Synthesis of Selenium-Quinone Hybrid Compounds with Potential Antitumor Activity via Rh-Catalyzed C-H Bond Activation and Click Reactions
Abstract
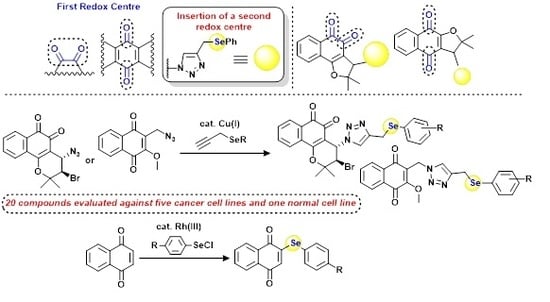
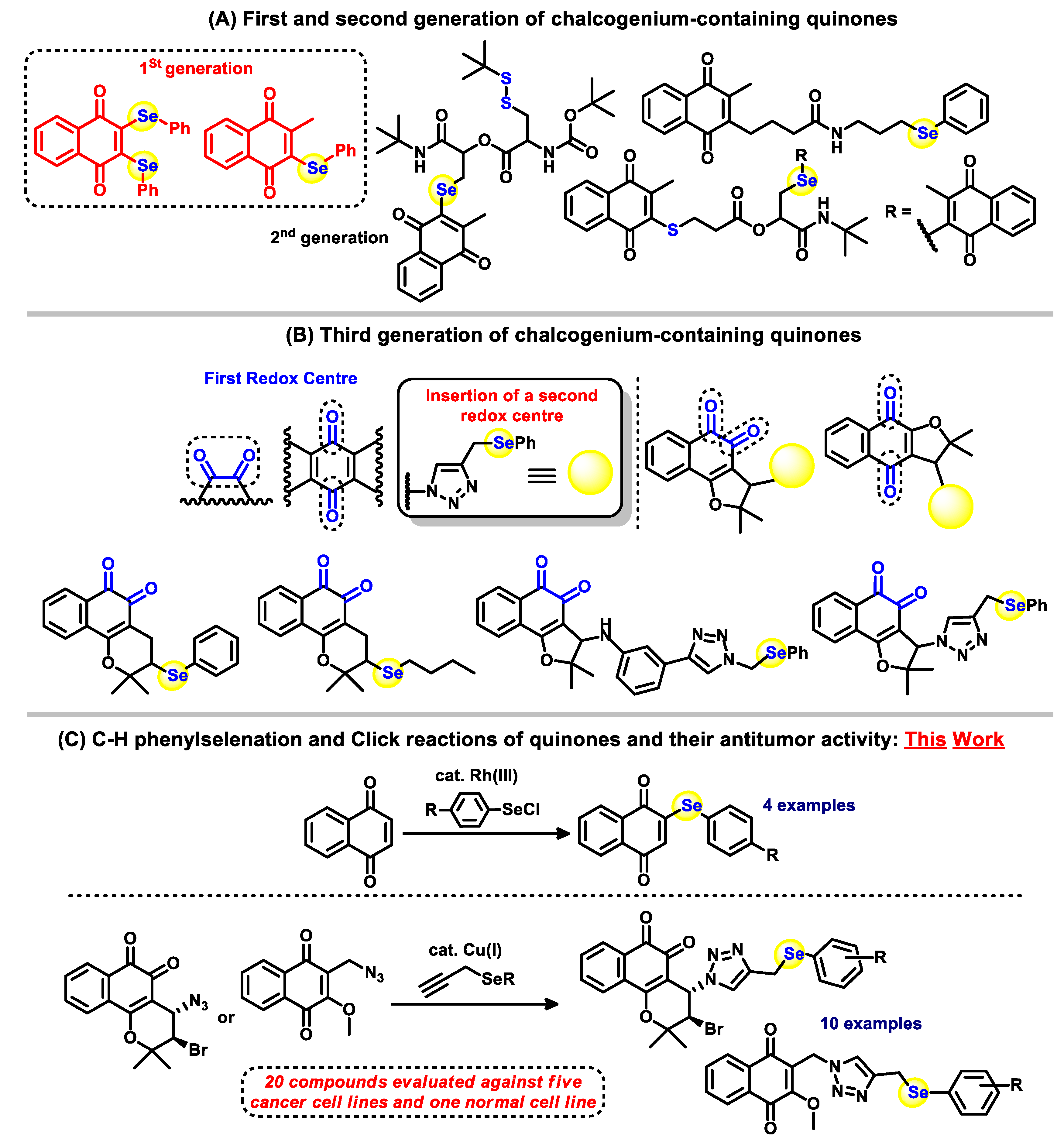
:1. Introduction
2. Results and Discussion
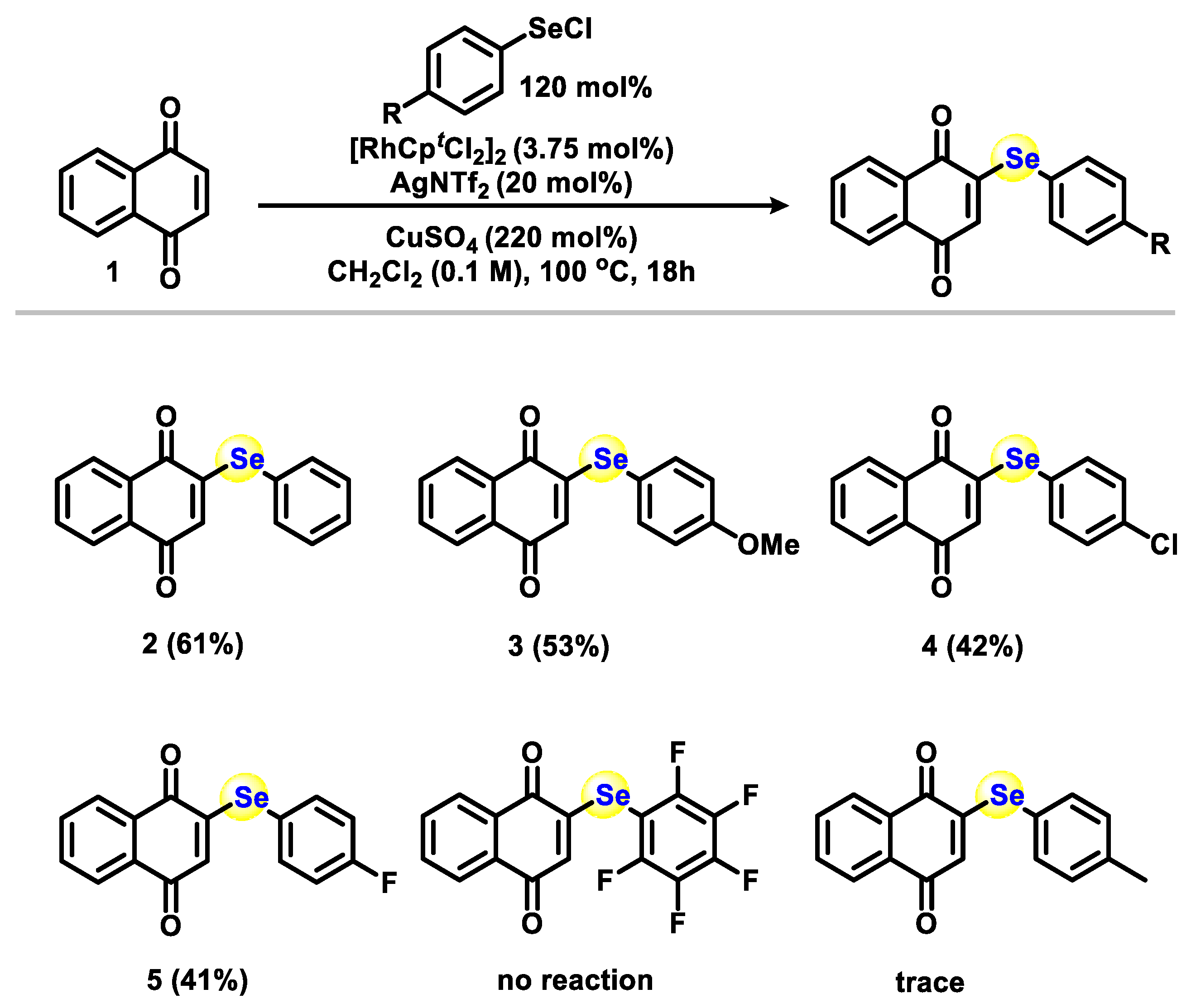
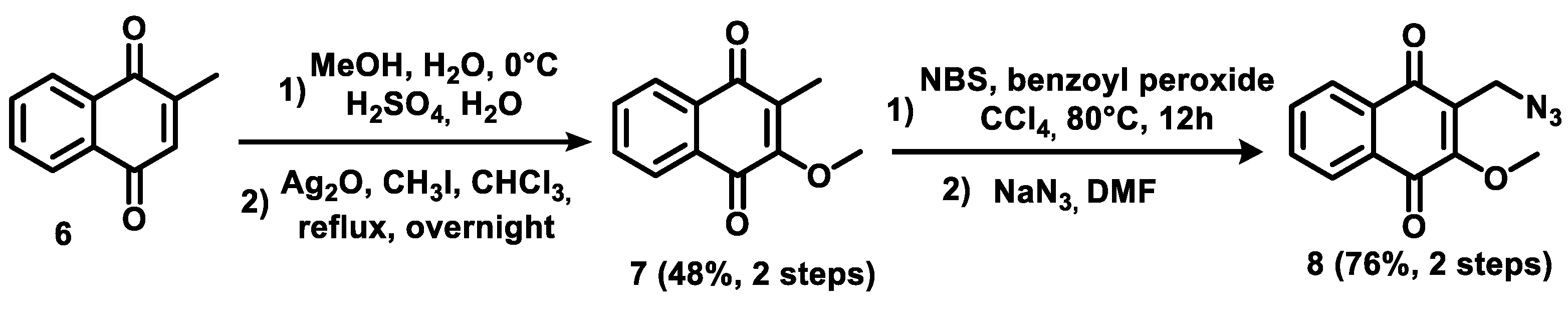
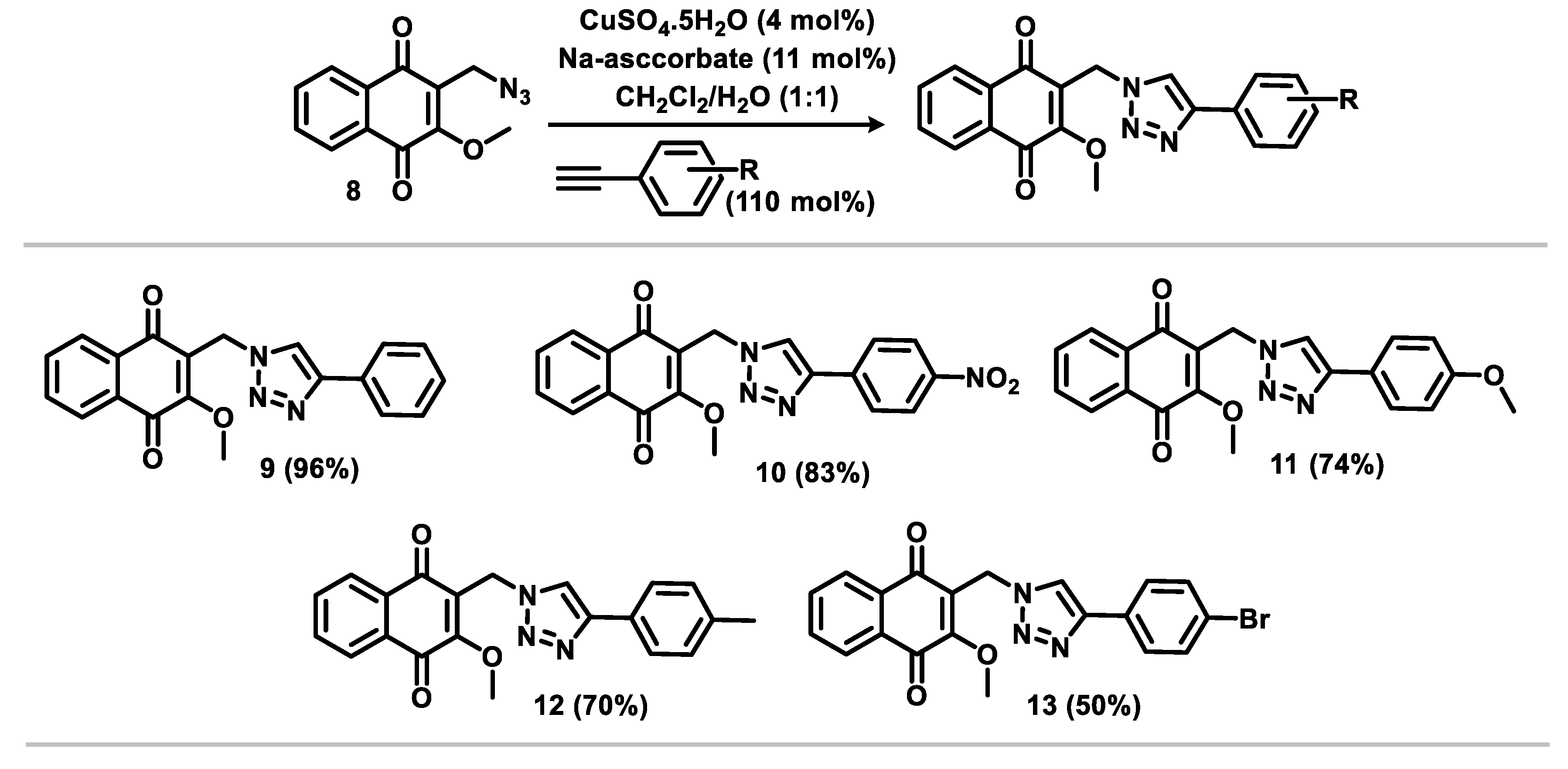
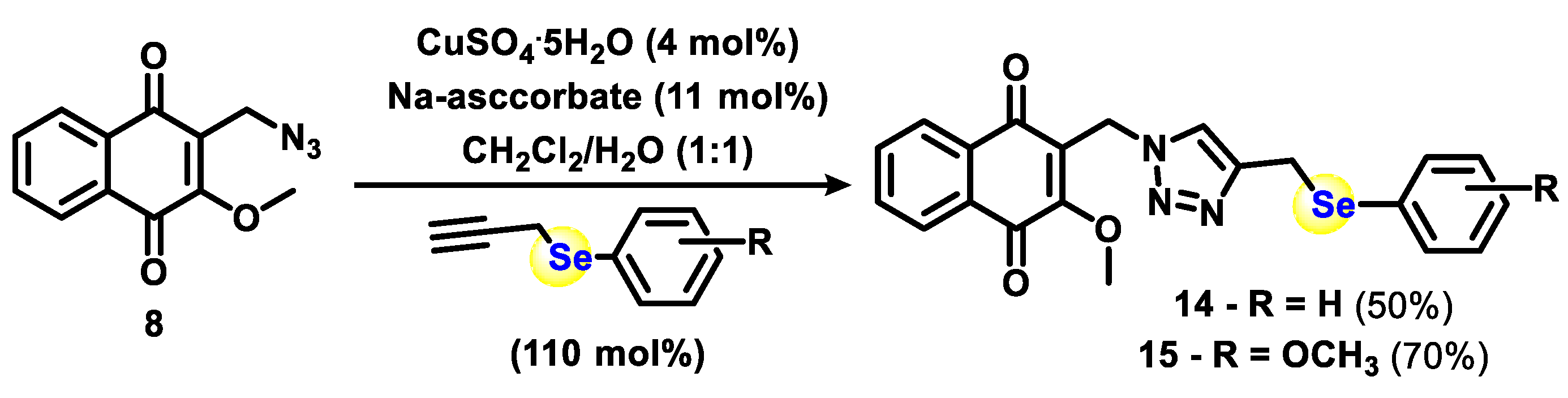
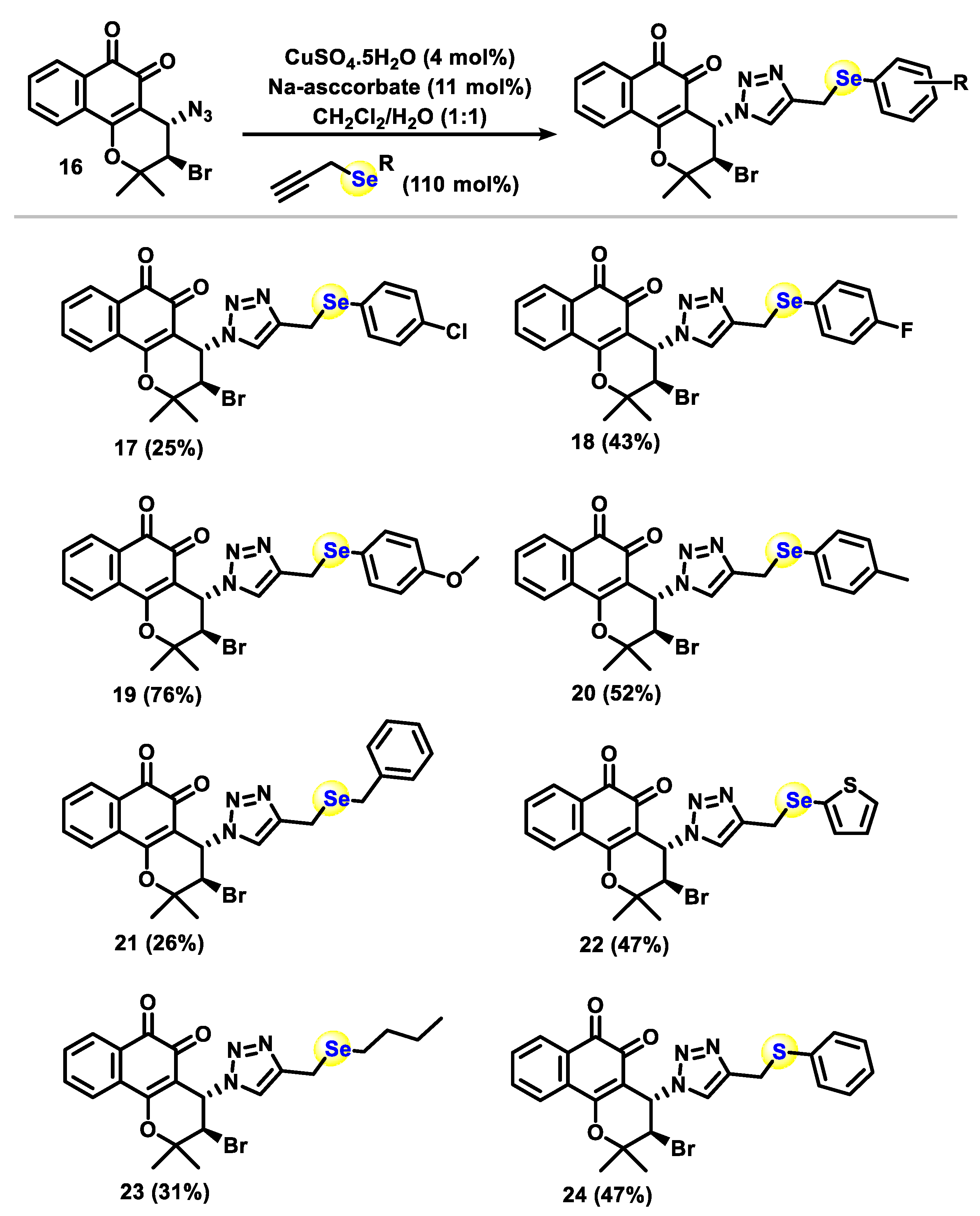
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. General Information
3.1.1. General Procedure for Phenylselenation at the 2-Position
3.1.2. General Procedure for the Synthesis of Triazoles
3.1.3. Activity Against Cancer Cell Lines
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| IC50 | Half Maximal Inhibitory Concentration |
| CuAAC | Copper(I) Catalyzed Azide-Alkyne Cycloaddition |
| NBS | N-bromosuccinimide |
| DMF | Dimethylformamide |
| CI | Confidence Interval |
| µM | Micromole |
| NMR | Nuclear Magnetic Resonance |
| EtOAc | Ethyl Acetate |
| DMSO | Dimethyl sulfoxide |
References
- Stewart, B.W.; Wild, C.P. World Cancer Report; IARC Publications: Lyon, France, 2014. [Google Scholar]
- World Health Organization (WHO). Guide to Cancer Early Diagnosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Global Health Observatory: The Data Repository; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Barbounaki-Konstantakou, E. Chemotherapy; Beta Medical Arts: Athens, Greece, 1989. [Google Scholar]
- Epstein, R.J. Maintenance therapy to suppress micrometastasis: The new challenge for adjuvant cancer treatment. Clin. Cancer Res. 2005, 11, 5337–5341. [Google Scholar] [CrossRef] [PubMed]
- Rampling, R.; James, A.; Papanastassiou, V. The present and future management of malignant brain tumours: Surgery, radiotherapy, chemotherapy. J. Neurol. Neurosurg. Psychiatry 2004, 75, 24–30. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Atkin, P.M.; Buckle, A.E. Natural occurrence of the naphthoquinone mycotoxins, xanthomegnin, viomellein and vioxanthin in cereals and animal feldstuffs. J. Stored Prod. Res. 1986, 22, 81–84. [Google Scholar] [CrossRef]
- Machado, T.B.; Pinto, A.V.; Pinto, M.C.F.R.; Leal, I.C.R.; Silva, M.G.; Amaral, A.C.F.; Kuster, R.M.; Netto-dos Santos, K.R. In vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinones and their analogues, against methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2003, 21, 279–284. [Google Scholar] [CrossRef]
- Lester, R.L.; White, D.C.; Smith, S.L. The 2-Desmethyl vitamin K2’s. A new group of naphthoquinones isolated from Hemophilus parainfluenzae. Biochemistry 1964, 3, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.J.; Chang, F.C.; Lee, K.H.; Wang, J.P.; Teng, C.M.; Kuo, S.C. Synthesis and antiplatelet, antiinflammatory, and antiallergic activities of substituted 3-chloro-5,8-dimethoxy-1,4-naphthoquinone and related compounds. Bioorg. Med. Chem. 1998, 6, 2261–2269. [Google Scholar] [CrossRef]
- Tandon, V.K.; Chhor, R.B.; Singh, R.V.; Rai, S.; Yadav, D.B. Design, synthesis and evaluation of novel 1,4-naphthoquinone derivatives as antifungal and anticancer agents. Bioorg. Med. Chem. Lett. 2004, 14, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Ilina, T.V.; Semenova, E.A.; Pronyaeva, T.R.; Pokrovskii, A.G.; Nechepurenko, I.V.; Shults, E.E.; Andreeva, O.I.; Kochetkov, S.N.; Tolstikov, G.A. Inhibition of HIV-1 reverse transcriptase by aryl-substituted naphtho- and anthraquinones. Dokl. Biochem. Biophys. 2002, 382, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Kuo, H.S.; Hsiao, C.L.; Lin, Y.L. Efficient synthesis of ‘redox-switched’ naphthoquinone thiol-crown ethers and their biological activity evaluation. Bioorg. Med. Chem. 2002, 10, 1947–1952. [Google Scholar] [CrossRef]
- Lien, J.C.; Huang, L.J.; Wang, J.P.; Teng, C.M.; Lee, K.H.; Kuo, S.C. Synthesis and antiplatelet, antiinflammatory and antiallergic activities of 2,3-disubstituted 1,4-naphthoquinones. Chem. Pharm. Bull. 1996, 44, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Hsu, C.T.; Hsu, H.T.; Su, J.S.; Chen, T.Y.; Tarn, W.Y.; Kuo, Y.H.; Whang-Peng, J.; Liu, L.F.; Hwang, J. Isodiospyrin as a novel human DNA topoisomerase I inhibitor. Biochem. Pharmacol. 2003, 66, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Wurm, G.; Schwandt, S. Methylated 2-aryl-1,4-naphthoquinone derivatives with diminished antioxidative activity. Pharmazie 2003, 58, 531–538. [Google Scholar] [PubMed]
- Song, G.Y.; Kim, Y.; You, Y.J.; Cho, H.; Kim, S.H.; Sok, D.E.; Ahn, B.Z. Naphthazarin derivatives (VI): Synthesis, inhibitory effect on DNA topoisomerase-I and antiproliferative activity of 2- or 6-(1-oxyiminoalkyl)-5,8-dimethoxy-1,4-naphthoquinones. Arch. Pharm. 2000, 333, 87–92. [Google Scholar] [CrossRef]
- De Castro, S.L.; Emery, F.S.; da Silva Júnior, E.N. Synthesis of quinoidal molecules: Strategies towards bioactive compounds with an emphasis on lapachones. Eur. J. Med. Chem. 2013, 69, 678–700. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, P.; Somanathan, R. Recent developments in the mechanism of anticancer agents based on electron transfer, reactive oxygen species and oxidative stress. Anticancer Agents Med. Chem. 2011, 11, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Sunassee, S.N.; Veale, C.G.L.; Shunmoogam-Gounden, N.; Osoniyi, O.; Hendricks, D.T.; Caira, M.R.; la Mare, J.A.; Edkins, A.L.; Pinto, A.V.; da Silva Júnior, E.N.; et al. Cytotoxicity of lapachol, β-lapachone and related synthetic 1,4-naphthoquinones against oesophageal cancer cells. Eur. J. Med. Chem. 2013, 62, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.; Gopinath, G.; Banerji, A.; Dinakar, A.; Srinivas, G. Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol. Carcinog. 2004, 40, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.S.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolau, K.C. The Chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 1999, 38, 270–300. [Google Scholar] [CrossRef]
- Li, C.J.; Li, Y.Z.; Pinto, A.V.; Pardee, A.B. Potent inhibition of tumor survival in vivo by β-lapachone plus taxol: Combining drugs imposes different artificial checkpoints. Proc. Natl. Acad. Sci. USA 1999, 96, 13369–13374. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Choi, D.Y.; Chung, H.S.; Seo, H.G.; Woo, H.J.; Choi, B.T.; Choi, Y.H. β-lapachone induces growth inhibition and apoptosis in bladder cancer cells by modulation of Bcl-2 family and activation of caspases. Exp. Oncol. 2006, 28, 30–35. [Google Scholar] [PubMed]
- Li, Y.Z.; Sun, X.G.; Lamont, J.T.; Pardee, A.B.; Li, C.J. Selective killing of cancer cells by β-lapachone: Direct checkpoint activation as a strategy against cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- da Silva Júnior, E.N.; Guimarães, T.T.; Menna-Barreto, R.F.S.; Pinto, M.C.F.R.; de Simone, C.A.; Pessoa, C.; Cavalcanti, B.C.; Sabino, J.R.; Andrade, C.K.Z.; Goulart, M.O.F.; et al. The evaluation of quinonoid compounds against Trypanosoma cruzi: Synthesis of imidazolic anthraquinones, nor-β-lapachone derivatives and β-lapachone-based 1,2,3-triazoles. Bioorg. Med. Chem. 2010, 18, 3224–3230. [Google Scholar] [CrossRef] [PubMed]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- Verrax, J.; Beck, R.; Dejeans, N.; Glorieux, C.; Sid, B.; Pedrosa, R.C.; Benites, J.; Vásquez, D.; Valderrama, J.A.; Calderon, P.B. Redox-active quinones and ascorbate: An innovative cancer therapy that exploits the vulnerability of cancer cells to oxidative stress. Anticancer Agents Med. Chem. 2011, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chem. Eur. J. 2010, 16, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Doering, M.; Ba, L.A.; Lilienthal, N.; Nicco, C.; Scherer, C.; Abbas, M.; Zada, A.A.P.; Coriat, R.; Burkholz, T.; Wessjohann, L.; et al. Synthesis and selective anticancer activity of organochalcogen based redox catalysts. J. Med. Chem. 2010, 53, 6954–6963. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, S.; Shaaban, S.; Ba, L.A.; Burkholz, T.; Schneider, T.; Diesel, B.; Kiemer, A.K.; Roseler, A.; Becker, K.; Reichrath, J.; et al. Exploring synthetic avenues for the effective synthesis of selenium- and tellurium-containing multifunctional redox agents. Org. Biomol. Chem. 2009, 7, 4753–4762. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of sodium selenite in prevention and treatment of cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.A.; Brandão, I.R.; Valença, W.O.; de Simone, C.A.; Cavalcanti, B.C.; Pessoa, C.; Carneiro, T.R.; Braga, A.L.; da Silva Júnior, E.N. Hybrid compounds with two redox centres: Modular synthesis of chalcogen-containing lapachones and studies on their antitumor activity. Eur. J. Med. Chem. 2015, 101, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, E.H.G.; Silvers, M.A.; Jardim, G.A.M.; Resende, J.M.; Cavalcanti, B.C.; Bomfim, I.S.; Pessoa, C.; de Simone, C.A.; Botteselle, G.V.; Braga, A.L.; et al. Synthesis and antitumor activity of selenium-containing quinone-based triazoles possessing two redox centres, and their mechanistic insights. Eur. J. Med. Chem. 2016, 122, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Roudesly, F.; oble, J.; Poli, G. Metal-catalyzed C-H activation/functionalization: The fundamentals. J. Mol. Catal. A Chem. 2017, 426, 275–296. [Google Scholar] [CrossRef]
- Gensch, T.; Hopkinson, M.N.; Glorius, F.; Wencel-Delord, J. Mild metal-catalyzed C–H activation: Examples and concepts. Chem. Soc. Rev. 2106, 45, 2900–2936. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 2016, 138, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Bahia, S.B.B.B.; Reis, W.J.; Jardim, G.A.M.; Souto, F.T.; De Simone, C.A.; Gatto, C.C.; Menna-Barreto, R.F.S.; De Castro, S.L.; Cavalcanti, B.C.; Pessoa, C.; et al. Molecular hybridization as a powerful tool towards multitarget quinoidal systems: Synthesis, trypanocidal and antitumor activities of naphthoquinone-based 5-iodo-1,4-disubstituted-, 1,4- and 1,5-disubstituted-1,2,3-triazoles. Med. Chem. Commun. 2016, 7, 1555–1563. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Calado, H.D.R.; Cury, L.A.; da Silva Júnior, E.N. Synthesis of a phenazine-based 1,2,3-triazole from naturally occurring naphthoquinone designed as a probe for Cd ions. Eur. J. Org. Chem. 2015, 38, 703–709. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Reis, W.J.; Ribeiro, M.F.; Ottoni, F.M.; Alves, R.J.; Silva, T.L.; Goulart, M.O.F.; Braga, A.L.; Menna-Barreto, R.F.S.; Salomão, K.; et al. On the investigation of hybrid quinones: Synthesis, electrochemical studies and evaluation of trypanocidal Activity. RSC Adv. 2015, 5, 78047–78060. [Google Scholar] [CrossRef]
- Cruz, E.H.G.; Carvalho, P.H.P.R.; Correa, J.R.; Silva, D.A.C.; Diogo, E.B.T.; Souza Filho, J.D.; Coelho, B.; Pessoa, C.; Oliveira, H.C.B.; Guido, B.C.; et al. Design, synthesis and application of fluorescent 2,1,3-benzothiadiazole-triazole-linked biologically active lapachone derivatives. New J. Chem. 2014, 38, 2569–2580. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; da Silva Júnior, E.N.; Bower, J.F. Overcoming naphthoquinone deactivation: Rhodium-catalyzed C-5 selective C–H iodination as a gateway to functionalized derivatives. Chem. Sci. 2016, 7, 3780–3784. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Bower, J.F.; da Silva Júnior, E.N. Rh-Catalyzed Reactions of 1,4-Benzoquinones with Electrophiles: C-H Iodination, Bromination, and Phenylselenation. Org. Lett. 2016, 18, 4454–4457. [Google Scholar] [CrossRef] [PubMed]
- Jardim, G.A.M.; Silva, T.L.; Goulart, M.O.F.; de Simone, C.A.; Barbosa, J.M.C.; Salomão, K.; de Castro, S.L.; Bower, J.F.; da Silva Júnior, E.N. Rhodium-catalyzed C-H bond activation for the synthesis of quinonoid compounds: Significant Anti-Trypanosoma cruzi activities and electrochemical studies of functionalized quinones. Eur. J. Med. Chem. 2017, 136, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Kathawate, L.; Gejji, S.P.; Yeole, S.D.; Verma, P.L.; Puranik, V.G.; Salunke-Gawali, S. The first naphthosemiquinone complex of K+ with vitamin K3 analog: Experiment and density functional theory. J. Mol. Struct. 2015, 1088, 56–63. [Google Scholar] [CrossRef]
- Biyogo, A.M.; Khoumeri, O.; Terme, T.; Curti, C.; Vanelle, P. Synthesis of new 2,4-disubstituted 2,3-dihydrobenzo[g]indol-5-ones by an SRN1 strategy. Synthesis 2015, 47, 2647–2653. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Fokin, V.V.; Green, L.G.; Rostovtsev, V.V. A stepwise Hüisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, K.; Skiera, I.; Piasecka, M.; Paryzek, Z. Alkaloids and isoprenoids modification by copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition (Click Chemistry): Toward new functions and molecular architectures. Chem. Rev. 2016, 116, 5689–5743. [Google Scholar] [CrossRef] [PubMed]
- Iha, R.K.; Wooley, K.L.; Nystro, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of orthogonal “click” chemistries in the synthesis of functional soft materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [PubMed]
- Maarseveen, J.H.; Hiemstra, H.; Bock, V.D. CuI-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 1, 51–68. [Google Scholar]
- Pinto, C.N.; Dantas, A.P.; De Moura, K.C.G.; Emery, F.S.; Polequevitch, P.F.; Pinto, M.C.F.R.; De Castro, S.L.; Pinto, A.V. Chemical Reactivity Studies with Naphthoquinones from Tabebuia with Anti-trypanosomal Efficacy. Arzneimittelforschung 2000, 50, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.V.; Pinto, C.N.; Pinto, M.C.; Rita, R.S.; Pezzella, C.A.C.; de Castro, S.L. Trypanocidal activity of synthetic heterocyclic derivatives of active quinones from Tabebuia sp. Arzneimittelforschung 1997, 47, 74–79. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are available from the C. Jacob ([email protected]) and E. N. da Silva Júnior ([email protected]) laboratories. |
| Compounds | HL-60 | HCT-116 | SF295 | NCIH-460 | PC3 | L929 |
|---|---|---|---|---|---|---|
| 2 | 1.96 (1.66–2.33) | >10 | >10 | >10 | >10 | >10 |
| 3 | 4.68 (3.93–5.58) | >10 | >10 | >10 | >10 | >10 |
| 4 | 2.25 (1.88–2.71) | >10 | >10 | >10 | >10 | >10 |
| 5 | 2.16 (1.81–2.59) | >10 | >10 | >10 | >10 | >10 |
| 8 | >10 | >10 | >10 | >10 | >10 | >10 |
| 9 | 5.39 (3.78–7.73) | >10 | >10 | >10 | >10 | >10 |
| 10 | >10 | >10 | >10 | >10 | >10 | >10 |
| 11 | 2.32 (1.72–3.25) | >10 | >10 | >10 | >10 | >10 |
| 12 | >10 | >10 | >10 | >10 | >10 | >10 |
| 13 | >10 | >10 | >10 | >10 | >10 | >10 |
| 14 | >10 | >10 | >10 | >10 | >10 | >10 |
| 15 | >10 | >10 | >10 | >10 | >10 | >10 |
| 17 | 0.81 (0.73–0.86) | >8.0 | 2.60 (2.47–2.72) | 2.06 (1.95–2.22) | 2.03 (1.90–2.25) | 0.52 (0.44–0.81) |
| 18 | 0.59 (0.45–0.70) | 0.37 (0.24–0.56) | 1.48 (1.34–1.63) | 1.32 (1.23–1.53) | 1.06 (0.90–1.27) | 0.36 (0.30–0.49) |
| 19 | 1.28 (1.16–1.31) | >8.0 | 1.75 (1.57–1.82) | 2.33 (2.23–2.45) | 1.55 (1.40–1.94) | 0.68 (0.53–0.82) |
| 20 | 1.00 (0.93–1.07) | 2.03 (1.79–2.28) | 3.12 (2.91–3.24) | 3.26 (3.15–3.42) | 2.70 (3.15–3.42) | 0.61 (0.58–0.68) |
| 21 | 1.00 (0.91–1.03) | >8.0 | 1.63 (1.42–1.84) | 1.49 (1.38–1.59) | 1.77 (1.66–1.98) | 1.28 (1.19–1.38) |
| 22 | 0.53 (0.48–0.59) | >8.0 | 2.13 (2.01–2.43) | 2.75 (2.54–3.00) | 2.47 (2.26–2.68) | 3.16 (3.04–3.27) |
| 23 | 0.71 (0.61–0.76) | 0.97 (0.88–1.10) | 3.43 (3.20–3.69) | 2.64 (2.44–2.90) | 1.64 (1.55–1.69) | 2.12 (1.92–2.27) |
| 24 | 1.94 (1.85–2.08) | 4.38 (4.24–4.54) | 2.34 (2.16–2.44) | 4.91 (4.70–5.26) | 2.10 (1.85–2.28) | 0.84 (0.71–1.06) |
| DOXO | 0.02 (0.01–0.02) | 0.21 (0.16–0.29) | 0.41 (0.21–0.47) | 0.15 (0.13–0.18) | 0.76 (0.59–0.93) | 1.72 (1.58–1.87) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardim, G.A.M.; Lima, D.J.B.; Valença, W.O.; Lima, D.J.B.; Cavalcanti, B.C.; Pessoa, C.; Rafique, J.; Braga, A.L.; Jacob, C.; Da Silva Júnior, E.N.; et al. Synthesis of Selenium-Quinone Hybrid Compounds with Potential Antitumor Activity via Rh-Catalyzed C-H Bond Activation and Click Reactions. Molecules 2018, 23, 83. https://doi.org/10.3390/molecules23010083
Jardim GAM, Lima DJB, Valença WO, Lima DJB, Cavalcanti BC, Pessoa C, Rafique J, Braga AL, Jacob C, Da Silva Júnior EN, et al. Synthesis of Selenium-Quinone Hybrid Compounds with Potential Antitumor Activity via Rh-Catalyzed C-H Bond Activation and Click Reactions. Molecules. 2018; 23(1):83. https://doi.org/10.3390/molecules23010083
Chicago/Turabian StyleJardim, Guilherme A. M., Daisy J. B. Lima, Wagner O. Valença, Daisy J. B. Lima, Bruno C. Cavalcanti, Claudia Pessoa, Jamal Rafique, Antonio L. Braga, Claus Jacob, Eufrânio N. Da Silva Júnior, and et al. 2018. "Synthesis of Selenium-Quinone Hybrid Compounds with Potential Antitumor Activity via Rh-Catalyzed C-H Bond Activation and Click Reactions" Molecules 23, no. 1: 83. https://doi.org/10.3390/molecules23010083