Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues
Abstract
:1. Introduction
2. Methods
| nanoparticles AND magnetic AND (central nervous system) |
| OR |
| nanoparticles AND magnetic AND (blood brain barrier) |
| OR |
| magnetoporation AND (central nervous system) |
| OR |
| magnetofaction AND (central nervous system). |
3. Results
- applications for CNS disease:
4. Discussion
4.1. General Properties of Magnetic Nanoparticles
- magnetic enhancing contrast agents;
- magnetic hyperthermia and heating;
- magnetic labeling and separation;
- magnetic vectors.
- magnetic force dominance;
- blood velocity dominance;
- boundary layer formation (when magnetic and drag force are comparable).
- the Magnetic-Richardson number, that represents the ratio between magnetic and drag forces;
- the mass-Péclet number, that represents the ratio between advection and diffusion rates;
- the Renkin-reduced diffusion coefficient, that represents the ratio between the diffusivity in the tissue and the total diffusivity in the blood.
4.2. Interplay between MNPs and Magnetic Fields
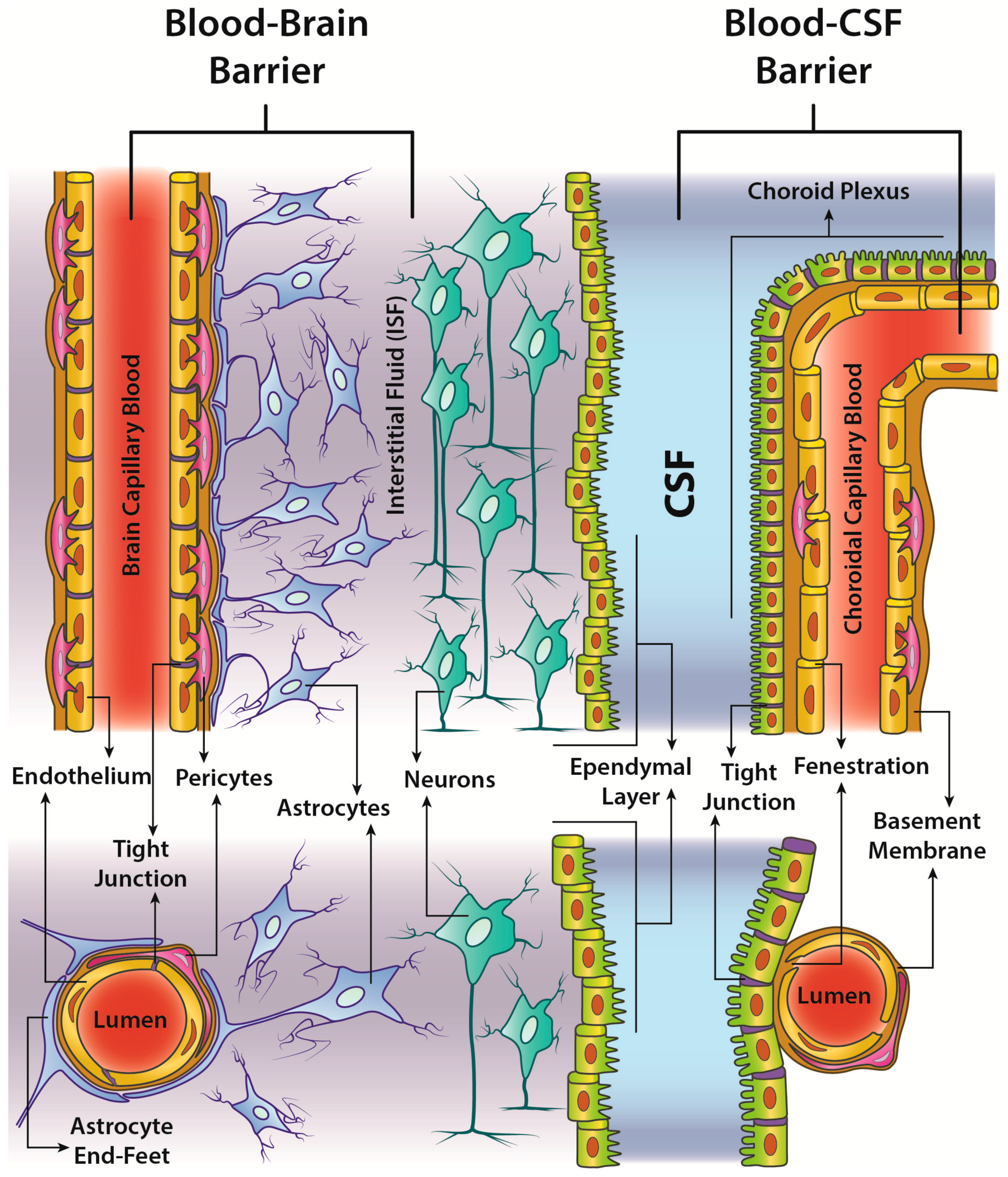
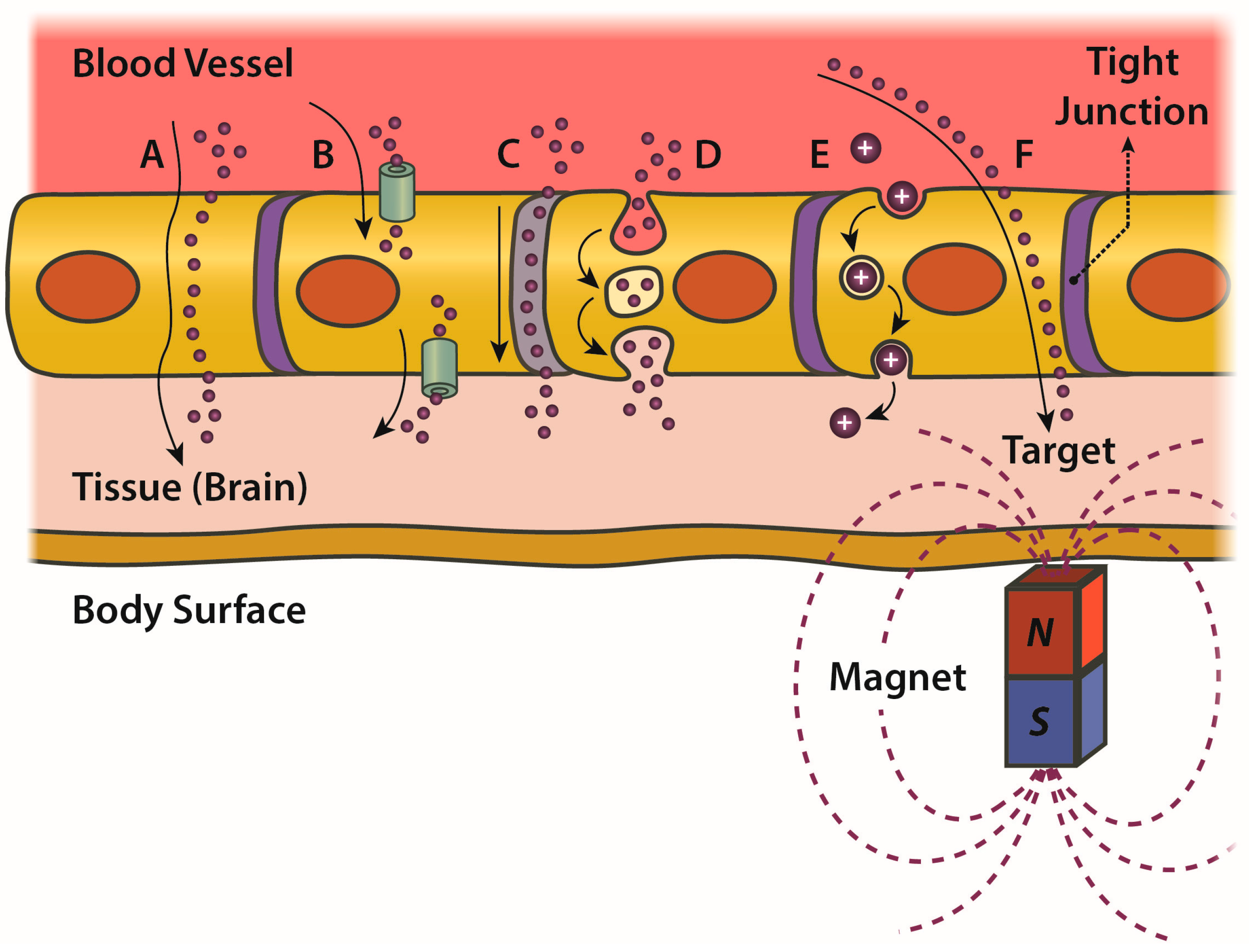
4.3. BBB: Critical Issues and Trespassing by MNPs
- passive transcellular diffusion (only O2, CO2 and small lipid-soluble substances),
- Carrier protein-Mediated Transport (CMT),
- Receptor-Mediated Transcytosis (RMT),
- Adsorptive-Mediated Transcytosis (AMT).
4.4. In Vitro and In Vivo Evidence of BBB Trespassing by MNPs under Static Magnetic Fields
- pass the BBB in the field direction,
- not modify the TEER (i.e., no damages to the barrier integrity),
- not alter the cell viability,
- be internalized by astrocytes.
4.5. Heating of MNPs by AMF: Applications for Increasing BBB Permeability and Drug Delivery
4.6. Magnetofection and CNS
4.7. MNP Biomedical Applications
4.8. Safety and Toxicity of MNPs
5. Challenges and Future Perspectives
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Neurological Disorders: Public Health Challenges; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed]
- Guiot, C.; Zullino, S.; Priano, L.; Cavalli, R. The physics of drug-delivery across the blood-brain barrier. Ther. Deliv. 2016, 7, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, S.; Gupta, G.K.; Avci, P.; Chandran, R.; Sadasivam, M.; Jorge, A.E.S.; Hamblin, M.R. Physical energy for drug delivery; poration, concentration and activation. Adv. Drug Deliv. Rev. 2014, 71, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Detappe, A.; LoPresti, M.; Kunjachan, S.; Mitrasinovic, S.; Goldman, S.; Chang, S.D.; Tillement, O. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro-Oncology 2016, 18, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Busquets, M.; Espargaró, A.; Sabaté, R.; Estelrich, J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials 2015, 5, 2231–2248. [Google Scholar] [CrossRef] [PubMed]
- David, A.E.; Cole, A.J.; Yang, V.C. Magnetically targeted nanoparticles for brain tumor therapy: What does the future hold? Nanomedicine 2011, 6, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Dilnawaz, F.; Sahoo, S.K. Therapeutic approaches of magnetic nanoparticles for the central nervous system. Drug Discov. Today 2015, 20, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M. Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Jayant, R.D.; Sagar, V.; Nair, M. The potential of magneto-electric nanocarriers for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, L.; Keates, A.C. Targeting cancer gene therapy with magnetic nanoparticles. Oncotarget 2012, 3, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, B.; Kulkarni, S.D.; Villar, P.S.; Smith, R.S.; Eberly, C.; Araneda, R.C.; Depireux, D.A.; Shapiro, B. Movement of magnetic nanoparticles in brain tissue: Mechanisms and impact on normal neuronal function. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, J.I.; Goya, G.F.; Calatayud, M.P.; Hereñú, C.B.; Reggiani, P.C.; Goya, R.G. Magnetic field-assisted gene delivery: Achievements and therapeutic potential. Curr. Gene Ther. 2012, 12, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.; Kulkarni, S.; Nacev, A.; Muro, S.; Stepanov, P.Y.; Weinberg, I.N. Open challenges in magnetic drug targeting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C.; Velasco-Aguirre, C.; Gallardo-Toledo, E.; Araya, E.; Kogan, M.J. Metal Nanoparticles as Targeted Carriers Circumventing the Blood-Brain Barrier. Int. Rev. Neurobiol. 2016, 130, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Girouard, H.; Carret, A.-S.; Martel, S. Toward nonsystemic delivery of therapeutics across the blood-brain barrier. Nanomedicine 2015, 10, 2129–2131. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Thomsen, M.S.; Moos, T. Targeted drug delivery to the brain using magnetic nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. The Behaviors of Ferro-Magnetic Nano-Particles In and Around Blood Vessels under Applied Magnetic Fields. J. Magn. Magn. Mater. 2011, 323, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Nacev, A.; Beni, C.; Bruno, O.; Shapiro, B. Magnetic nanoparticle transport within flowing blood and into surrounding tissue. Nanomedicine 2010, 5, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Pedram, M.Z.; Shamloo, A.; GhafarZadeh, E.; Alasty, A. Modeling and simulation of crossing magnetic nanoparticles through Blood Brain Barrier (BBB). In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 27–31 August 2014; pp. 5280–5283. [Google Scholar]
- Pedram, M.Z.; Shamloo, A.; Alasty, A.; Ghafar-Zadeh, E. Optimal Magnetic Field for Crossing Super-Para-Magnetic Nanoparticles through the Brain Blood Barrier: A Computational Approach. Biosensors 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Sagar, V.; Agudelo, M.; Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Raymond, A.; Samikkannu, T.; Nair, M.P. Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 2014, 25, 55101. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic Iron Oxide Nanoparticles Modified with Tween 80 Pass through the Intact Blood-Brain Barrier in Rats under Magnetic Field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Lueshen, E.; Venugopal, I.; Soni, T.; Alaraj, A.; Linninger, A. Implant-Assisted Intrathecal Magnetic Drug Targeting to Aid in Therapeutic Nanoparticle Localization for Potential Treatment of Central Nervous System Disorders. J. Biomed. Nanotechnol. 2015, 11, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Sagar, V.; Saxena, S.K.; Nair, M. Targeted Brain Derived Neurotropic Factors (BDNF) Delivery across the Blood-Brain Barrier for Neuro-Protection Using Magnetic Nano Carriers: An In-Vitro Study. PLoS ONE 2013, 8, e62241. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.L.; Kirthivasan, B.; Bommana, M.M.; Squillante, E.; Sadoqi, M. The formulation, characterization and in vivo evaluation of a magnetic carrier for brain delivery of NIR dye. Nanotechnology 2010, 21, 395102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Worden, M.; Wroczynskyj, Y.; Yathindranath, V.; van Lierop, J.; Hegmann, T.; Miller, D. Magnetic field enhanced convective diffusion of iron oxide nanoparticles in an osmotically disrupted cell culture model of the blood brain barrier. Int. J. Nanomed. 2014, 3013. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G.M.; Moos, T. Uptake and Transport of Superparamagnetic Iron Oxide Nanoparticles through Human Brain Capillary Endothelial Cells. ACS Chem. Neurosci. 2013, 4, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, I.; Habib, N.; Linninger, A. Intrathecal magnetic drug targeting for localized delivery of therapeutics in the CNS. Nanomedicine 2017, 12, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hu, J.; Zhang, L.; Zhang, L.; Sun, Y.; Ma, N.; Chen, X.; Gao, Z. Study of amphotericin B magnetic liposomes for brain targeting. Int. J. Pharm. 2014, 475, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chang, J.; Fu, X.; Liang, C.; Liang, S.; Yan, R.; Li, A. Nano-sized cationic polymeric magnetic liposomes significantly improves drug delivery to the brain in rats. J. Drug Target. 2012, 20, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shang, T.; Zhang, X.; Ye, T.; Wang, D.; Rei, L. Passage of Magnetic Tat-Conjugated Fe3O4@SiO2 Nanoparticles Across In Vitro Blood-Brain Barrier. Nanoscale Res. Lett. 2016, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Bae, Y.; Pittman, T.A.; Yokel, R.A. Alternating Magnetic Field-Induced Hyperthermia Increases Iron Oxide Nanoparticle Cell Association/Uptake and Flux in Blood-Brain Barrier Models. Pharm. Res. 2015, 32, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghoshmitra, S.; Cai, T.; Diercks, D.R.; Mills, N.C.; Hynds, D.L. Alternating Magnetic Field Controlled, Multifunctional Nano-Reservoirs: Intracellular Uptake and Improved Biocompatibility. Nanoscale Res. Lett. 2009, 5, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lu, I.-L.; Chiang, W.-H.; Lin, Y.-W.; Tsai, Y.-C.; Chen, H.-H.; Chang, C.-W.; Chiang, C.-S.; Chiu, H.-C. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J. Control. Release 2017, 254, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Nikkhah-Moshaie, R.; Sinha, R.; Bhardwaj, V.; Atluri, V.; Jayant, R.D.; Yndart, A.; Kateb, B.; Pala, N.; Nair, M. Investigation of ac-magnetic field stimulated nanoelectroporation of magneto-electric nano-drug-carrier inside CNS cells. Sci. Rep. 2017, 7, 45663. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Maji, S.; Maiti, T.K.; Chattopadhyay, S. A Smart Magnetically Active Nanovehicle for on-Demand Targeted Drug Delivery: Where van der Waals Force Balances the Magnetic Interaction. ACS Appl. Mater. Interfaces 2015, 7, 24229–24241. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, S.N.; Girouard, H.; Carret, A.-S.; Martel, S. Remote control of the permeability of the blood-brain barrier by magnetic heating of nanoparticles: A proof of concept for brain drug delivery. J. Control. Release 2015, 206, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.F.; Pickard, M.R.; Chari, D.M. Magnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fields. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Lamkowsky, M.-C.; Geppert, M.; Schmidt, M.M.; Dringen, R. Magnetic field-induced acceleration of the accumulation of magnetic iron oxide nanoparticles by cultured brain astrocytes. J. Biomed. Mater. Res. Part A 2012, 100, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Song, H.P.; Yang, J.Y.; Lo, S.L.; Wang, Y.; Fan, W.M.; Tang, X.S.; Xue, J.M.; Wang, S. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials 2010, 31, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Soto-Sánchez, C.; Martínez-Navarrete, G.; Humphreys, L.; Puras, G.; Zarate, J.; Pedraz, J.L.; Fernández, E. Enduring high-efficiency in vivo transfection of neurons with non-viral magnetoparticles in the rat visual cortex for optogenetic applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Villate-Beitia, I.; Puras, G.; Soto-Sánchez, C.; Agirre, M.; Ojeda, E.; Zarate, J.; Fernández, E.; Pedraz, J.L. Non-viral vectors based on magnetoplexes, lipoplexes and polyplexes for VEGF gene delivery into central nervous system cells. Int. J. Pharm. 2017, 521, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, K.; Zhao, Z.; Zhang, Y.; Sun, T.; Zhang, F.; Wu, J.; Fu, Y.; Du, Y.; Zhang, L.; et al. Brain-Targeted Delivery of Trans-Activating Transcriptor-Conjugated Magnetic PLGA/Lipid Nanoparticles. PLoS ONE 2014, 9, e106652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Muroski, M.E.; Petit, D.C.M.C.; Mansell, R.; Vemulkar, T.; Morshed, R.A.; Han, Y.; Balyasnikova, I.V.; Horbinski, C.M.; Huang, X.; et al. Rotating magnetic field induced oscillation of magnetic particles for in vivo mechanical destruction of malignant glioma. J. Control. Release 2016, 223, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Eslaminejad, T.; Nematollahi-Mahani, S.N.; Ansari, M. Glioblastoma Targeted Gene Therapy Based on pEGFP/p53-Loaded Superparamagnetic Iron Oxide Nanoparticles. Curr. Gene Ther. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.G.; Nagaoka, Y.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Aptamer conjugated magnetic nanoparticles as nanosurgeons. Nanotechnology 2010, 21, 455102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liang, C.; Li, A.; Chang, J.; Wang, H.; Yan, R.; Zhang, J.; Tai, J. Magnetic paclitaxel nanoparticles inhibit glioma growth and improve the survival of rats bearing glioma xenografts. Anticancer Res. 2010, 30, 2217–2223. [Google Scholar] [PubMed]
- Atluri, V.; Jayant, R.; Pilakka-Kanthikeel, S.; Garcia, G.; Thangavel, S.; Yndart, A.; Kaushik, A.; Nair, M. Development of TIMP1 magnetic nanoformulation for regulation of synaptic plasticity in HIV-1 infection. Int. J. Nanomed. 2016, 11, 4287–4298. [Google Scholar] [CrossRef] [PubMed]
- Jayant, R.; Atluri, V.; Agudelo, M.; Sagar, V.; Kaushik, A.; Nair, M. Sustained-release nanoART formulation for the treatment of neuroAIDS. Int. J. Nanomed. 2015, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Guduru, R.; Liang, P.; Hong, J.; Sagar, V.; Khizroev, S. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat. Commun. 2013, 4, 1707. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Kaushik, A.; Lapierre, J.; Dever, S.M.; El-Hage, N.; Nair, M. Electro-Magnetic Nano-Particle Bound Beclin1 siRNA Crosses the Blood-Brain Barrier to Attenuate the Inflammatory Effects of HIV-1 Infection in Vitro. J. Neuroimmune Pharmacol. 2017, 12, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Sagar, V.; Atluri, V.S.R.; Pilakka-Kanthikeel, S.; Nair, M. Magnetic nanotherapeutics for dysregulated synaptic plasticity during neuroAIDS and drug abuse. Mol. Brain 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Sagar, V.; Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Ding, H.; Arias, A.Y.; Jayant, R.D.; Kaushik, A.; Nair, M. Therapeutical Neurotargeting via Magnetic Nanocarrier: Implications to Opiate-Induced Neuropathogenesis and NeuroAIDS. J. Biomed. Nanotechnol. 2015, 11, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Romero, G.; Christiansen, M.G.; Mohr, A.; Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 2015, 347, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Guduru, R.; Liang, P.; Hong, J.; Rodzinski, A.; Hadjikhani, A.; Horstmyer, J.; Levister, E.; Khizroev, S. Magnetoelectric “spin” on stimulating the brain. Nanomedicine 2015, 10, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.; Qadri, S.M.; Zhang, Q.; Castellanos Rubio, I.; del Pino, P.; Pralle, A. Magnetothermal genetic deep brain stimulation of motor behaviors in awake, freely moving mice. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Kelly, L.; Latcha, K.N.; Schmidt, S.F.; Yu, X.; Nectow, A.R.; Sauer, J.; Dyke, J.P.; Dordick, J.S.; Friedman, J.M. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature 2016, 531, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Carlo, D. Remote Neural Stimulation Using Magnetic Nanoparticles. Curr. Med. Chem. 2017, 24, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Di Carlo, D. Magnetic Nanoparticle-Based Mechanical Stimulation for Restoration of Mechano-Sensitive Ion Channel Equilibrium in Neural Networks. Nano Lett. 2017, 17, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Kunze, A.; Murray, C.; Di Carlo, D. Induction of Calcium Influx in Cortical Neural Networks by Nanomagnetic Forces. ACS Nano 2016, 10, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Guduru, R.; Hong, J.; Liang, P.; Nair, M.; Khizroev, S. Magneto-Electric Nano-Particles for Non-Invasive Brain Stimulation. PLoS ONE 2012, 7, e44040. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, Y.-J.; Kim, Y.-H.; Roh, J.; Kim, E.-C.; Lee, H.J.; Kim, S.U.; Yoon, B.-W. Long-Term Effects of Magnetically Targeted Ferumoxide-Labeled Human Neural Stem Cells in Focal Cerebral Ischemia. Cell Transplant. 2015, 24, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Kim, Y.-J.; Kim, Y.; Roh, J.; Kim, S.U.; Yoon, B.-W. Using a Neodymium Magnet to Target Delivery of Ferumoxide-Labeled Human Neural Stem Cells in a Rat Model of Focal Cerebral Ischemia. Hum. Gene Ther. 2010, 21, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Hoshiar, A.K.; Do, T.D.; Noh, Y.; Shah, S.A.; Khan, M.S.; Yoon, J.; Kim, M.O. Osmotin-loaded magnetic nanoparticles with electromagnetic guidance for the treatment of Alzheimer’s disease. Nanoscale 2017, 9, 10619–10632. [Google Scholar] [CrossRef] [PubMed]
- Do, T.D.; Ul Amin, F.; Noh, Y.; Kim, M.O.; Yoon, J. Guidance of Magnetic Nanocontainers for Treating Alzheimer’s Disease Using an Electromagnetic, Targeted Drug-Delivery Actuator. J. Biomed. Nanotechnol. 2016, 12, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Rijo, P.; Reis, C.P. Nanotechnological strategies for nerve growth factor delivery: Therapeutic implications in Alzheimer’s disease. Pharmacol. Res. 2017, 120, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Zhang, L.-K.; Zhang, L.; Zhuang, S.; Zhan, X.; Chen, W.-Y.; Du, S.; Yin, L.; You, R.; Li, C.-H.; Guan, Y.-Q. Inhibition by Multifunctional Magnetic Nanoparticles Loaded with Alpha-Synuclein RNAi Plasmid in a Parkinson’s Disease Model. Theranostics 2017, 7, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Umarao, P.; Bose, S.; Bhattacharyya, S.; Kumar, A.; Jain, S. Neuroprotective Potential of Superparamagnetic Iron Oxide Nanoparticles Along with Exposure to Electromagnetic Field in 6-OHDA Rat Model of Parkinson’s Disease. J. Nanosci. Nanotechnol. 2016, 16, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Vio, V.; Marchant, M.J.; Araya, E.; Kogan, M.K. Metal Nanoparticles for the Treatment and Diagnosis of Neurodegenerative Brain Diseases. Curr. Pharm. Des. 2017, 23, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Cupaioli, F.A.; Zucca, F.A.; Boraschi, D.; Zecca, L. Engineered nanoparticles. How brain friendly is this new guest? Prog. Neurobiol. 2014, 119–120, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and Metabolism of Iron Oxide Nanoparticles in Brain Cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.W.; Arrott, A.; Watson, J.H.L. Magnetism in Medicine. J. Appl. Phys. 1960, 31, S404–S405. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Liu, H.-L.; Hua, M.-Y.; Yang, H.-W.; Huang, C.-Y.; Chu, P.-C.; Lyu, L.-A.; Tseng, I.-C.; Feng, L.-Y.; Tsai, H.-C.; et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol. 2010, 12, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Huang, X. Improved otutcome of targeted delivery of chemotherapy drugs to the brain using a combined strategy of ultrasound, magnetic targeting and drug-loaded nanoparticles. Ther. Deliv. 2011, 2, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Hua, M.-Y.; Yang, H.-W.; Huang, C.-Y.; Chu, P.-C.; Wu, J.-S.; Tseng, I.-C.; Wang, J.-J.; Yen, T.-C.; Chen, P.-Y.; et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl. Acad. Sci. USA 2010, 107, 15205–15210. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; David, A.E.; Huang, Y.; Yang, V.C. Glioma selectivity of magnetically targeted nanoparticles: A role of abnormal tumor hydrodynamics. J. Control. Release 2007, 122, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zullino, S.; Soster, M.; Khadjavi, A.; Gabriele, D.; Ciprian, R.; Albertini, F.; Cavalli, R.; Guiot, C. Characterization of superparamagnetic, oxygen loaded NanoBubbles for hyperthermia and radiotherapy. Radiother. Oncol. 2015, 115, S576. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Jones, R.M.; Barrett, E.; Schwab, A.; Head, E.; Hynynen, K. Investigation of the Safety of Focused Ultrasound-Induced Blood-Brain Barrier Opening in a Natural Canine Model of Aging. Theranostics 2017, 7, 3573–3584. [Google Scholar] [CrossRef] [PubMed]
- Janowski, M.; Walczak, P.; Pearl, M.S. Predicting and optimizing the territory of blood-brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J. Cereb. Blood Flow Metab. 2016, 36, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Bynoe, M.S.; Viret, C.; Yan, A.; Kim, D.-G. Adenosine receptor signaling: A key to opening the blood-brain door. Fluids Barriers CNS 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Akilo, O.D.; Choonara, Y.E.; Strydom, A.M.; du Toit, L.C.; Kumar, P.; Modi, G.; Pillay, V. AN in vitro evaluation of a carmustine-loaded Nano-co-Plex for potential magnetic-targeted intranasal delivery to the brain. Int. J. Pharm. 2016, 500, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, W.; Wang, S.; Wang, X.; Liao, Z.; Kang, C.; Han, L.; Chang, J.; Wang, G.; Pu, P. Smart multifunctional core-shell nanospheres with drug and gene co-loaded for enhancing the therapeutic effect in a rat intracranial tumor model. Nanoscale 2012, 4, 6501–6508. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.-B.; Martel, S. Steering of aggregating magnetic microparticles using propulsion gradients coils in an MRI Scanner. Magn. Reson. Med. 2010, 63, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.-B.; Martel, S. Magnetic microparticle steering within the constraints of an MRI system: Proof of concept of a novel targeting approach. Biomed. Microdevices 2007, 9, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef]
- Bu, L.; Xie, J.; Chen, K.; Huang, J.; Aguilar, Z.P.; Wang, A.; Sun, K.W.; Chua, M.-S.; So, S.; Cheng, Z.; et al. Assessment and comparison of magnetic nanoparticles as MRI contrast agents in a rodent model of human hepatocellular carcinoma. Contrast Media Mol. Imaging 2012, 7, 363–372. [Google Scholar] [CrossRef] [PubMed]

| IONs | MNPs | Magnetic | Mechanism | Model | Effect | Preparation | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe3O4 | PEGylated fluorescent liposomes + Transferrin Diameter = 130 nm | Static 0.08 T 24 h | Transferrin (RMT) + magnetic force promote crossing | In vitro BBB human endothelium + astrocytes | +50–100% transmigration 2 pg Fe/cell uptake | Coprecipitation aqueous 2 h stability | TEER and cell viability unchanged at 48 h | [26] |
| Fe3O4 | Polysorbate 80 Diameter = 11 nm (hydro 29 nm) ζ = 19 mV | Static 0.3 T 2 h | Poly adsorb protein (RMT) + magnetic force promote crossing | In vivo rat BBB | Accumulation in near cortex 0.6 mg Fe/g tissue uptake 9-fold increase | Mixing 0.2 g Tween 80 with 0.4 PEG IONs | Cell viability unchanged at 72 h | [27] |
| Fe3O4 | Silica-coated nanocapsule Diameter = 100–150 nm Ms = 5-fold SPION | Static 1000 Oe 1 week RF (100 MHz) | Cell membrane translocation | In vivo mice BBB | 25-fold increased concentration | Emulsion polymerization | Slight reversible astrogliosis No immunotoxicity | [28] |
| Fe3O4 | Gold coated Diameter = 25–40 nm Ms = 30 emu/g | Static 0.01 T 6 h | Accumulation by magnetic force | In vitro human CSFBB + in vivo rat CSFBB | Up to 50% MRI signal difference confirmed local histological accumulation | Coprecipitation aqueous | High cell viability | [29,34] |
| Fe3O4 | BDNF binded Diameter = 60 nm | Weak static magnet exposition | Magnetic force | In vitro BBB human endothelium + astrocytes | 73% BDNF cross 3.5-fold increase Suppress apoptosis Spine loss reversed | Coprecipitation | High cell viability and TEER unchanged | [30] |
| Fe2O3 | Oleic acid coated Diameter = 220–250 nm ζ = −4 to −17 mV | Static 8000 Gauss | Passive diffusion or RMT + magnetic force | In vivo rats | Indocyanine green load increased brain concentration 5% tot dose | Thermal decomposition | - | [31] |
| Fe3O4 | Aminosilane or EDT coating Diameter = hydro 25 or 29 nm ζ = 21 or −39 mV | Static 0.06–0.1 T 24 h | Improve concentration after mannitol opening BBB | In vitro mice endothelium | Flux increase to 44% for EDT after osmotic opening | Aqueous phase reduction/hydrolysis | No change in permeability | [32] |
| Fe3O4 | Lipophilic fluorescence dye covered by α-d-glucose units Ms = 350 kA/m Diameter = hydro 117 nm ζ = −17 mV | Static 5 h | Magnetic force | In vitro BBB human endothelium + rat astroglia | 11, 8, and 29 fold uptake increase of 35, 70, and 140 μg/mL | - | TEER and cell viability unchanged at 29 h | [33] |
| Fe3O4 | Amphotericin B magnetic liposomes Diameter = 240 nm Ms = 32 memu/g | Static | Magnetic force | In vivo rats | Histological accumulation 400 ng/g brain (after 30 min) | Film dispersion–ultrasonication | Reduced death with magnetic field | [35] |
| Fe3O4 | Cationic polymeric liposome Diameter = 20 nm | Static 0.5 T | Magnetic force | In vivo rats | Paclitaxel concentration 3-fold histological accumulation | Thin-layer evaporation | - | [36] |
| Fe3O4 | SiO2-coated+Amino Tat peptide Diameter = 100 nm ζ = 42 mV Ms = 19 emu/g | Static 2 h | Magnetic force + transport Tat | In vitro BBB human endothelium + glioma | Cell internalization 2.6-fold increase Permeability 2.3-fold increase | Alkaline co-precipitation | TEER moderate decrease High cell viability | [37] |
| Fe3O4 | Cross-linked poly(ethylene glycol)-poly(aspartate) or citrate-coated Diameter = 25 nm or 90 nm | Alternate 33.4 kA/m at 300 kHz | Temperature opening BBB | In vitro mice or dogs | 2–3 -fold flux increase 3-fold cell uptake increase | Co-precipitation | No cell death | [38] |
| Fe3O4 | Poly(maleic acid-co-olefin) coated Diameter = 11–13 nm | Alternate 7.6 kA/m at 150 kHz | Temperature opening BBB | In vivo rats | Histological accumulation only after RF | - | Reversibility of opening No brain immune response | [43] |
| MNPs | Magnetic | Mechanism | Model | Results | Toxicity | Ref. |
|---|---|---|---|---|---|---|
| Au + Ni80Fe20 (permalloy) 1 μm radius disk-shaped | Dynamic 1 T at 20 Hz rotating | Vortex shaped rotation | In vivo mice glioma | Increased survival | No change in histology No side effects | [50] |
| pEGFP/p53 conjugated | Static | Gene therapy + magnetofection | In vitro BBB + glioblastoma | Increased induced apoptosis | - | [51] |
| Aptamer conjugated dextran coated | Alternate 9.55 kA/m at 1 Hz | 3D Rotating nanosurgeons | In vitro glioblastoma | Increased induced apoptosis | - | [52] |
| Octadecyl-quaternized carboxymethyl chitosan | Static 0.5 T | Delivery loaded paclitaxel | In vivo rats glioma | Increased survival Prolonged bioavailability | Reduced side-effects | [53] |
| Inhibitor of metalloproteinase-1 conjugated | Static 0.8 T | Crossing BBB and regulation of metalloproteinases | In vitro BBB + HIV infection | Recovery in spine density ROS and HIV infection level decrease | Unchanged TEER and cells viability | [54] |
| Bilayers: Tenofovir + dextran Sulphate + vorinostat | Static 0.08 T 6 h | Crossing BBB and antiretroviral therapy | In vitro BBB + HIV infection | HIV infection level decrease Prolonged bioavailability | Unchanged TEER and cells viability | [55] |
| Azidothymidine 5′-triphosphate loaded CoFe2O4@BaTiO3 | Static 22 Oe/cm 6 h Alternate 66 Oe at 100 Hz 5 min | Crossing BBB and controlled release of antiviral drug | In vitro BBB + HIV infection | Functional and structural integrity of the drug after the release | High cell viability | [56] |
| Beclin1 siRNA binded CoFe2O4@BaTiO3 | Static 0.8 T 3 h | Crossing BBB and regulate autophagy | In vitro BBB + HIV infection | Attenuate HIV-1 replication and viral-induced inflammation | Unchanged TEER and occludin expression | [57] |
| Morphine antagonist, CTOP conjugated | Static 0.5 T | Crossing BBB and drug delivery | In vitro BBB + HIV infection | Recovery in spine density Prevention of morphine induced apoptosis | High cell viability Unchanged TEER | [59] |
| PEG shell | Alternate 15 kA/m 500 KHz | Heat-sensitive capsaicin receptor TRPV1 activated by magnetothermal genetic stimulation | In vitro neurons + in vivo mice | On demand activation of neurons in deep nuclei (VTA) | Lower glial activation and macrophage accumulation compared to implant | [60] |
| CoFe2O4-BaTiO3 GMO coated | Static 3000 Oe/cm Alternate 100 Oe at 0–20 Hz | Crossing and concentrate in brain then modulate neural activity | In vitro + in vivo mice | EEG detectable modulation activity of 1 mV | No toxicity for astrocytes and blood cells in vitro | [61] |
| Co-ferrite core and Mn-ferrite shell Polymer PMA coated | Alternate 7–30 kA/m at 412–570 KHz | Heat-sensitive capsaicin receptor TRPV1 activated by magnetothermal genetic stimulation | In vitro neurons + in vivo mice | On demand evoked motor cortex ambulation, striatum rotation or freezing | - | [62] |
| GFP-tagged ferritin | Alternate 23–31 mT at 465 kHz | Heat-sensitive capsaicin receptor TRPV1 activated by magnetothermal genetic stimulation | In vitro neurons + in vivo mice | Glucose-sensing hypothalamus neurons modulate feed behavior | - | [63] |
| Starch-coated | Static 150 mT | Magnetic force open neuron channels | In vitro neuron | Mechanical opening of N-type mechanosensitive Ca2+ channels | Reversibility of opening | [65] |
| Starch and chitosan coated | Static 150 mT | Magnetic force open neuron channels | In vitro neuron | Mechanical opening of N-type mechanosensitive Ca2+ channels | Unchanged cell viability, reversibility of opening | [66] |
| Ferumoxide-labeled human neural stem cells | Static 0.32 T | Magnetic targeting | In vivo stroke rats | Better targeting and recovery in a stroke model | Unchanged differentiation into neurons or astrocytes | [68,69] |
| Dextran-coated | Alternate 1–6 A at 0.25–2 Hz 10 min | Osmotin load targeting in hippocampus and delivery | In vitro + in vivo AD rats | Memory improvement Reduced protein accumulation and synaptotoxicity | Unchanged viability No apoptosis No BBB leakage | [70,71] |
| Oleic acid-coated | 3 days | Gene therapy delivery Alpha-Synuclein RNAi Plasmid | In vitro + in vivo PD mice | Motor improvement Reduced neurodegeneration | No organ damage Normal blood test 12 days | [73] |
| Uncoated Fe3O4 | Alternate 2 h/d for 1 week | Synergic effect of magnetic stimulation and MNPs | In vivo PD rats | Motor improvement/recover feeding behavior Reduced ROS and lesion volume | Normal mitochondrial activity | [74] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules 2018, 23, 9. https://doi.org/10.3390/molecules23010009
D’Agata F, Ruffinatti FA, Boschi S, Stura I, Rainero I, Abollino O, Cavalli R, Guiot C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules. 2018; 23(1):9. https://doi.org/10.3390/molecules23010009
Chicago/Turabian StyleD’Agata, Federico, Federico Alessandro Ruffinatti, Silvia Boschi, Ilaria Stura, Innocenzo Rainero, Ornella Abollino, Roberta Cavalli, and Caterina Guiot. 2018. "Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues" Molecules 23, no. 1: 9. https://doi.org/10.3390/molecules23010009
APA StyleD’Agata, F., Ruffinatti, F. A., Boschi, S., Stura, I., Rainero, I., Abollino, O., Cavalli, R., & Guiot, C. (2018). Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Molecules, 23(1), 9. https://doi.org/10.3390/molecules23010009







