Selenazolinium Salts as “Small Molecule Catalysts” with High Potency against ESKAPE Bacterial Pathogens
Abstract
:1. Introduction
2. Results and Discussion
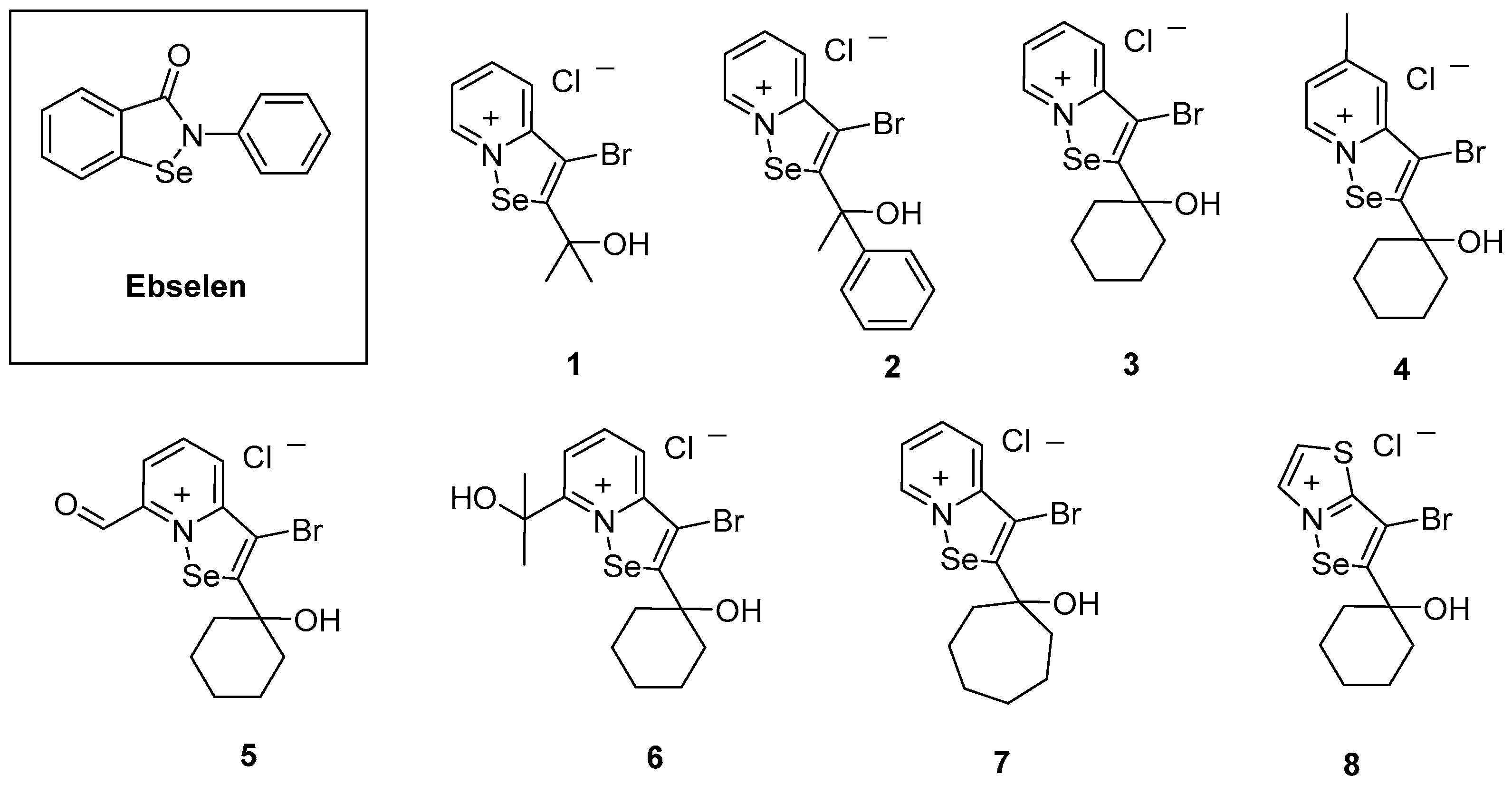
2.1. Chemical Synthesis
2.2. In Vitro Antibacterial Activity
2.2.1. Antibacterial Activity against S. aureus Strains
2.2.2. Antibacterial Activity against Gram-Negative Bacteria
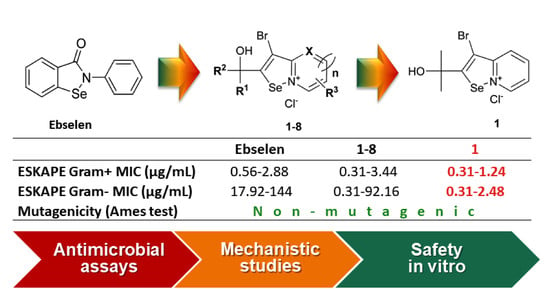
2.3. Results of Pharmaceutical Safety
2.4. Studies on the Possible Mode of Antimicrobial Action
2.4.1. Evaluation of ROS Formation
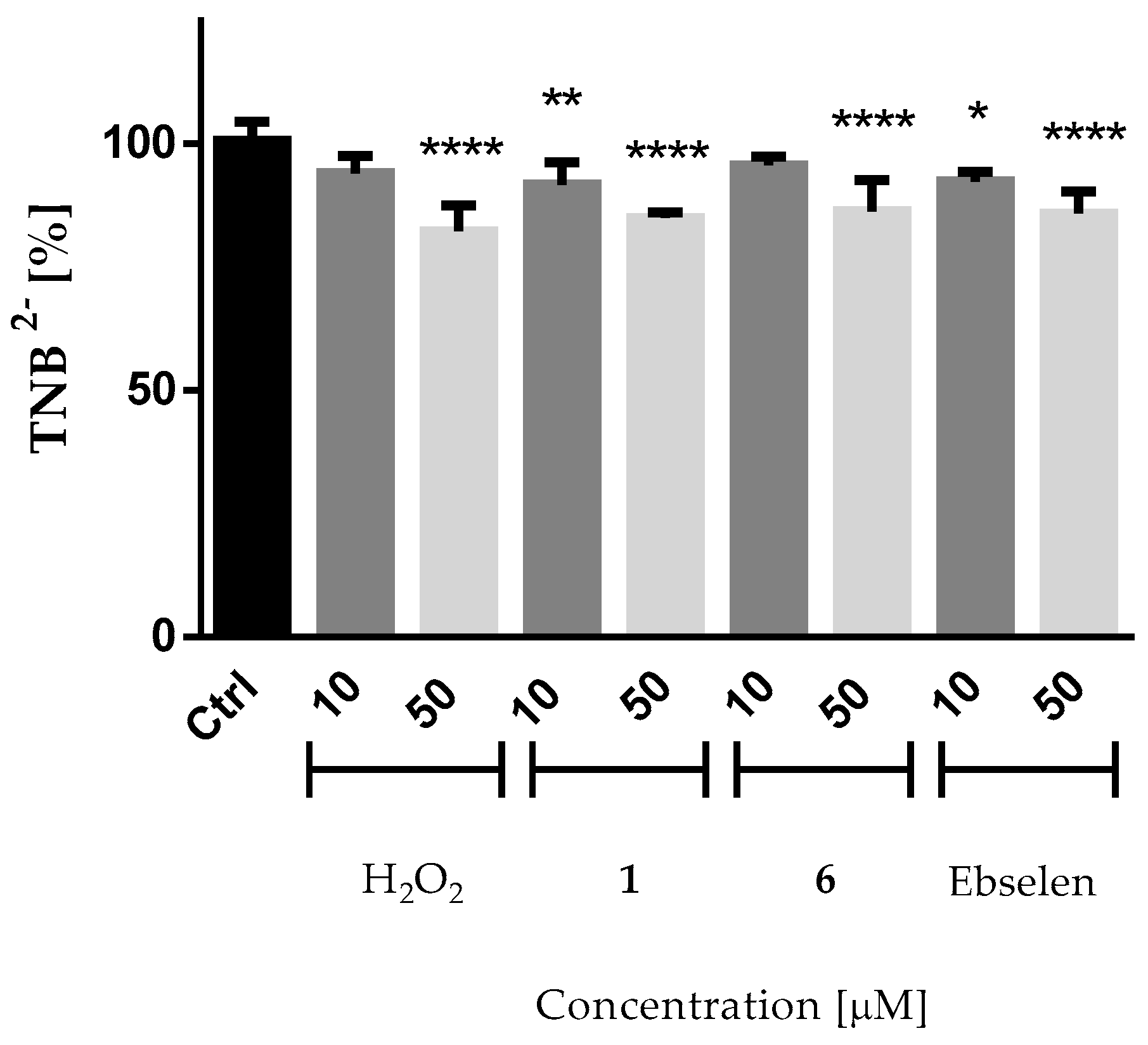
2.4.2. Reactivity of the Compounds with Thiol Groups
2.5. Structure–Activity Relationship Discussion
3. Materials and Methods
3.1. Microbiological Assays
3.1.1. Chemical Compounds
3.1.2. Bacterial Strains
3.1.3. Antimicrobial Susceptibility Testing
3.1.4. Determination of Intracellular Oxidative Stress Levels via the DCFH-DA Assay
3.1.5. DTNB Assay
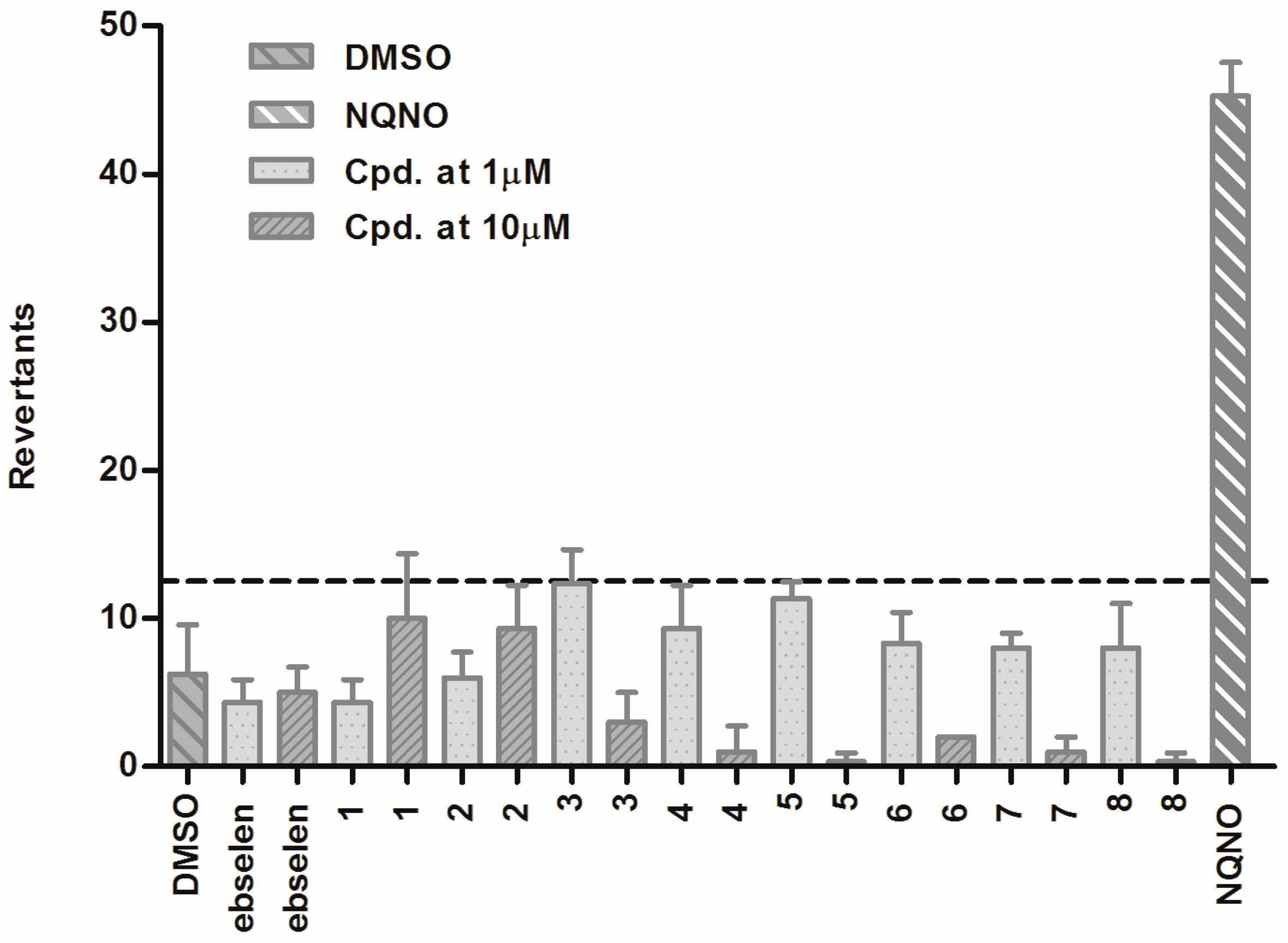
3.1.6. Ames Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, V.; Genilloud, O.; Horbal, L.; Marcone, G.L.; Marinelli, F.; Paitan, Y.; Ron, E.Z. Antibacterial discovery and development: From gene to product and back. Biomed. Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.M.; Kaye, K.S.; Cohen, D.A.; Marchaim, D. Appropriate antimicrobial therapy in the era of multidrug-resistant human pathogens. Clin. Microbiol. Infect. 2015, 21, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Merelli, M.; Temperoni, C.; Astilean, A. New antibiotics for bad bugs: Where are we? Ann. Clin. Microb. Antimcrob. 2013, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.G.; Long, T.E. Allicin-inspired thiolated fluoroquinolones as antibacterials against ESKAPE pathogens. Bioorg. Med. Chem. Lett. 2016, 26, 5545–5549. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spelberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ′20 progress—Development of new drugs active against Gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E. Development of novel antibacterial drugs to combat multiple resistant organisms. Langenbeck Arch. Surg. 2015, 400, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Memmi, G.; Filipe, S.R.; Pinho, M.G.; Fu, Z.; Cheung, A. Staphylococcus aureus PBP4 is essential for β-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob. Agents Chemother. 2008, 52, 3955–3966. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M. Worldwide attention to resistant bacteria. Iran. J. Clin. Infect. Dis. 2009, 4, 1–2. [Google Scholar]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Bliziotis, I.A. Pandrug-resistant Gram-negative bacteria: The dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 2007, 29, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Discovery research: The scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 2011, 66, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Piętka-Ottlik, M.; Wojtowicz-Młochowska, H.; Kołodziejczyk, K.; Piasecki, E.; Młochowski, J. New organoselenium compounds active against pathogenic bacteria, fungi and viruses. Chem. Pharm. Bull. 2008, 56, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.N.; Osman, H.; Werngren, J.; Hoffner, S.; Engman, L.; Holmgren, A. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochim. Biophys. Acta 2016, 1860, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Minnerup, J.; Sutherland, B.A.; Buchan, A.M.; Kleinschnitz, C. Neuroprotection for stroke: Current status and future perspectives. Int. J. Mol. Sci. 2012, 13, 11753–11772. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Redox signalling via the cellular thiolstat. Biochem. Soc. Trans. 2011, 39, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Terentis, A.C.; Freewan, M.; Plaza, T.S.S.; Raftery, M.J.; Stocker, R.; Thomas, S.R. The selenazal drug ebselen potently inhibits indoleamine 2,3-dioxygenase by targeting enzyme cysteine residues. Biochemistry 2010, 49, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing clinical molecule ebselen to combat drug-resistant pathogens. PLoS ONE 2015, 10, e0133877. [Google Scholar] [CrossRef] [PubMed]
- Arsenyan, P.; Vasiljeva, J.; Belyakov, S.; Liepinsh, E.; Petrova, M. Fused selenazolinium salt derivatives with a Se-N+ bond: Preparation and properties. Eur. J. Org. Chem. 2015, 2015, 5842–5855. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Jockenhöfer, F.; Gollnick, H.; Herberger, K.; Isbary, G.; Renner, R.; Stücker, M.; Valesky, E.; Wollina, U.; Weichenthal, M.; Karrer, S.; et al. Aktuelle nachweisraten multiresistenter Gram-negativer bakterien (3MRGN, 4MRGN) bei patienten mit chronischem Ulcus cruris. Der Hautarz.t 2014, 65, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Kamber, M.; Fluckiger-Isler, S.; Engelhardt, G.; Jaeckh, R.; Zeiger, E. Comparison of the Ames II and traditional Ames test responses with respect to mutagenicity, strain specificities, need for metabolism and correlation with rodent carcinogenicity. Mutagenesis 2009, 24, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger-Isler, S.; Baumeister, M.; Braun, K.; Gervais, V.; Hasler-Nguyen, N.; Reimann, R.; Van Gompel, J.; Wunderlich, H.G.; Engelhardt, G. Assessment of the performance of the Ames II assay: A collaborative study with 19 coded compounds. Mutat. Res. 2004, 558, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Sadek, B.; Saad, A.; Latacz, G.; Kuder, K.; Olejarz, A.; Karcz, T.; Stark, H.; Kiec-Kononowicz, K. Non-imidazole-based histamine H3 receptor antagonists with anticonvulsant activity in different seizure models in male adult rats. Drug Des. Dev. Ther. 2016, 10, 3879–3898. [Google Scholar] [CrossRef] [PubMed]
- Umbuzeiro, G.D.; Rech, C.M.; Correia, S.; Bergamasco, A.M.; Cardenette, G.H.L.; Fluckiger-Isler, S.; Kamber, M. Comparison of the Salmonella/microsome microsuspension assay with the new microplate fuctuation protocol for testing the mutagenicity of environmental samples. Environ. Mol. Mutagen. 2010, 51, 31–38. [Google Scholar]
- Zeiger, E. Bacterial mutation assays. Methods Mol. Biol. 2013, 1044, 3–26. [Google Scholar] [PubMed]
- Marć, M.A.; Domínguez-Álvarez, E.; Słoczyńska, K.; Żmudzki, P.; Chłoń-Rzepa, G.; Pękala, E. In vitro biotransformation, safety, and chemopreventive action of novel 8-methoxy-purine-2,6-dione derivatives. Appl. Biochem. Biotechnol. 2017, in press. [Google Scholar]
- De Cássia Ribeiro Gonçalves, R.; Rezende Kitagawa, R.; Aparecida Varanda, E.; Stella Gonçalves Raddi, M.; Andrea Leite, C.; Regina Pombeiro Sponchiado, S. Effect of biotransformation by liver S9 enzymes on the mutagenicity and cytotoxicity of melanin extracted from Aspergillus nidulans. Pharm. Biol. 2016, 54, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Battaglia, E.; Burkholz, T.; Peng, D.; Bagrel, D.; Montenarh, M. Control of oxidative posttranslational cysteine modifications: From intricate chemistry to widespread biological and medical applications. Chem. Res. Toxicol. 2012, 25, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Viswanathan, U.M.; Khairan, K.; Buric, T.; Saidu, N.E.B.; Xu, Z.J.; Hanf, B.; Bazukyan, I.; Trchounian, A.; Hannemann, F.; et al. Synthesis of amphiphilic, chalcogen-based redox modulators with in vitro cytotoxic activity against cancer cells, macrophages and microbes. Med. Chem. Commun. 2014, 5, 25–31. [Google Scholar] [CrossRef]
- Manikova, D.; Letavayova, L.M.; Vlasakova, D.; Kosik, P.; Estevam, E.C.; Nasim, M.J.; Gruhlke, M.; Slusarenko, A.; Burkholz, T.; Jacob, C.; et al. Intracellular diagnostics: Hunting for the mode of action of redox-modulating selenium compounds in selected model systems. Molecules 2014, 19, 12258–12279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidu, N.E.B.; Touma, R.; Abu Asali, I.; Jacob, C.; Montenarh, M. Diallyl tetrasulfane activates both the eIF2α and Nrf2/HO-1 pathways. Biochim. Biophys. Acta 2013, 1830, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Saidu, N.E.B.; Abu Asali, I.; Czepukojc, B.; Seitz, B.; Jacob, C.; Montenarh, M. Comparison between the effects of diallyl tetrasulfide on human retina pigment epithelial cells (ARPE-19) and HCT116 cells. Biochim. Biophys. Acta 2013, 1830, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Allah, D.R.; Schwind, L.; Abu Asali, I.; Nasim, M.J.; Jacob, C.; Gotz, C.; Montenarh, M. A scent of therapy: Synthetic polysulfanes with improved physico-chemical properties induce apoptosis in human cancer cells. Int. J. Oncol. 2015, 47, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, T.; Chen, H.; Xu, Y.N.; Yu, L.F.; Liu, T.; Tang, J.; Yi, Z.; Yang, C.G.; Xue, W.; et al. The synthesis and antistaphylococcal activity of 9,13-disubstituted berberine derivatives. Eur. J. Med. Chem. 2017, 127, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Göker, H.; Karaaslan, C.; Püsküllü, M.O.; Yildiz, S.; Duydu, Y.; Üstündağ, A.; Yalcin, C.Ö. Synthesis and in vitro activity of polyhalogenated 2-phenylbenzimidazoles as a new class of anti-MRSA and anti-VRE agents. Chem. Biol. Drug Des. 2016, 87, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.R.; McCarroll, A.J.; McGarry, D.; Kirkham, J.; Pichowicz, M.; Walker, R.; Warrilow, C.; Salisbury, A.M.; Savage, V.J.; Moyo, E.; et al. Discovery and structure-activity relationships of a novel isothiazolone class of bacterial type II topoisomerase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, R.; LaVoi, T.M.; Santos, R.G.; Morales, A.; Nefzi, A.; Welmaker, G.S.; Medina-Franco, J.L.; Giulianotti, M.A.; Houghten, R.A.; Shaw, L.N. Combinatorial libraries as a tool for the discovery of novel, broad-spectrum antibacterial agents targeting the ESKAPE pathogens. J. Med. Chem. 2015, 58, 3340–3355. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Document M07-A9 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Estevam, E.C.; Witek, K.; Faulstich, L.; Nasim, M.J.; Latacz, G.; Domínguez-Álvarez, E.; Kieć-Kononowicz, K.; Demasi, M.; Handzlik, J.; Jacob, C. Aspects of a distinct cytotoxicity of selenium salts and organic selenides in living cells with possible implications for drug design. Molecules 2015, 20, 13894–13912. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Winther, J.R.; Thorpe, C. Quantification of thiols and disulfides. Biochim. Biophys. Acta 2014, 1840, 838–846. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Bacterial Strain | Relevant Phenotype * |
|---|---|
| Staphylococcus aureus | |
| ATCC 25923 | Reference strain, CC5, MSSA |
| MM-O058 | Clinical isolate, CC121, MSSA, MDR |
| MM-N072 | Clinical isolate, CC152, MSSA, MDR |
| HEMSA 5 | Clinical isolate, MRSA, XDR |
| LG-N017 | Clinical isolate, CC5, MRSA, MDR |
| MM-O021 | Clinical isolate, CC8, MRSA, MDR |
| R45-CC45 | Clinical isolate, CC45, MRSA |
| R46-CC22 | Clinical isolate, CC22, MRSA |
| USA300 LAC | Clinical isolate, CC8, CA-MRSA, MDR |
| 5328 | Clinical isolate, CC398, LA-MRSA |
| Mu50 | Clinical isolate, CC5, MRSA, VISA |
| Bacterial Strain | Relevant Phenotype |
|---|---|
| Klebsiella pneumoniae | |
| NRZ-00103 | Reference strain, KPC-2, MDR, 4 MRGN |
| KP 21513017 | Clinical isolate |
| KP 1963584 | Clinical isolate, OXA-2, MDR, 4 MRGN |
| Acinetobacter spp. | |
| AC 2151300 | Clinical isolate, A. ursingii |
| AC 1995594 | Clinical isolate, A. baumannii complex |
| AB 4184/2/5 | Clinical isolate, A. baumannii |
| Pseudomonas aeruginosa | |
| ATCC 27853 | Reference strain |
| PA T18 | Clinical isolate, MDR, 3 MRGN |
| PA 54 | Clinical isolate, MDR |
| PA 58 | Clinical isolate, MDR |
| Escherichia coli | |
| NCTC 13351 | Reference strain, TEM-3 |
| EC 2151612 | Clinical isolate, MDR, 3 MRGN |
| EC 1995591 | Clinical isolate, MDR, 3 MRGN |
| EC 1227107 | Clinical isolate, MDR, 3 MRGN |
| S. aureus Strain | MIC (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OXA | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Ebselen | |
| ATCC 25923 | 0.25 | 1.24 | 1.72–3.44 | 1.4 | 1.45 | 0.72–1.44 | 0.36–0.72 | 1.44–2.88 | 2.88 | 0.56 |
| MM-O058 * | 0.25 | 0.62 | 0.86 | 0.35–0.7 | 0.36–0.73 | 0.72 | 0.36–0.72 | 0.36–0.72 | 0.36–0.72 | 0.56–1.12 |
| MM-N072 * | 0.25 | 0.62–1.24 | 3.44 | 0.7–1.4 | 0.73–1.46 | 0.72 | 0.36–0.72 | 0.72 | 1.44–2.88 | 0.56–1.12 |
| USA300 LAC * | 12 | 0.62 | 3.44 | 0.7 | 0.73 | 0.72–1.44 | 0.72–1.44 | 0.36–0.72 | 0.72–1.44 | 0.56–1.12 |
| 5328 | 4 | 0.62–1.24 | 3.44 | 1.4 | 1.45 | 1.44–2.88 | 0.72–1.44 | 0.72–1.44 | 1.44–2.88 | 1.12–2.24 |
| LG-N017 * | 12 | 0.62–1.24 | 0.86–1.72 | 0.35–0.7 | 0.73–1.45 | 0.72–1.44 | 0.36–0.72 | 0.36–1.44 | 0.72–1.44 | 1.12–2.24 |
| MM-O021 * | 64 | 0.31–0.62 | 1.72–3.44 | 0.35–0.7 | 0.36–0.73 | 0.36–0.72 | 0.36–0.72 | 0.36–0.72 | 0.72–1.44 | 0.56–1.12 |
| R45 CC22 | 64 | 0.31 | 0.86–1.72 | 0.35 | 0.36–0.73 | 0.36–0.72 | 0.36 | 0.36–0.72 | 0.36–0.72 | 1.12 |
| R45 CC45 | 4 | 0.31–0.62 | 1.72–3.44 | 0.7–1.4 | 0.36–0.73 | 0.72 | 0.36–0.72 | 0.72 | 1.44 | 0.56 |
| HEMSA 5 ** | 128 | 1.24 | 1.72 | 1.4 | 1.45 | 1.44 | 0.72 | 0.72–1.44 | 2.88 | 2.8 |
| Mu50 *** | 256 | 0.31–0.62 | 0.43–0.86 | 0.7–1.4 | 0.36–0.73 | 0.36–0.72 | 0.36 | 0.36–0.72 | 0.72–1.44 | 0.28 |
| Bacterial Strain | MIC (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Ebselen | ||
| K. pneumoniae | 1 | 1.24 * | 14 | 1.4–2.8 | 2.88–5.76 | 46 | 46 | 2.88 | 5.76 | ≥143 |
| 2 | 0.62–1.24 | 6.88–14 | 1.4 | 2.88 | 35 | 17 | 2.88 | 5.76 | 108 | |
| 3 | 0.62 | 6.88 | 1.4 | 2.88 | 17 | 17 | 1.44–2.88 | 2.88 | 72 | |
| Acinetobacter spp. | 4 | 0.31–0.62 | 0.86 | 0.35–0.7 | 0.36–0.72 | 2.88 | 1.44 | 0.36–0.72 | 1.44 | 18 |
| 5 | 0.62 | 1.72–3.44 | 1.4 | 0.72 | 2.88–5.76 | 2.88 | 0.72 | 1.44–2.88 | 27 | |
| 6 | 0.31 | 0.86 | 0.35 | 0.36 | 2.88 | 0.72–1.44 | 0.36 | 1.44 | 18 | |
| P. aeruginosa | 7 | 2.48 | 110 | 5.60–11 | 5.76–12 | 69 | 138 | 17 | 23 | 72 |
| 8 | 0.31 | 1.72 | 0.7 | 1.44 | 12 | 12 | 0.72 | 1.44–2.88 | 18 | |
| 9 | 0.62–1.24 | 21 | 1.4–2.8 | 5.76 | 12 | 69 | 5.76 | 5.76–12 | 108 | |
| 10 | 0.62 | 6.88–14 | 1.4 | 2.88 | 17 | 23 | 2.88 | 5.76 | 27 | |
| E. coli | 11 | 1.24–2.48 | 6.88–14 | 1.4 | 2.88 | 17 | 12 | 2.88 | 5.76 | 108 |
| 12 | 1.24–2.48 | 6.88 | 1.4 | 2.88 | 17 | 12 | 1.44–2.88 | 5.76 | 72 | |
| 13 | 2.48 | 6.88–14 | 1.44–2.8 | 2.88 | 23 | 12 | 2.88 | 5.76 | 72 | |
| 14 | 1.24–2.48 | 14 | 2.8 | 5.76 | 23 | 17 | 2.88 | 5.76–12 | 54 | |
| Cpd. | MI (1 µM) | B | MI (10 µM) | B |
|---|---|---|---|---|
| Ebselen | 0.69 | 0.26 | 0.80 | 0.46 |
| 1 | 0.69 | 0.35 | 1.60 | 1.00 |
| 2 | 0.96 | 0.83 | 1.49 | 1.00 |
| 3 | 1.97 | 0.99 | 0.48 | 0.06 |
| 4 | 1.49 | 0.71 | 0.16 | 0.00 |
| 5 | 1.81 | 0.96 | 0.05 | 0.00 |
| 6 | 1.33 | 0.97 | 0.32 | 0.00 |
| 7 | 1.28 | 0.94 | 0.16 | 0.00 |
| 8 | 1.28 | 0.98 | 0.05 | 0.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witek, K.; Nasim, M.J.; Bischoff, M.; Gaupp, R.; Arsenyan, P.; Vasiljeva, J.; Marć, M.A.; Olejarz, A.; Latacz, G.; Kieć-Kononowicz, K.; et al. Selenazolinium Salts as “Small Molecule Catalysts” with High Potency against ESKAPE Bacterial Pathogens. Molecules 2017, 22, 2174. https://doi.org/10.3390/molecules22122174
Witek K, Nasim MJ, Bischoff M, Gaupp R, Arsenyan P, Vasiljeva J, Marć MA, Olejarz A, Latacz G, Kieć-Kononowicz K, et al. Selenazolinium Salts as “Small Molecule Catalysts” with High Potency against ESKAPE Bacterial Pathogens. Molecules. 2017; 22(12):2174. https://doi.org/10.3390/molecules22122174
Chicago/Turabian StyleWitek, Karolina, Muhammad Jawad Nasim, Markus Bischoff, Rosmarie Gaupp, Pavel Arsenyan, Jelena Vasiljeva, Małgorzata Anna Marć, Agnieszka Olejarz, Gniewomir Latacz, Katarzyna Kieć-Kononowicz, and et al. 2017. "Selenazolinium Salts as “Small Molecule Catalysts” with High Potency against ESKAPE Bacterial Pathogens" Molecules 22, no. 12: 2174. https://doi.org/10.3390/molecules22122174