Preparation of Ruthenium Dithiolene Complex/Polysiloxane Films and Their Responses to CO Gas
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
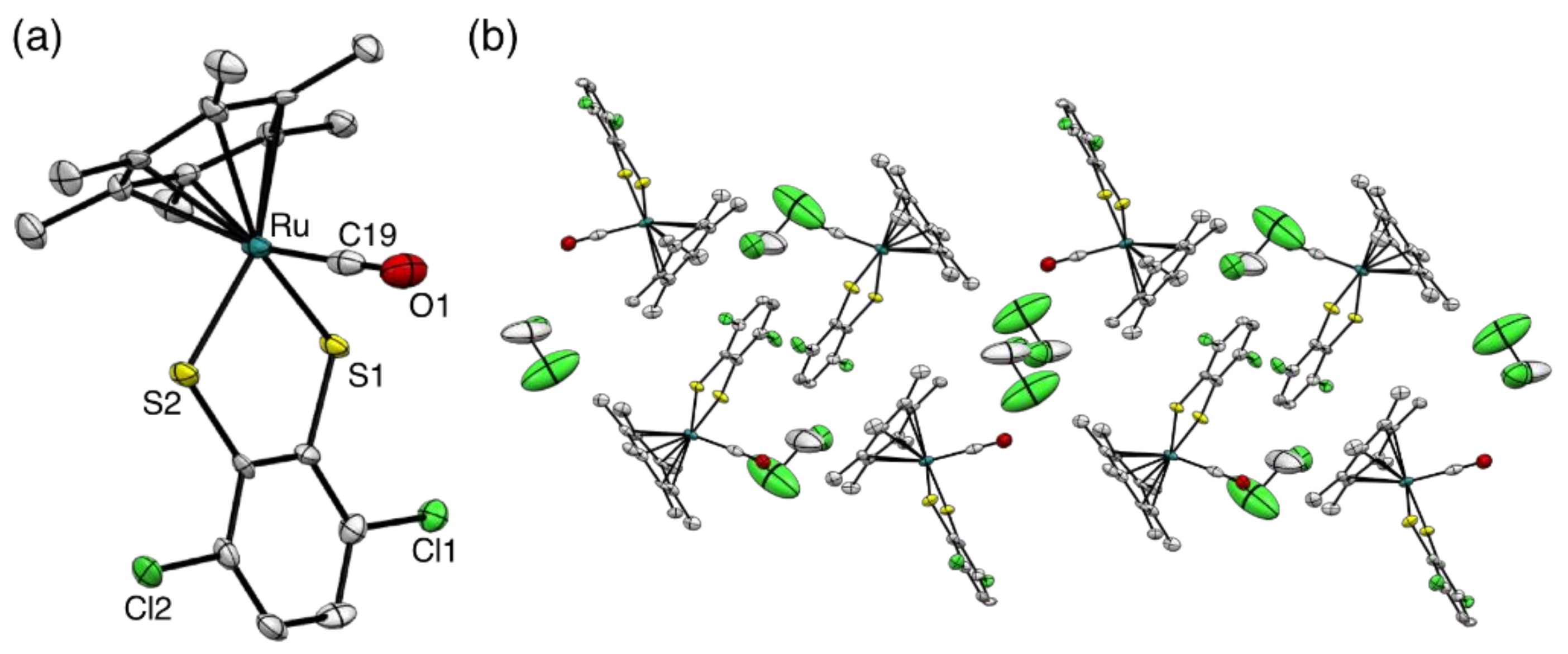
2.2. Molecular Structures
2.3. Optical Properties and Thermal Characteristics
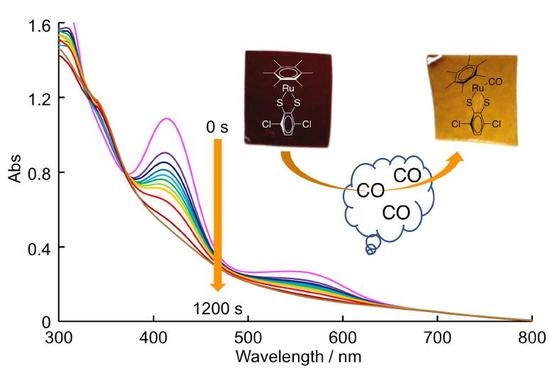
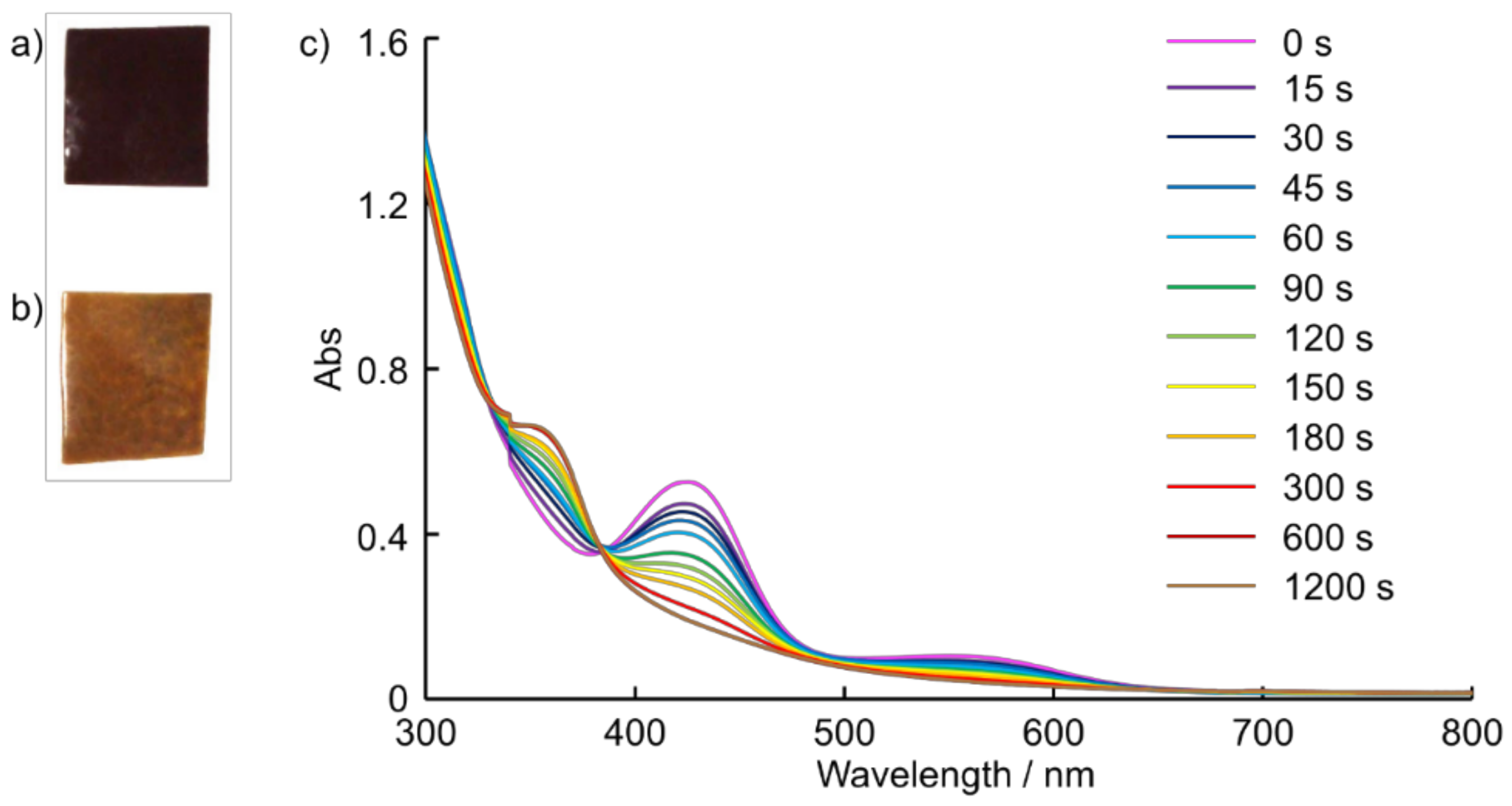
2.4. Preparation of Composite Films and Their Reactivity with CO Gas
3. Materials and Methods
3.1. General
3.2. X-ray Crystallography
3.3. Synthesis
3.3.1. General Procedure for the Synthesis of Dihalogen-Substituted Benzendithiols 3b–e
3.3.2. General Procedure for the Synthesis of Ruthenium Dithiolene Complexes 1b–e
3.3.3. General Procedure for the Reaction of Ruthenium Carbonyl Complexes 2b–e
3.3.4. Preparation of Polysiloxane
3.3.5. Preparation of Self-Standing Composite Film
3.3.6. Reaction of a Composite Film with CO Gas
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mark, J.E.; Allcock, H.R.; West, R. Inorganic Polymers; Prentice Hall: Englewood Cliffs, NJ, USA, 1992; p xiv; 272p. [Google Scholar]
- Wisian-Neilson, P.; Allcock, H.R.; Wynne, K.J.; Division of Polymer Chemistry, American Chemical Society; American Chemical Society. Meeting. In Inorganic and Organometallic Polymers II: Advanced Materials and Intermediates, Proceedings of the 205th National Meeting of the American Chemical Society, Denver, CO, USA, 28 March–2 April 1993; ACS: Washington, DC, USA, 1994; Developed from a Symposium Sponsored by the Division of Polymer Chemistry, Inc.; p xiii; 536p. [Google Scholar]
- Pittman, C.U.; American Chemical Society. Meeting. In Metal-Containing Polymeric Materials; Plenum Press: New York, NY, USA, 1996; p x; 518p. [Google Scholar]
- Archer, R.D. Inorganic and Organometallic Polymers; Wiley-VCH: New York, NY, USA, 2001; p xii; 247p. [Google Scholar]
- Akitsu, T.; Itoh, T. Polarized spectroscopy of hybrid materials of chiral Schiff base cobalt(II), nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes in PMMA films. Polyhedron 2010, 29, 477–487. [Google Scholar] [CrossRef]
- Aritake, Y.; Takanashi, T.; Yamazaki, A.; Akitsu, T. Polarized spectroscopy and hybrid materials of chiral Schiff base Ni(II), Cu(II), Zn(II) complexes with included or separated azo-groups. Polyhedron 2011, 30, 886–894. [Google Scholar] [CrossRef]
- Yamazaki, A.; Akitsu, T. Polarized spectroscopy and polarized UV light-induced molecular orientation of chiral diphenyl Schiff base Ni(II) and Cu(II) complexes and azobenzene in a PMMA film. RSC Adv. 2012, 2, 2975–2980. [Google Scholar] [CrossRef]
- Ito, M.; Akitsu, T. Polarized UV light induced molecular arrangement depending on flexibility of chiral Schiff base Ni(II), Cu(II), and Zn(II) complexes by azobenzene in PMMA matrix. Contemp. Eng. Sci. 2014, 7, 869–877. [Google Scholar] [CrossRef]
- Hariu, N.; Ito, M.; Akitsu, T. Linearly, circularly, or non-polarized light induced supramolecular arrangement of diastereomer Schiff base Ni(II), Cu(II), and Zn(II) complexes by azobenzene in PMMA matrix. Contemp. Eng. Sci. 2015, 8, 57–70. [Google Scholar] [CrossRef]
- Morisue, M.; Hoshino, Y.; Shimizu, M.; Nakanishi, T.; Hasegawa, Y.; Hossain, M.A.; Sakurai, S.; Sasaki, S.; Uemura, S.; Matsui, J. Supramolecular polymer of near-infrared luminescent porphyrin glass. Macromolecules 2017, 50, 3186–3192. [Google Scholar] [CrossRef]
- Morisue, M.; Ueno, I.; Nakanishi, T.; Matsui, T.; Sasaki, S.; Shimizu, M.; Matsui, J.; Hasegawa, Y. Amorphous porphyrin glasses exhibit near-infrared excimer luminescence. RSC Adv. 2017, 7, 22679–22683. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Catalysis in C1 chemistry: Future and prospect. Catal. Lett. 1993, 22, 67–91. [Google Scholar] [CrossRef]
- Bao, J.; Tsubaki, N. Design and synthesis of powerful capsule catalysts aimed at applications in C1 chemistry and biomass conversion. Chem. Rec. 2018, 18, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, T.; Dai, Y.; An, Y.; Yu, F.; Zhong, L.; Li, S.; Sun, Y. Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts. Catal. Today 2017, in press. [Google Scholar] [CrossRef]
- Tao, D.J.; Chen, F.F.; Tian, Z.Q.; Huang, K.; Mahurin, S.M.; Jiang, D.E.; Dai, S. Highly efficient carbon monoxide capture by carbanion-functionalized ionic liquids through C-site interactions. Angew. Chem. 2017, 56, 6843–6847. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.A.; Xiao, D.J.; Gonzalez, M.I.; Darago, L.E.; Herm, Z.R.; Grandjean, F.; Long, J.R. Reversible CO scavenging via adsorbate-dependent spin state transitions in an iron(II)-triazolate metal-organic framework. J. Am. Chem. Soc. 2016, 138, 5594–5602. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Kosaka, W.; Matsuda, R.; Hori, A.; Hijikata, Y.; Belosludov, R.V.; Sakaki, S.; Takata, M.; Kitagawa, S. Self-accelerating co sorption in a soft nanoporous crystal. Science 2014, 343, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.D.; Hudson, M.R.; Mason, J.A.; Chavan, S.; Crocella, V.; Howe, J.D.; Lee, K.; Dzubak, A.L.; Queen, W.L.; Zadrozny, J.M.; et al. Reversible co binding enables tunable CO/H2 and CO/N2 separations in metal-organic frameworks with exposed divalent metal cations. J. Am. Chem. Soc. 2014, 136, 10752–10761. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Amalraj, F.; Radhakrishnan, S. CO sensor based on polypyrrole functionalized with iron porphyrin. Synth. Met. 2009, 159, 1019–1023. [Google Scholar] [CrossRef]
- Mosciano, F.; Magna, G.; Catini, A.; Pomarico, G.; Martinelli, E.; Paolesse, R.; Di Natale, C. Room temperature co detection by hybrid porphyrin-ZnO nanoparticles. Proced. Eng. 2015, 120, 71–74. [Google Scholar] [CrossRef]
- Yamazaki, S.; Yao, M.; Asahi, M.; Sato, H.; Yamano, A.; Ioroi, T. Characterization of a Rh(III) porphyrin-CO complex: Its structure and reactivity with an electron acceptor. Dalton Trans. 2015, 44, 13823–13827. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.T.; Malliakas, C.D.; Harris, T.D. Co. binding at a four-coordinate cobaltous porphyrin site in a metal-organic framework: Structural, EPR, and gas adsorption analysis. Inorg. Chem. 2017, 56, 4655–4662. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Sagawa, T.; Gunji, T. Carbon monoxide addition to ruthenium-dithiolene complex and polysiloxane hybrid film formation. Chem. Asian J. 2015, 10, 1881–1883. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Kondo, M.; Sato, H.; Gunji, T. Fine electronic state tuning of cobaltadithiolene complexes by substituent groups on the benzene ring. Polyhedron 2016, 117, 265–272. [Google Scholar] [CrossRef]
- Gilliam, O.R.; Johnson, C.M.; Gordy, W. Microwave spectroscopy in the region from two to three millimeters. Phys. Rev. 1950, 78, 140–144. [Google Scholar] [CrossRef]
- Mashima, K.; Kaneko, S.-I.; Tani, K.; Kaneyoshi, H.; Nakamura, A. Synthesis and reactions of coordinatively unsaturated 16-electron chalcogenolate complexes, Ru(EAr)2(η6-arene) and cationic binuclear chalcogenolate complexes, [(η6-arene)Ru(μ-EPh)3Ru(η6-arene)]PF6. J. Organomet. Chem. 1997, 545–546, 345–356. [Google Scholar] [CrossRef]
- Mashima, K.; Kaneyoshi, H.; Kaneko, S.-I.; Mikami, A.; Tani, K.; Nakamura, A. Chemistry of coordinatively unsaturated bis(thiolato)ruthenium(II) complexes (η6-arene)Ru(SAr)2[SAr = 2,6-dimethylbenzenethiolate, 2,4,6-triisopropylbenzenethiolate; (SAr)2= 1,2-benzenedithiolate; arene = benzene, p-cymene, hexamethylbenzene]. Organometallics 1997, 16, 1016–1025. [Google Scholar] [CrossRef]
- Kajitani, M.; Igarashi, A.; Hatano, H.; Akiyama, T.; Sugimori, A.; Matsumoto, S.; Iguchi, Y.; Boennemann, H.; Shimizu, K.; Satô, G.P. Formation constants of some phosphine and phosphite adducts of (η5-cyclopentadienyl) (substituted 1,2-ethylenedichalcogenolato) cobalt(III) complexes and their 1H, 13C and 31P NMR spectra. J. Organomet. Chem. 1995, 485, 31–36. [Google Scholar] [CrossRef]
- Bennett, M.A.; Huang, T.N.; Matheson, T.W.; Smith, A.K.; Ittel, S.; Nickerson, W. 16. (η6-hexamethylbenzene)ruthenium complexes. In Inorganic Syntheses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 74–78. [Google Scholar]
- Sheldrick, G.M. SADABS (V2.10); Program for Siemens area detector absorption correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXS–97 (6.14 8/06/00); Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL–97 (6.14 8/06/00); Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
Sample Availability: Not available. |




| 2a | 2b | 2c | 2d | 2e | |
|---|---|---|---|---|---|
| C–O distance (Å) | 1.133 (7) | 1.122 (10) | 1.150 (14) | 1.09 (3) a | 1.22 (3) b |
| Ru–C–O angle (°) | 178.8 (6) | 178.2 (8) | 178.6 (10) | 174 (2) a | 179 (2) b |
| νCO (cm−1) | 1952 | 1968 | 1976 | 1972 | 1979 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukada, S.; Sagawa, T.; Yamamoto, K.; Gunji, T. Preparation of Ruthenium Dithiolene Complex/Polysiloxane Films and Their Responses to CO Gas. Molecules 2018, 23, 845. https://doi.org/10.3390/molecules23040845
Tsukada S, Sagawa T, Yamamoto K, Gunji T. Preparation of Ruthenium Dithiolene Complex/Polysiloxane Films and Their Responses to CO Gas. Molecules. 2018; 23(4):845. https://doi.org/10.3390/molecules23040845
Chicago/Turabian StyleTsukada, Satoru, Takuya Sagawa, Kazuki Yamamoto, and Takahiro Gunji. 2018. "Preparation of Ruthenium Dithiolene Complex/Polysiloxane Films and Their Responses to CO Gas" Molecules 23, no. 4: 845. https://doi.org/10.3390/molecules23040845