Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lipase-Catalysed Synthesis of Hydroxytyrosol-Fatty Acid Conjugates
2.2. Characterisation of Hydroxytyrosyl Esters
2.3. Antioxidant Activity by Spectrophotometric Methods
2.3.1. DPPH Radical Scavenging Activity
2.3.2. ABTS Radical Scavenging Activity
2.3.3. Antioxidant Activity in a β-Carotene-Linoleate Model System
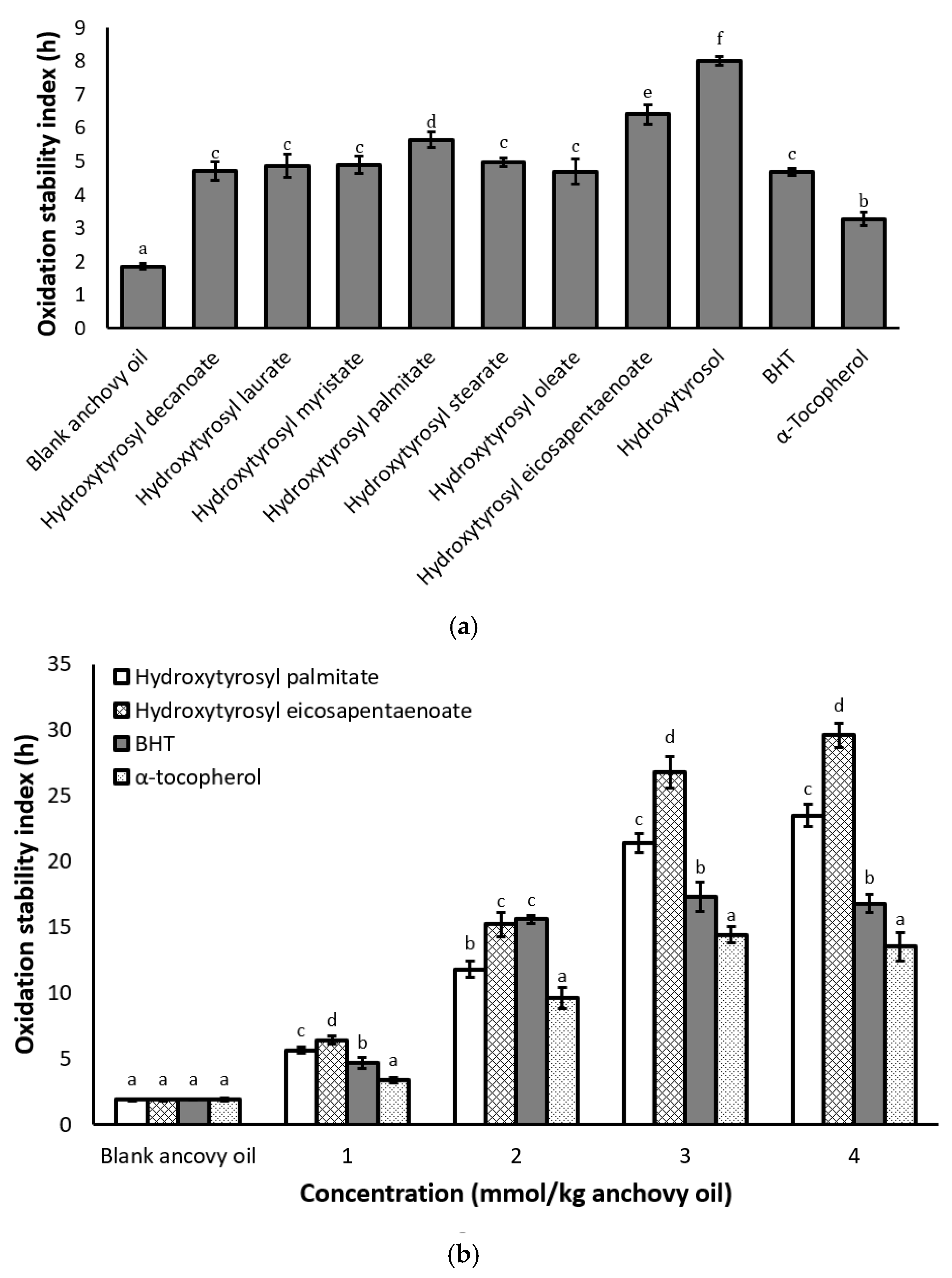
2.4. Antioxidant Activity Under Rancimat Conditions
2.5. Antioxidant Activity under Schaal Oven Conditions
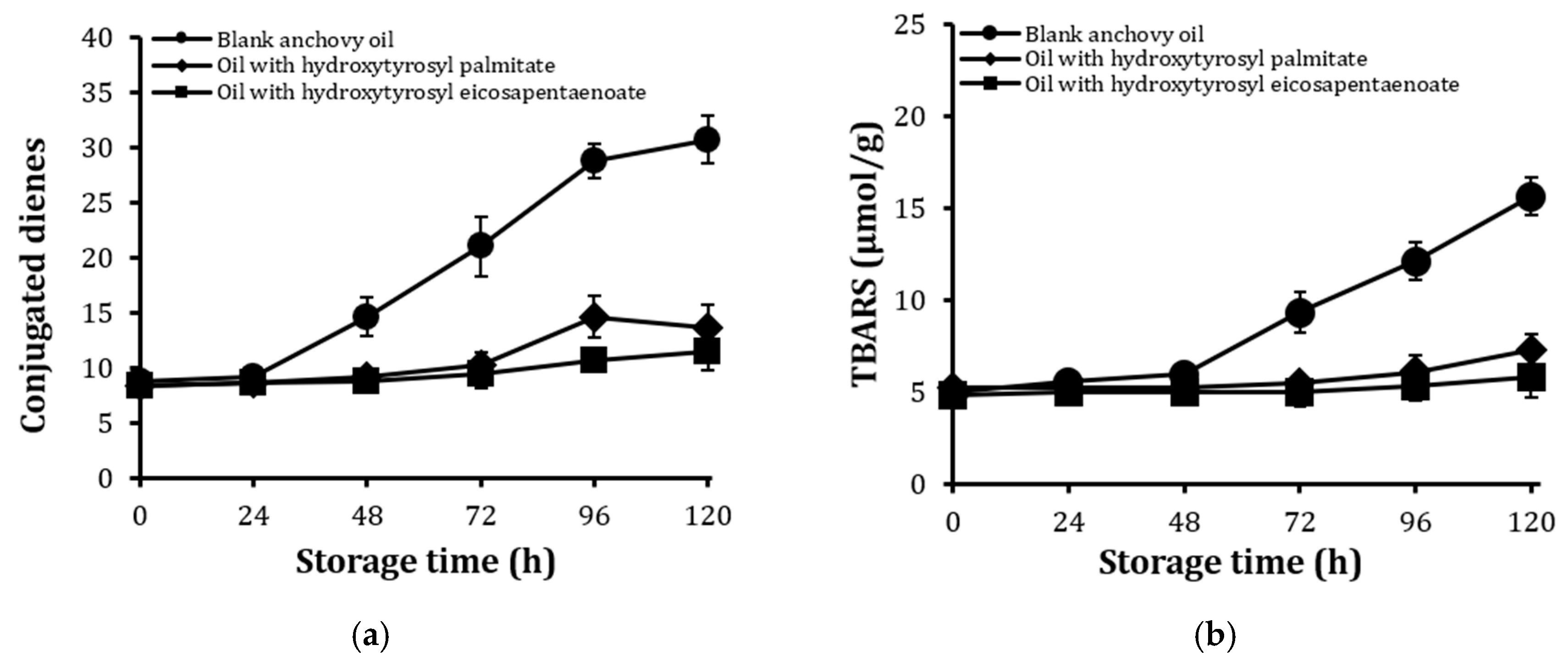
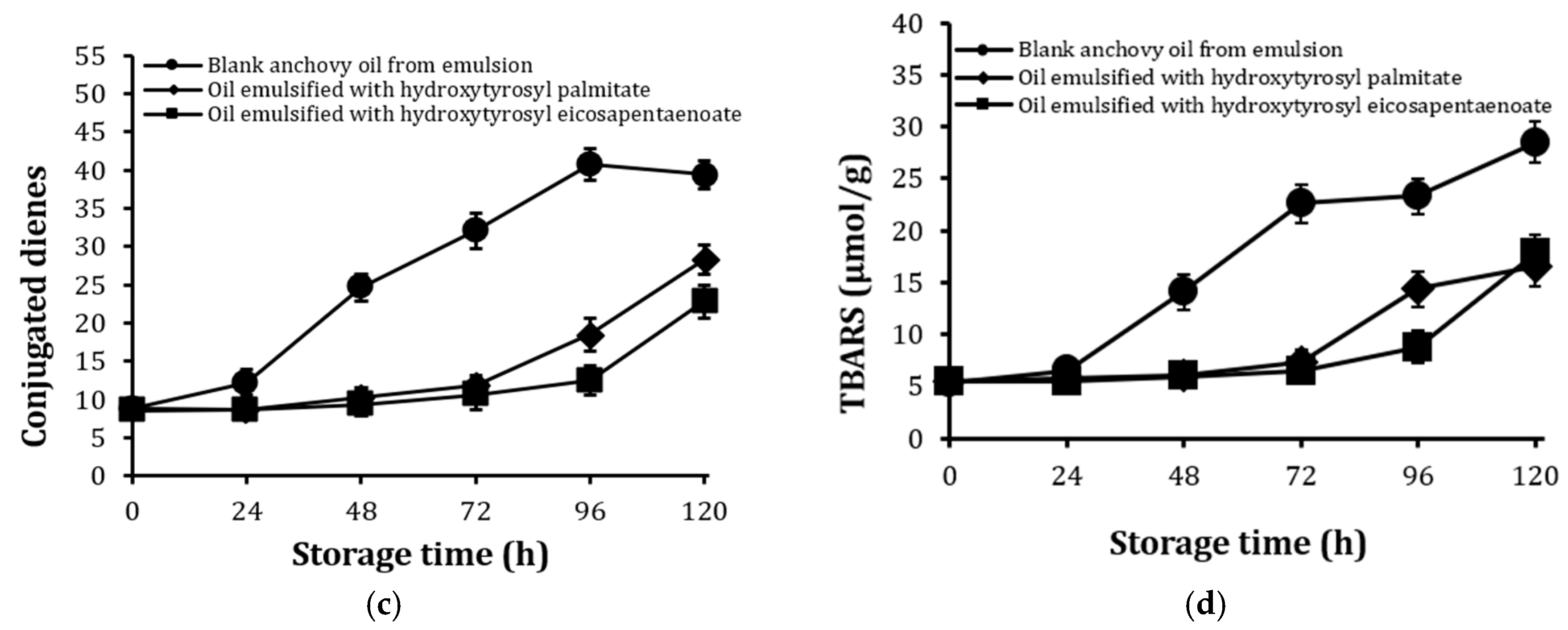
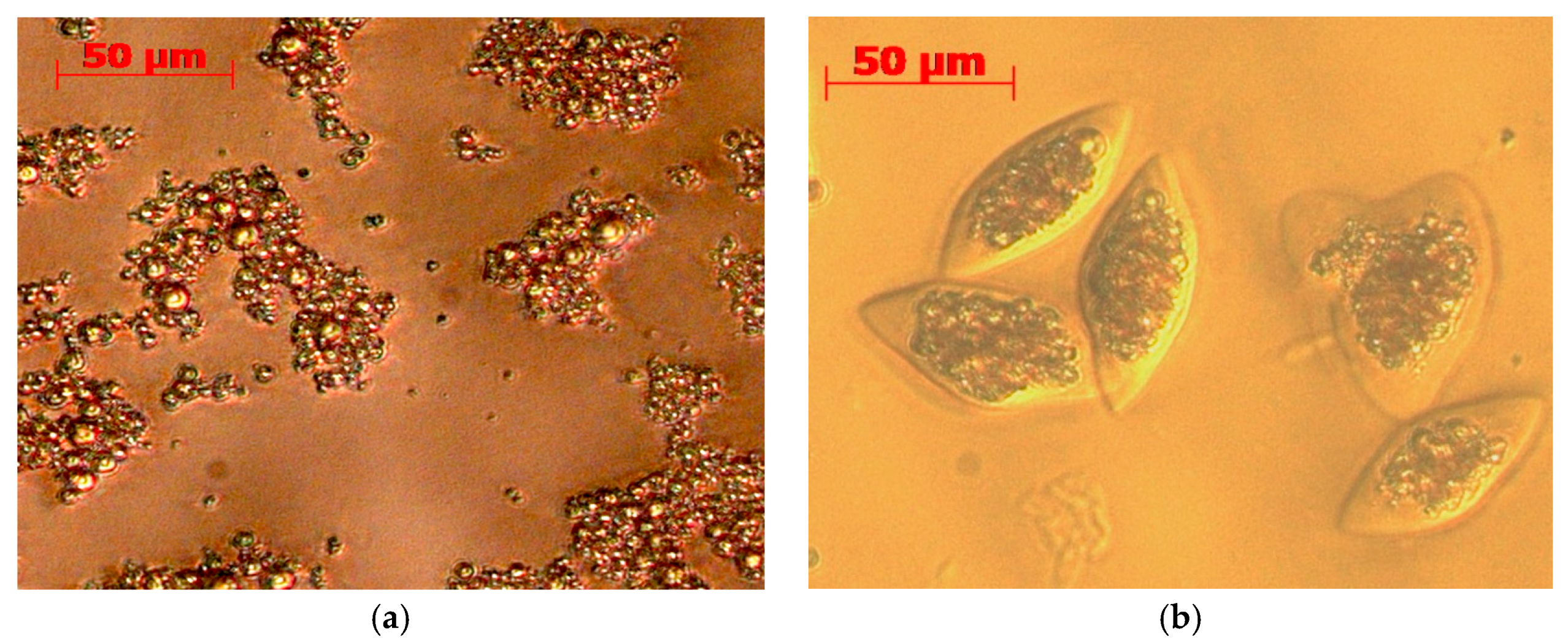
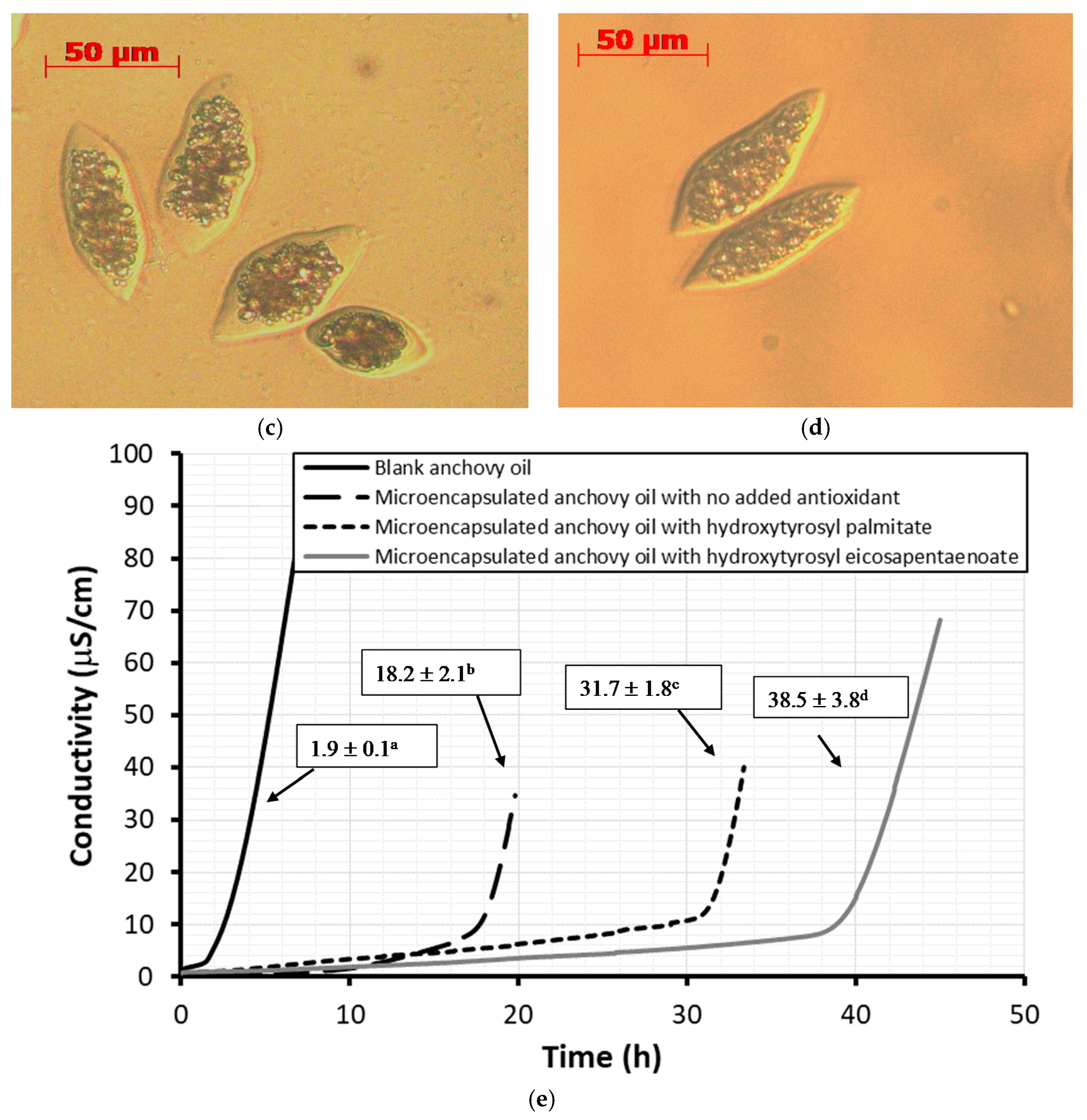
2.6. Stability Against Oxidation of Encapsulated Anchovy Oil
2.7. Stability of Conjugates After Extended Storage
3. Materials and Methods
3.1. Materials
3.2. Enzymatic Synthesis of Hydroxytyrosyl Esters
3.2.1. Optimizing Synthesis Conditions
3.2.2. Scaled-up Synthesis of Hydroxytyrosyl Esters
3.3. Identification, Purification and Characterisation of Hydroxytyrosyl Esters
3.4. Measurement of Antioxidant Activity by Spectrophotometric Methods
3.4.1. DPPH Radical Scavenging Method
3.4.2. ABTS Radical Scavenging Method
3.4.3. β-Carotene Bleaching Method
3.5. Accelerated Antioxidant Tests
3.5.1. Preparation of Bulk Oil and Oil-in-Water Emulsion Containing Hydroxytyrosyl Esters
3.5.2. Measurement of Antioxidant Activity Using the Schaal Oven Test
3.5.3. Measurement of Antioxidant Activity by Rancimat Method
3.6. Microencapsulation by Coacervation
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pazos, M.; Alonso, A.; Sánchez, I.; Medina, I. Hydroxytyrosol prevents oxidative deterioration in foodstuffs rich in fish lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.W.; Caccetta, R.A.A.; Puddey, I.B.; Croft, K.D. Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2000, 27, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No. 258/97. EFSA J. 2017, 15, 4728. [Google Scholar] [CrossRef]
- Bouallagui, Z.; Bouaziz, M.; Lassoued, S.; Engasser, J.M.; Ghoul, M.; Sayadi, S. Hydroxytyrosol acyl esters: Biosynthesis and activities. Appl. Biochem. Biotechnol. 2011, 163, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Madrona, A.; Bravo, L.; Espartero, J.L.; Alcudia, F.; Cert, A.; Mateos, R. Antioxidant activity evaluation of alkyl hydroxytyrosyl ethers, a new class of hydroxytyrosol derivatives. Food Chem. 2009, 115, 86–91. [Google Scholar] [CrossRef]
- Trujillo, M.; Mateos, R.; Collantes de Teran, L.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Barrow, C.J. Lipase-catalysed incorporation of EPA into emu oil: Formation and characterisation of new structured lipids. J. Funct. Foods 2015, 19, 801–809. [Google Scholar] [CrossRef]
- Akanbi, T.O.; Barrow, C.J. Candida antarctica lipase a effectively concentrates DHA from fish and thraustochytrid oils. Food Chem. 2017, 229, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Comelles, F.; Alcántara, D.; Maldonado, O.S.; Curcuroze, M.; Parra, J.L.; Morales, J.C. Surface-active properties of lipophilic antioxidants tyrosol and hydroxytyrosol fatty acid esters: A potential explanation for the nonlinear hypothesis of the antioxidant activity in oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 8021–8026. [Google Scholar] [CrossRef] [PubMed]
- Medina, I.; Lois, S.; Alcántara, D.; Lucas, R.; Morales, J.C. Effect of lipophilization of hydroxytyrosol on its antioxidant activity in fish oils and fish oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 9773–9779. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Akoh, C.C. Enzymatic synthesis of tyrosol-based phenolipids: Characterization and effect of alkyl chain unsaturation on the antioxidant activities in bulk oil and oil-in-water emulsion. J. Am. Oil Chem. Soc. 2016, 93, 329–337. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Rupasinghe, H. Long chain fatty acid acylated derivatives of quercetin-3-O-glucoside as antioxidants to prevent lipid oxidation. Biomolecules 2014, 4, 980–993. [Google Scholar] [CrossRef] [PubMed]
- de Pinedo, A.T.; Penalver, P.; Rondon, D.; Morales, J. Efficient lipase-catalyzed synthesis of new lipid antioxidants based on a catechol structure. Tetrahedron 2005, 61, 7654–7660. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Sun, Y.-X.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Foods 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of polyamide oxidative fluorescence test on lipid emulsions: Contrast in relative effectiveness of antioxidants in bulk versus dispersed systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the polar paradox theory: A critical overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shahidi, F. Acidolysis of p-coumaric acid with omega-3 oils and antioxidant activity of phenolipid products in in vitro and biological model systems. J. Agric. Food Chem. 2013, 62, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Trujillo, M.; Pereira-Caro, G.; Madrona, A.; Cert, A.; Espartero, J.L. New lipophilic tyrosyl esters. Comparative antioxidant evaluation with hydroxytyrosyl esters. J. Agric. Food Chem. 2008, 56, 10960–10966. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Shahidi, F. Chemoenzymatic synthesis of phytosteryl ferulates and evaluation of their antioxidant activity. J. Agric. Food Chem. 2011, 59, 12375–12383. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Iatropoulos, M.; Whysner, J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]
- Martin-Rubio, A.; Sopelana, P.; Ibargoitia, M.; Guillén, M.D. Prooxidant effect of α-tocopherol on soybean oil. Global monitoring of its oxidation process under accelerated storage conditions by 1h nuclear magnetic resonance. Food Chem. 2018, 245, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Shahidi, F. Oxidative stability of stripped and nonstripped borage and evening primrose oils and their emulsions in water. J. Am. Oil Chem. Soc. 2000, 77, 963–969. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Prior, E.; Aeschbach, R. Evaluation of antioxidant activity of rosemary extracts, carnosol and carnosic acid in bulk vegetable oils and fish oil and their emulsions. J. Sci. Food Agric. 1996, 72, 201–208. [Google Scholar] [CrossRef]
- Hamam, F.; Shahidi, F. Synthesis of structured lipids via acidolysis of docosahexaenoic acid single cell oil (DHASCO) with capric acid. J. Agric. Food Chem. 2004, 52, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin–sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Vongsvivut, J.; Adhikari, B.; Barrow, C.J. Microencapsulation of tuna oil fortified with the multiple lipophilic ingredients vitamins A, D3, E, K2, curcumin and coenzyme Q10. J. Funct. Foods 2015, 19, 893–901. [Google Scholar] [CrossRef]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Adcock, J.L.; Barrow, C.J. Selective concentration of EPA and DHA using thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chem. 2013, 138, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, T.O.; Barrow, C.J. Compositional information useful for authentication of krill oil and the detection of adulterants. Food Anal. Methods 2018, 11, 178–187. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1, 1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Optimization of the extraction of antioxidative constituents of six barley cultivars and their antioxidant properties. J. Agric. Food Chem. 2006, 54, 8048–8057. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
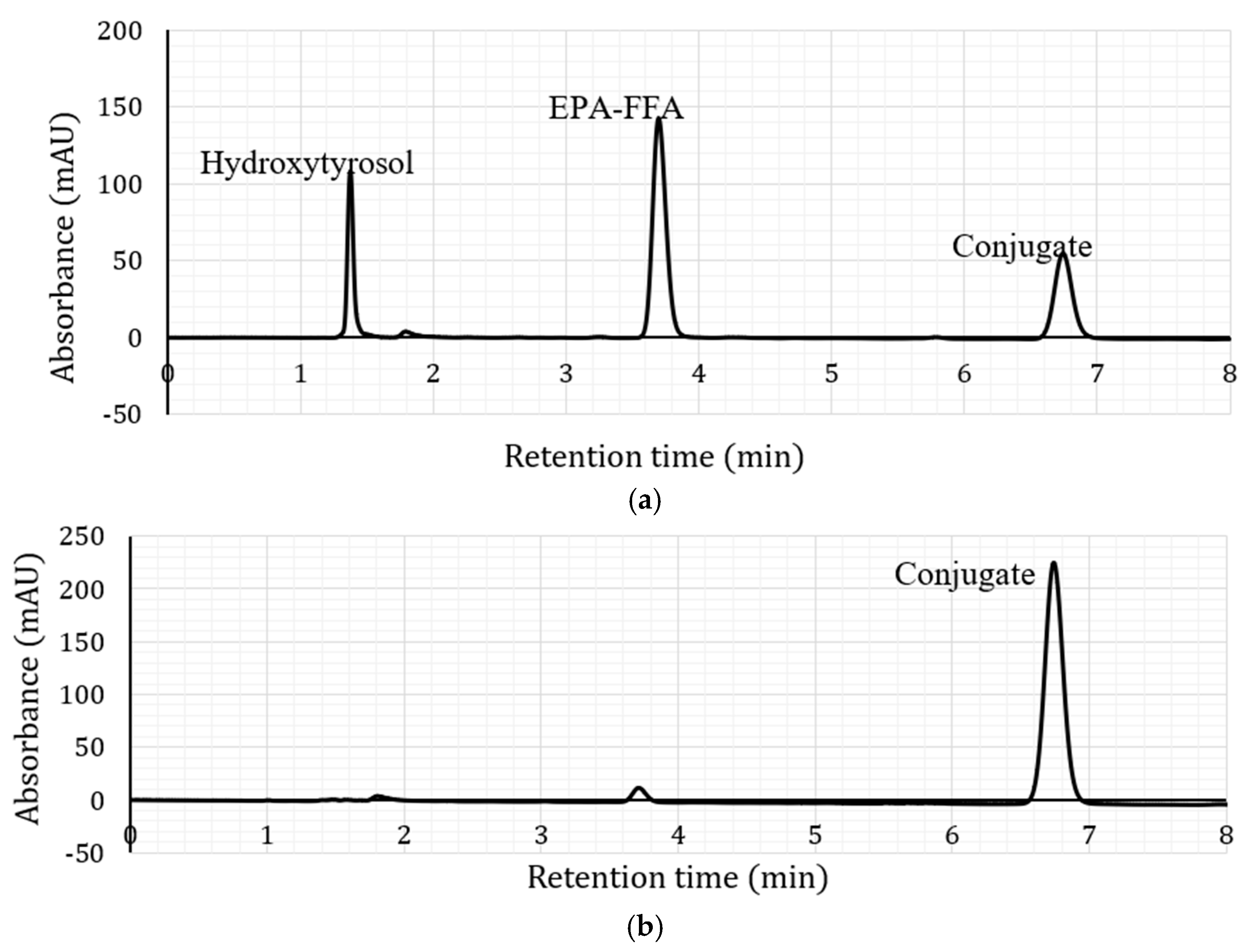
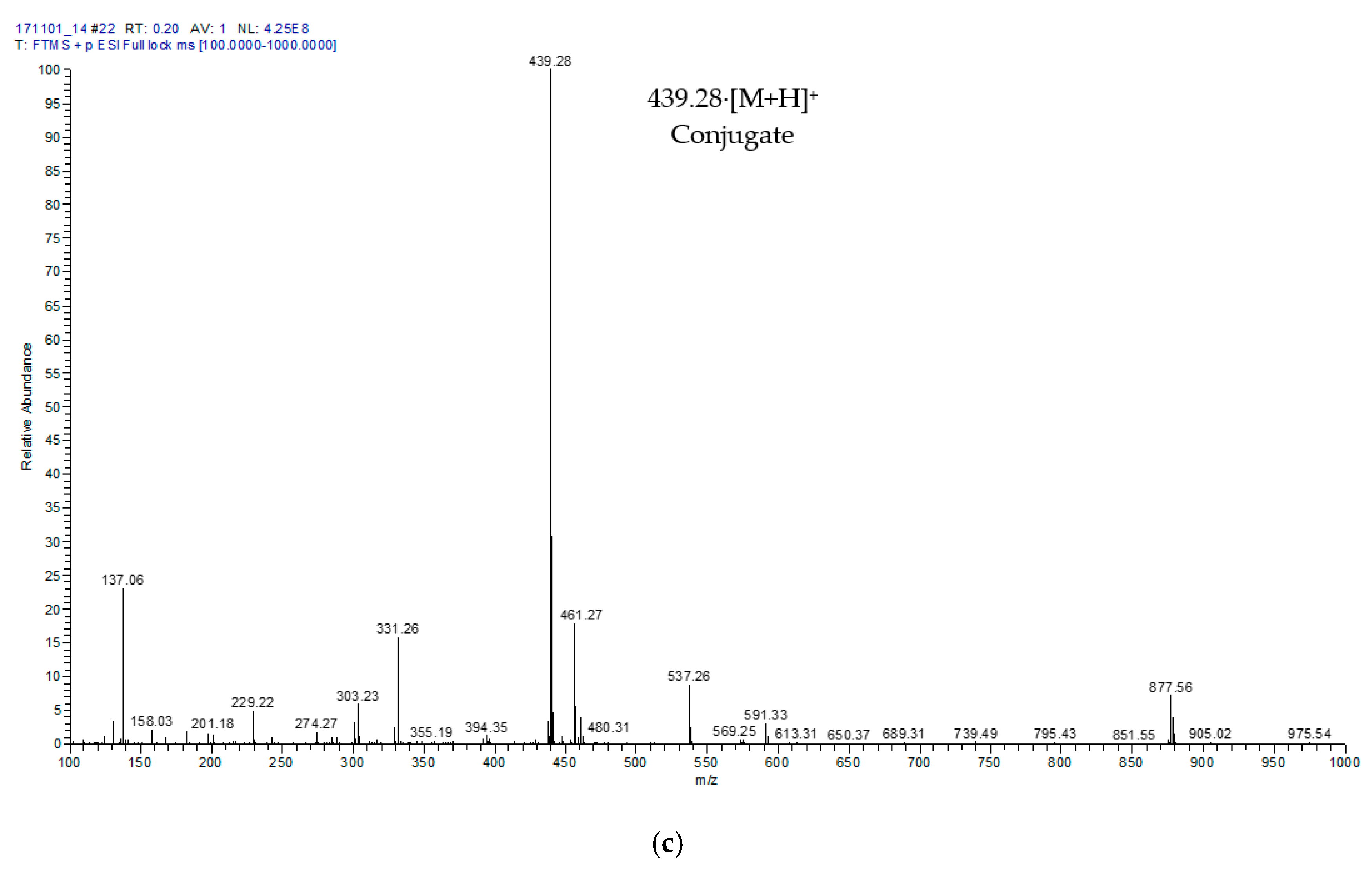
| Compound | Conversion (%) * | LCMS | 1H NMR Chemical Shift (δ, ppm) ** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fragment | m/z | A (CH2) | B (CH2) | C (CH2) | Allylic | Olefinic | Bis-Allylic | D (CH3) | ||
| Hydroxytyrosyl decanoate | 85.61 ± 2.9 e | [M + H]+ | 309.42 | 2.29 | 1.62 | 1.28 | - | - | - | 0.90 |
| Hydroxytyrosyl laurate | 81.61 ± 1.6 d | [M + H]+ | 337.48 | 2.29 | 1.63 | 1.29 | - | - | - | 0.90 |
| Hydroxytyrosyl myristate | 76.72 ± 2.7 c | [M + H]+ | 365.53 | 2.32 | 1.63 | 1.29 | - | - | - | 0.90 |
| Hydroxytyrosyl palmitate | 75.13 ± 3.9 c | [M + H]+ | 393.58 | 2.31 | 1.64 | 1.28 | - | - | - | 0.93 |
| Hydroxytyrosyl stearate | 61.82 ± 3.7 b | [M + H]+ | 421.64 | 2.33 | 1.66 | 1.28 | - | - | - | 0.96 |
| Hydroxytyrosyl oleate | 45.91 ± 0.5 a | [M + H]+ | 419.62 | 2.35 | 1.65 | 1.30 | 2.05 | 5.36 | - | 0.97 |
| Hydroxytyrosyl eicosapentaenoate | 68.13 ± 3.5 c | [M + H]+ | 439.28 | 2.34 | 1.75 | 1.29 | 2.09 | 5.37 | 2.87 | 0.99 |
| Test Compound | DPPH EC50 (μM) | ABTS EC50 (μM) | β-Carotene Inhibition Rate (%) |
|---|---|---|---|
| Hydroxytyrosyl decanoate | 6.37 ± 0.82 a | 2.02 ± 0.41 a | 57.09 ± 3.10 b |
| Hydroxytyrosyl laurate | 6.55 ± 0.54 a | 2.12 ± 0.22 a | 59.99 ± 3.08 c |
| Hydroxytyrosyl myristate | 6.42 ± 0.26 a | 2.22 ± 0.16 a | 61.73 ± 1.60 c |
| Hydroxytyrosyl palmitate | 6.71 ± 0.42 a | 2.19 ± 0.25 a | 61.45 ± 2.79 c |
| Hydroxytyrosyl stearate | 7.04 ± 0.33 a | 2.64 ± 0.55 a | 61.15 ± 2.38 c |
| Hydroxytyrosyl oleate | 6.85 ± 0.61 a | 2.05 ± 0.12 a | 62.39 ± 2.10 c |
| Hydroxytyrosyl eicosapentaenoate | 6.65 ± 0.25 a | 2.85 ± 0.28 a | 72.21 ± 3.07 d |
| Hydroxytyrosol | 6.06 ± 0.31 a | 2.45 ± 0.15 a | 45.80 ± 2.81 a |
| BHT | 6.13 ± 0.62 a | 3.88 ± 0.38 b | 56.09 ± 3.65 b |
| α-tocopherol | 9.87 ± 1.03 b | 5.82 ± 0.65 c | 62.43 ± 1.10 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akanbi, T.O.; Barrow, C.J. Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules. Molecules 2018, 23, 275. https://doi.org/10.3390/molecules23020275
Akanbi TO, Barrow CJ. Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules. Molecules. 2018; 23(2):275. https://doi.org/10.3390/molecules23020275
Chicago/Turabian StyleAkanbi, Taiwo Olusesan, and Colin James Barrow. 2018. "Lipase-Produced Hydroxytyrosyl Eicosapentaenoate is an Excellent Antioxidant for the Stabilization of Omega-3 Bulk Oils, Emulsions and Microcapsules" Molecules 23, no. 2: 275. https://doi.org/10.3390/molecules23020275






