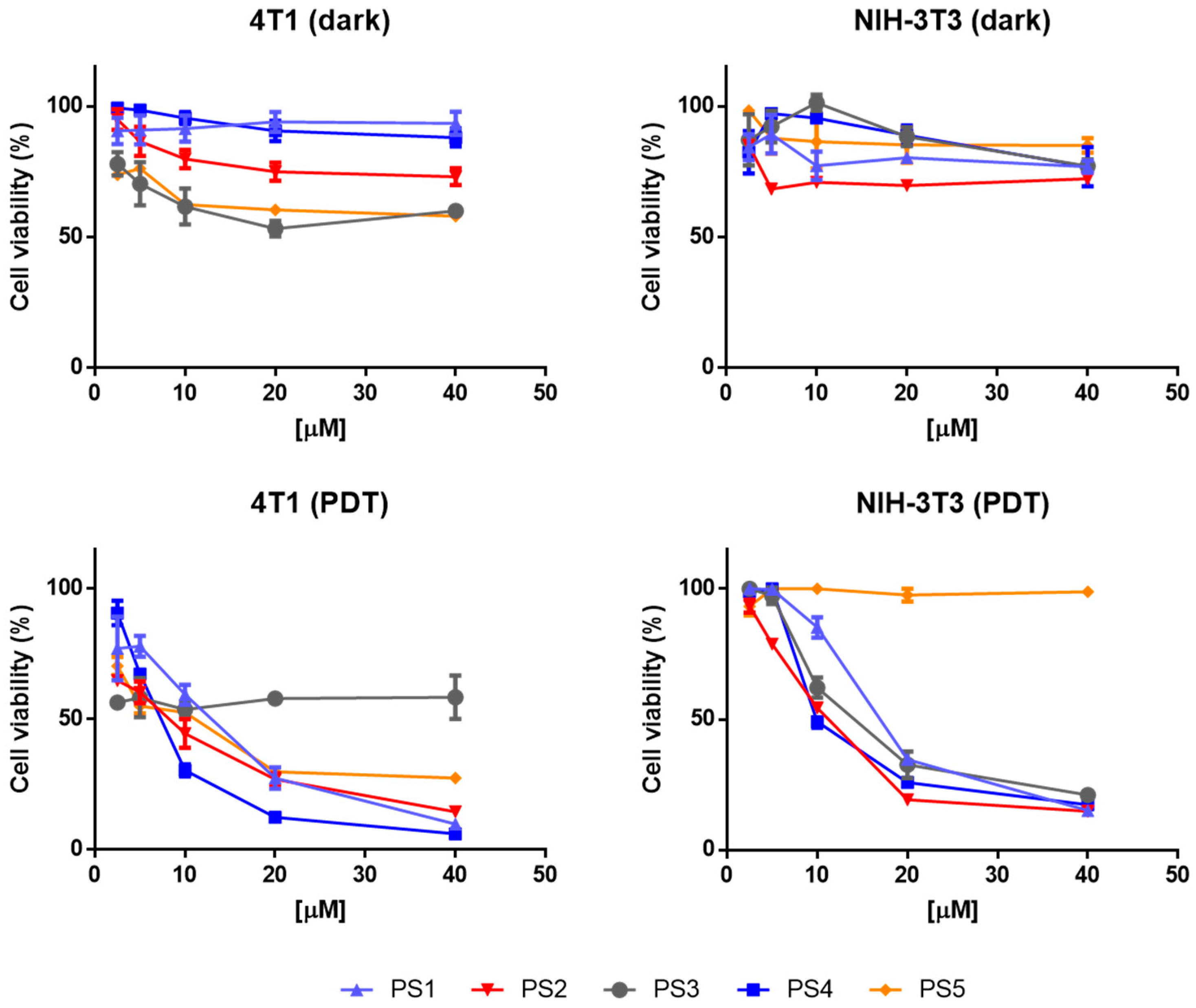
2.4. Photodynamic Activity Against Cells In Vitro
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of 3-(Naphthalen-1-ylamino)propanoic Acid (2, C13H13NO2)
3.2.2. Synthesis of 5-(Diethylamino)-2-nitrosophenol Hydrochloride (4, C10H15ClN2O2)
3.2.3. Synthesis of N-Ethyl-N-(5-(3-methoxy-3-oxopropylamino)-9H-benzo[a]phenoxazin-9-ylidene)ethanaminium Chloride (PS1, C24H26ClN3O3)
3.2.4. Synthesis of N-(2-Morpholinoethyl)-3-(naphthalen-1-ylamino)propanamide (5, C19H25N3O2)
3.2.5. Synthesis of N-Ethyl-N-(5-(3-(2-morpholinoethylamino)-3-oxopropylamino)-9H-benzo[a]phenoxazin-9-ylidene)ethanaminium Chloride (PS2, C29H36ClN5O3)
3.2.6. Synthesis of tert-Butyl 2-(3-(naphthalen-1-ylamino)propanamido)ethylcarbamate (6, C20H27N3O3)
3.2.7. Synthesis of N-(2-(3-(Naphthalen-1-ylamino)propanamido)ethyl)-5-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide (7, C25H33N5O3S)
3.2.8. Synthesis of N-Ethyl-N-(5-(3-oxo-3-(2-(5-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)ethylamino)propylamino)-9H-benzo[a]phenoxazin-9-ylidene)ethanaminium Chloride (PS3, C35H44ClN7O4S)
3.2.9. Synthesis of (3S,10R,13S,17S)-17-Acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 2-(3-(naphthalen-1-ylamino)propanamido)ethylcarbamate (9, C37H49N3O4)
3.2.10. Synthesis of N-(5-(3-(2-(((3S,10R,13S,17S)-17-Acetyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yloxy)carbonylamino)ethylamino)-3-oxopropylamino)-9H-benzo[a]phenoxazin-9-ylidene)-N-ethylethanaminium Chloride (PS4, C47H60ClN5O5)
3.2.11. Synthesis of N,N′-(Ethane-1,2-diyl)bis(3-(naphthalen-1-ylamino)propanamide) (10, C28H30N4O2)
3.2.12. Synthesis of di(N-(5-(3-(2-Aminoethylamino)-3-oxopropylamino)-9H-benzo[a]phenoxazin-9-ylidene))-N,N′-ethylethanaminium Dichloride (PS5, C48H52Cl2N8O4)
3.3. General Spectroscopic Measurements
3.4. ROS Detection
3.5. Phototoxicity Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic therapy with endogenous protoporphyrin. IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, C.S.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J.; van den Bergh, H.; Wagnières, G.; Burgess, K.; Lee, H.B. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yu, B.; Han, G.; Liu, M.; Shan, B.; Dong, G.; Miao, Z.; Jia, N.; Tan, Z.; Li, B.; et al. Chlorin p6-Based Water-Soluble Amino Acid Derivatives as Potent Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2016, 59, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Muehlmann, L.A.; Rodrigues, M.C.; Longo, J.P.; Garcia, M.P.; Py-Daniel, K.R.; Veloso, A.B.; de Souza, P.E.; da Silva, S.W.; Azevedo, R.B. Aluminium-phthalocyanine chloride nanoemulsions for anticancer photodynamic therapy: Development and in vitro activity against monolayers and spheroids of human mammary adenocarcinoma MCF-7 cells. J. Nanobiotechnol. 2015, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Muehlmann, L.A.; Ma, B.C.; Longo, J.P.; Almeida Santos Mde, F.; Azevedo, R.B. Aluminum-phthalocyanine chloride associated to poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as a new third-generation photosensitizer for anticancer photodynamic therapy. Int. J. Nanomed. 2014, 9, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Muehlmann, L.A.; Longo, J.P.; Silva, R.C.; Graebner, I.B.; Degterev, I.A.; Lucci, C.M.; Azevedo, R.B.; Garcia, M.P. Photodynamic Therapy Based on Arrabidaeachica (Crajiru) Extract Nanoemulsion: In vitro Activity against Monolayers and Spheroids of Human Mammary Adenocarcinoma MCF-7 Cells. J. Nanomed. Nanotechnol. 2015, 6, 1000286. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.; Silva, J.R.; Fascineli, M.L.; de Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; Carneiro, F.P.; et al. Photodynamic therapy mediated by acai oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J. Photochem. Photobiol. B 2017, 166, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef] [PubMed]
- Leitão, M.P.S.; Rama Raju, B.; Naik, S.; Coutinho, P.J.G.; João Sousa, M.; Gonçalves, M.T. Synthesis and photophysical studies of new benzo[a]phenoxazinium chlorides as potential antifungal agents. Tetrahedron Lett. 2016, 57, 3936–3941. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Goncalves, M.S.T.; Coutinho, P.J.G.; Moura, J.C.V.P. Synthesis and spectral properties of long-wavelength flurescent dyes. J. Photochem. Photobiol. A 2007, 185, 220–230. [Google Scholar] [CrossRef]
- Frade, V.H.J.; Sousa, M.J.; Moura, J.C.V.P.; Gonçalves, M.S.T. Synthesis of naphtho[2,3-a]phenoxazinium chlorides: Structure–activity relationships of these heterocycles and benzo[a]phenoxazinium chlorides as new antimicrobials. Bioorg. Med. Chem. 2008, 16, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Mizukawa, Y.; Ge, J.F.; Bakar, A.; Itoh, I.; Scheurer, C.; Wittlin, S.; Brun, R.; Matsuoka, H.; Ihara, M. Novel synthetic route for antimalarial benzo[a]phenoxazine derivative SSJ-183 and two active metabolites. Bioorg. Med Chem. 2014, 22, 3749–3752. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Alves, C.T.; Rama Raju, B.; Gonçalves, M.S.; Coutinho, P.J.; Henriques, M.; Belo, I. Application of benzo[a]phenoxazinium chlorides in antimicrobial photodynamic therapy of Candida albicans biofilms. J. Photochem. Photobiol. B 2014, 141, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, J.W.; Song, X.; Demidova, T.N.; Jalil, F.; Hamblin, M.R. Synthesis and properties of benzo[a]phenoxaziniumchalcogen analogues as novel broad-spectrum antimicrobial photosensitizers. J. Med. Chem. 2006, 49, 5291–5299. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; McLean, A. Rational design of phenothiazinium derivatives and photoantimicrobial drug discovery. Dyes Pigment. 2017, 136, 590–600. [Google Scholar] [CrossRef]
- Cincotta, L.; Foley, J.W.; Cincotta, A.H. Novel red absorbing benzo[a]phenoxazinium and benzo[a]phenothiazinium photosensitizers: In vitro evaluation. Photochem. Photobiol. 1987, 46, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Frade, V.H.; Gonçalves, M.S.; Moura, J.C. Synthesis and fluorescence properties of side-chain carboxylated 5,9-diaminobenzo[a]phenoxazinium salts. Tetradedron Lett. 2005, 46, 4949–4952. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of fluorescent probes based on protection-deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Chen, J.; Chen, J.; Kuznetsova, L.; Wong, S.S.; Ojima, I. Mechanism-based tumor-targeting drug delivery system. Validation of efficient vitamin receptor-mediated endocytosis and drug release. Bioconjug. Chem. 2010, 21, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Gülmez, A.D.; Göksel, M.; Durmuş, M.J. Silicon(IV) phthalocyanine-biotin conjugates: Synthesis, photophysicochemical properties and in vitro biological activity for photodynamic therapy. Porphyr. Phthalocyanines 2017, 21, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Qiu, L.; Liu, Q.; Lv, G.; Zhao, X.; Wang, S.; Lin, J. Conjugate of biotin with silicon(IV) phthalocyanine for tumor-targeting photodynamic therapy. J. Photochem. Photobiol. B 2017, 174, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Shervington, L.A.; Smith, N.; Norman, E.; Ward, T.; Phillips, R.; Shervington, A. To determine the cytotoxicity of chlorambucil and one of its nitro-derivatives, conjugated to prasterone and pregnenolone, towards eight human cancer cell-lines. Eur. J. Med. Chem. 2009, 44, 2944–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.L.; Zhang, J.; Liu, C.L.; Liu, C.; Zhu, K.K.; Yang, F.F.; Liu, X.G.; Figueiró Longo, J.P.; Alexandre Muehlmann, L.; Azevedo, R.B.; et al. Design and synthesis of pregnenolone/2-cyanoacryloyl conjugates with dual NF-κB inhibitory and anti-proliferative activities. Bioorg. Med. Chem. Lett. 2017, 27, 4682–4686. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; El-Sayed, N.N.; Abdelaziz, M.A. The Knoevenagel reactions of pregnenolone with cyanomethylene reagents: Synthesis of thiophene, thieno[2,3-b]pyridine, thieno[3,2-d]isoxazole derivatives of pregnenolone and their in vitro cytotoxicity towards tumor and normal cell lines. Steroids 2013, 78, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Szalóki, G.; Pantzou, A.; Prousis, K.C.; Mavrofrydi, O.; Papazafiri, P.; Calogeropoulou, T. Design and synthesis of 21-alkynylaryl pregnenolone derivatives and evaluation of their anticancer activity. Bioorg. Med. Chem. 2014, 22, 6980–6988. [Google Scholar] [CrossRef] [PubMed]
- Takakusa, H.; Kikuchi, K.; Urano, Y.; Sakamoto, S.; Yamaguchi, K.; Nagano, T. Design and Synthesis of an Enzyme-Cleavable Sensor Molecule for Phosphodiesterase Activity Based on Fluorescence Resonance Energy Transfer. J. Am. Chem. Soc. 2002, 124, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Dong, H.; Hu, M.; Wang, J.; Zhang, H.; Zhu, H.; Sun, W.; Peng, X. Fluorescence imaging lysosomal changes during cell division and apoptosis observed using Nile Blue based near-infrared emission. Chem. Commun. 2014, 50, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Cassidy, C.M.; Woolfson, D.; Donnelly, R.F. Designing photosensitizers for photodynamic therapy: Strategies, challenges and promising developments. Future Med. Chem. 2009, 1, 667–691. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.M.; Bilgin, M.D.; Grossweiner, L.L. Singlet oxygen generation by photodynamic agents. J. Photochem. Photobiol. B 1997, 37, 131–140. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds PS1–PS5 are available from the authors. |



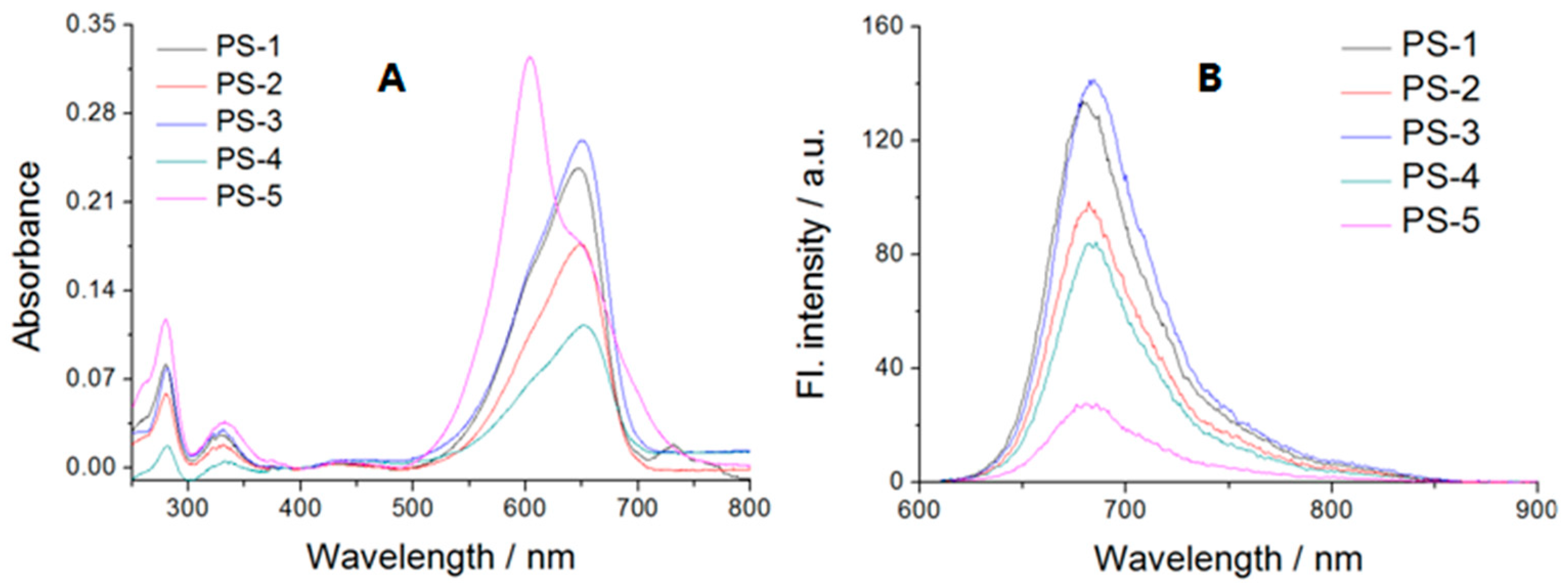
| Compound | λabs a | Ε b | λem a | Δλ a | φ |
|---|---|---|---|---|---|
| PS1 | 648 | 47,400 | 681 | 33 | 0.075 |
| PS2 | 649 | 35,400 | 682 | 33 | 0.077 |
| PS3 | 650 | 51,800 | 684 | 34 | 0.082 |
| PS4 | 651 | 22,600 | 686 | 35 | 0.116 |
| PS5 | 649 (604) | 35,600 (65,000) | 686 | 37 | 0.025 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Tavares de Sousa Júnior, W.; Mello da Silva, V.C.; Rodrigues, M.C.; Vasconcelos Morais, J.A.; Song, J.-L.; Cheng, Z.-Q.; Longo, J.P.F.; Bentes Azevedo, R.; Jiang, C.-S.; et al. Synthesis and Evaluation of New Potential Benzo[a]phenoxazinium Photosensitizers for Anticancer Photodynamic Therapy. Molecules 2018, 23, 1436. https://doi.org/10.3390/molecules23061436
Zhang J, Tavares de Sousa Júnior W, Mello da Silva VC, Rodrigues MC, Vasconcelos Morais JA, Song J-L, Cheng Z-Q, Longo JPF, Bentes Azevedo R, Jiang C-S, et al. Synthesis and Evaluation of New Potential Benzo[a]phenoxazinium Photosensitizers for Anticancer Photodynamic Therapy. Molecules. 2018; 23(6):1436. https://doi.org/10.3390/molecules23061436
Chicago/Turabian StyleZhang, Juan, Wellington Tavares de Sousa Júnior, Victor Carlos Mello da Silva, Mosar Correa Rodrigues, José Athayde Vasconcelos Morais, Jia-Li Song, Zhi-Qiang Cheng, João Paulo Figueiró Longo, Ricardo Bentes Azevedo, Cheng-Shi Jiang, and et al. 2018. "Synthesis and Evaluation of New Potential Benzo[a]phenoxazinium Photosensitizers for Anticancer Photodynamic Therapy" Molecules 23, no. 6: 1436. https://doi.org/10.3390/molecules23061436
APA StyleZhang, J., Tavares de Sousa Júnior, W., Mello da Silva, V. C., Rodrigues, M. C., Vasconcelos Morais, J. A., Song, J.-L., Cheng, Z.-Q., Longo, J. P. F., Bentes Azevedo, R., Jiang, C.-S., Alexandre Muehlmann, L., & Zhang, H. (2018). Synthesis and Evaluation of New Potential Benzo[a]phenoxazinium Photosensitizers for Anticancer Photodynamic Therapy. Molecules, 23(6), 1436. https://doi.org/10.3390/molecules23061436