Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights
Abstract
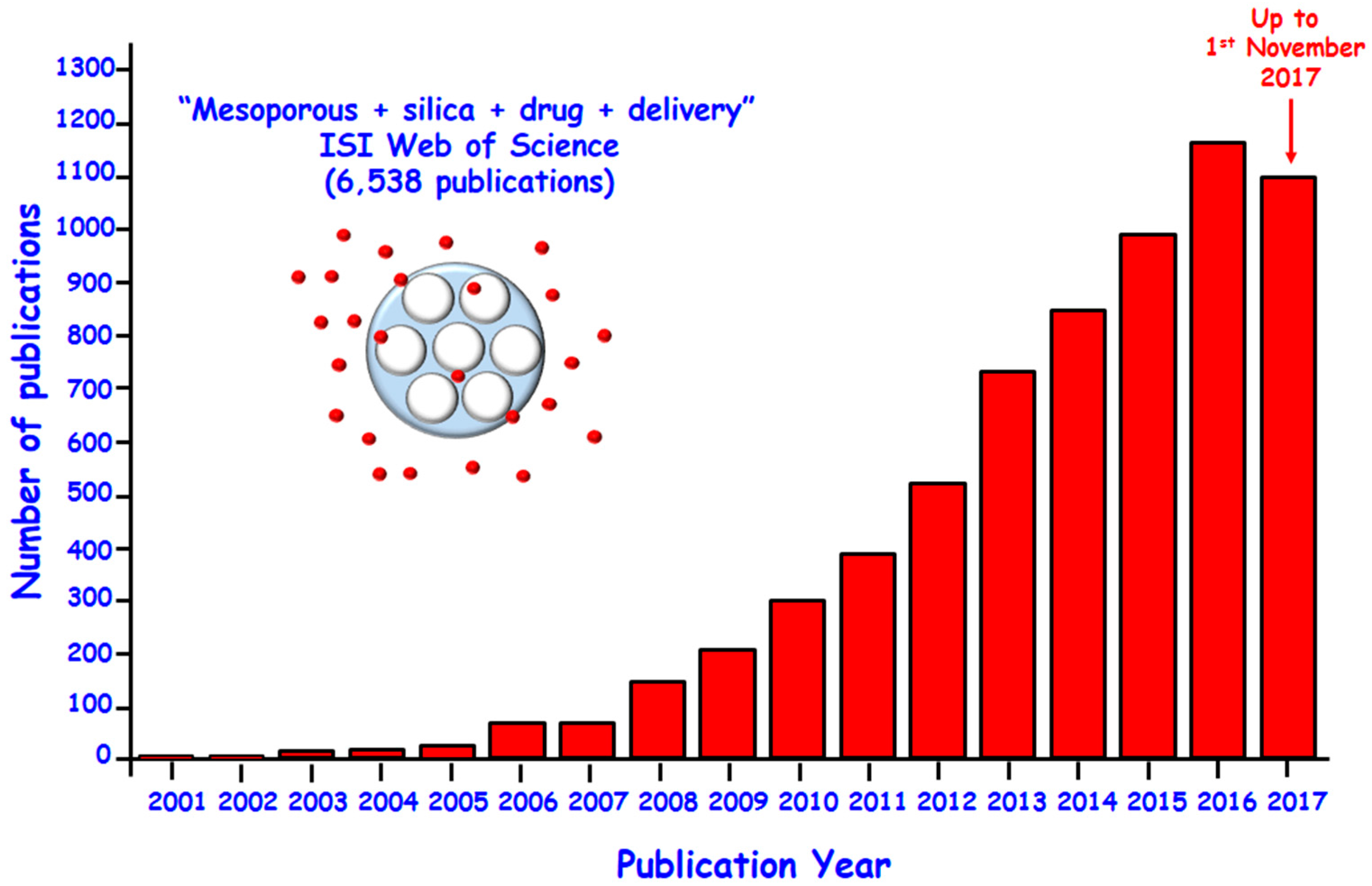
:1. Introduction
2. Mesoporous Silica Nanoparticles as Drug Delivery Systems
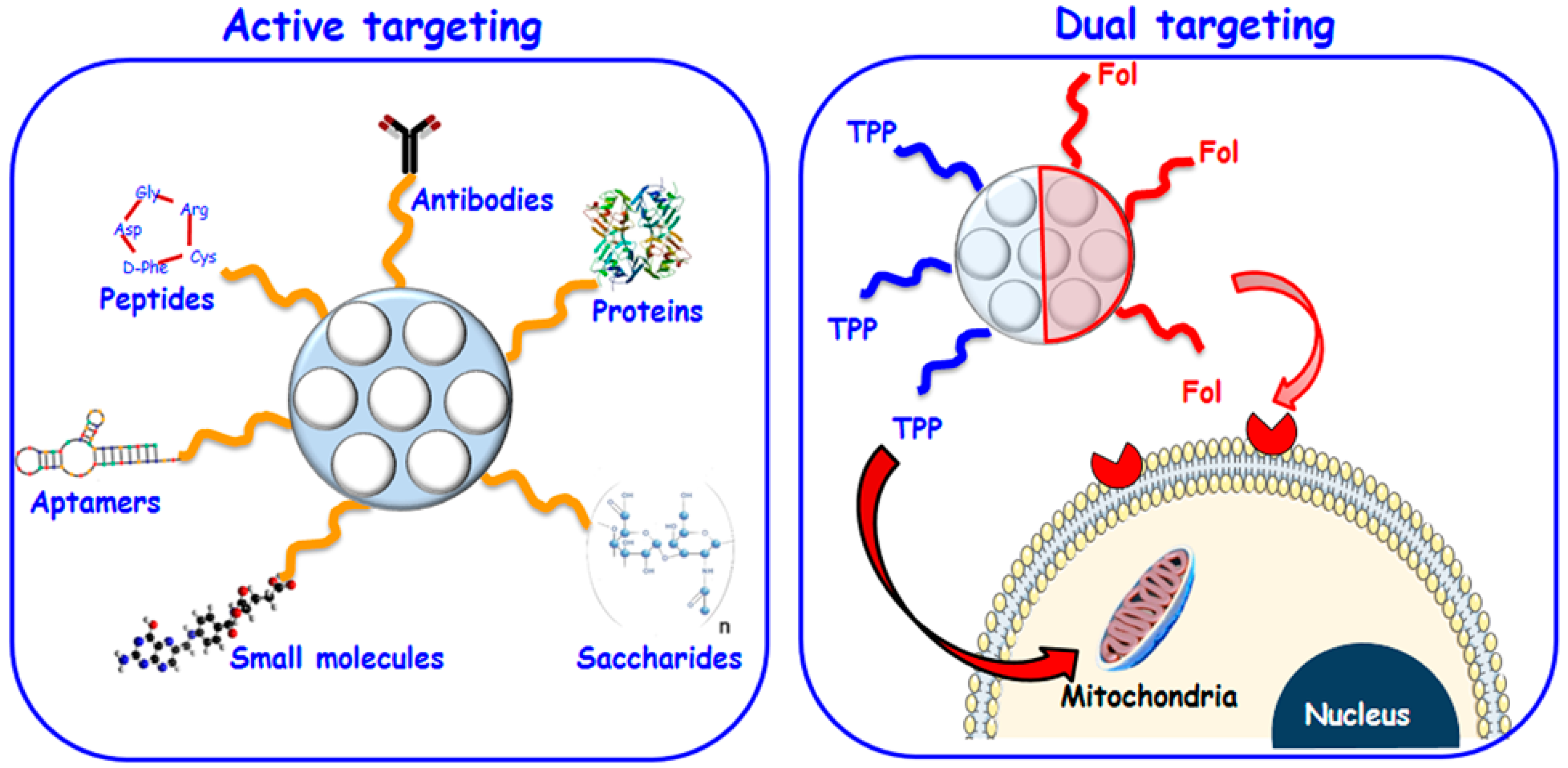
2.1. Selective Targeting for Localized Therapy
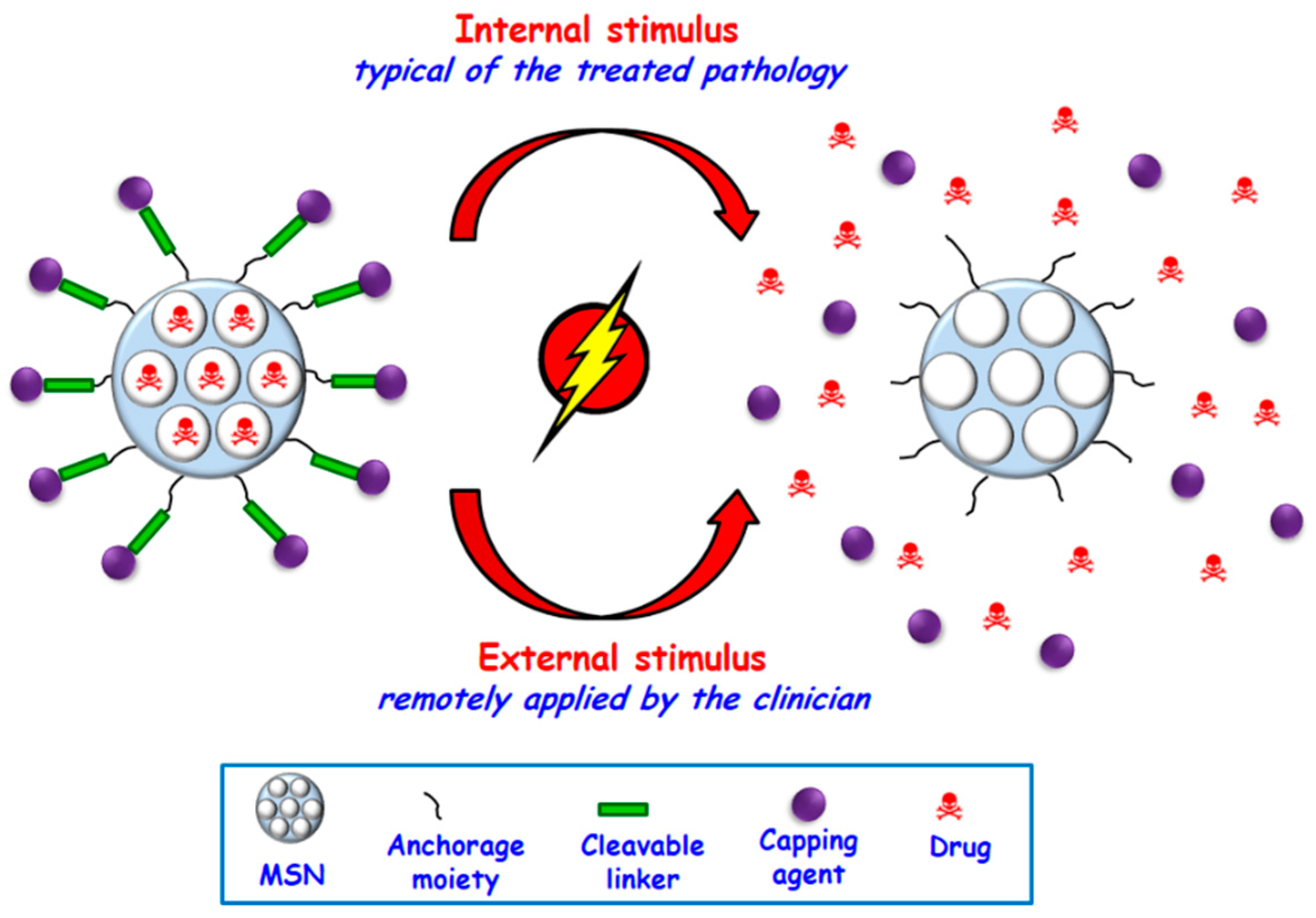
2.2. Controlled Dosage and Smart Behavior
2.2.1. Internal Stimuli-Responsive Drug Delivery MSNs
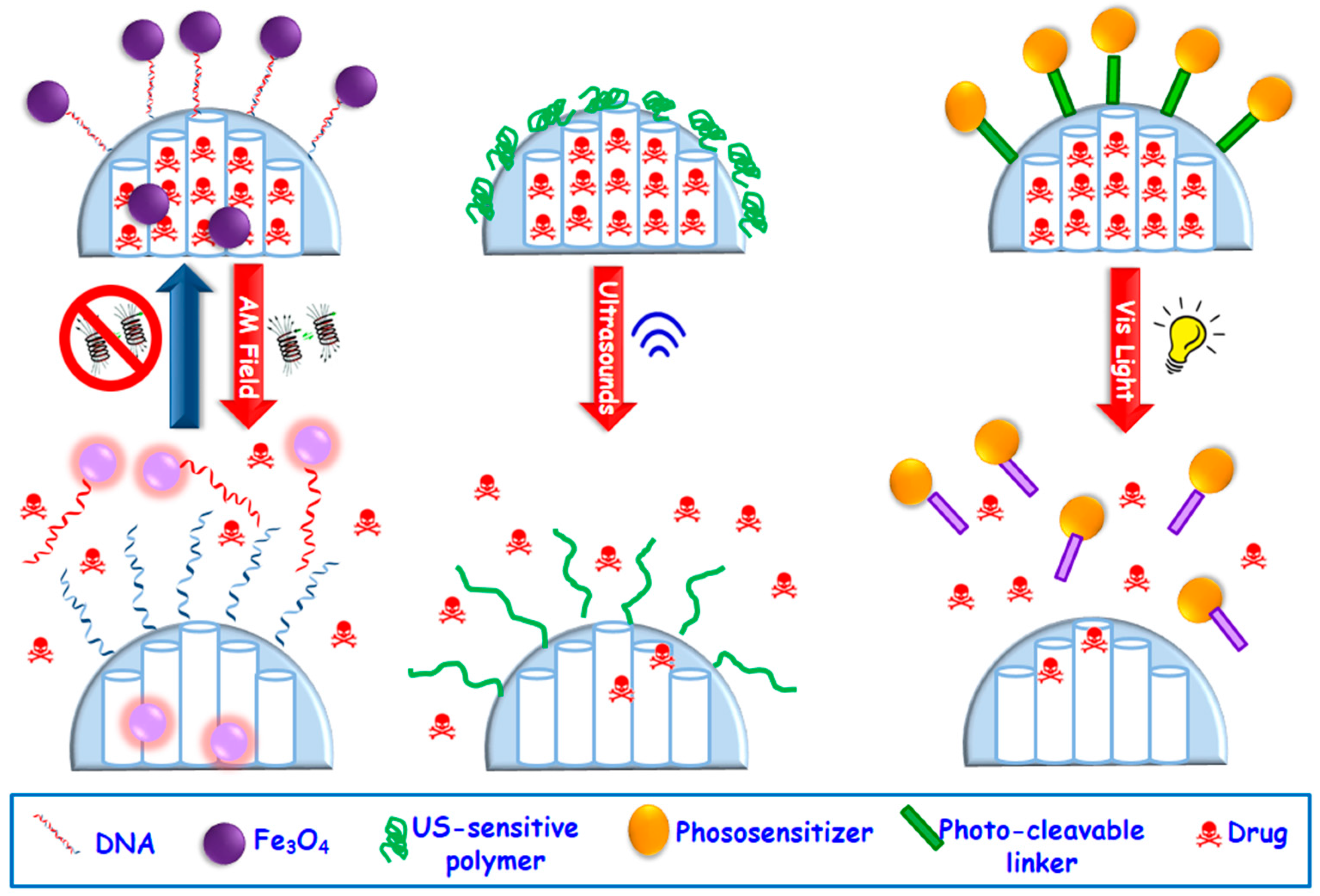
2.2.2. External Stimuli-Responsive Drug Delivery MSNs
- Magnetically-responsive MSNs. The benefits of using magnetic fields is due to the different effect that they can exert on MSNs, which can be magnetic guidance under a permanent magnetic field or a temperature increase upon application of an alternating magnetic (AM) field [76] This permits a wide range of possibilities for in the biomedical field. The most widely used magnetic nanoparticles for stimuli-responsive drug delivery are superparamagnetic iron oxide ones (SPIONs). These nanoparticles are able to convert the magnetic energy into heat obeying two mechanisms: (i) Brownian fluctuations provoked by the fast rotation of the magnetic nuclei, and (ii) Nell fluctuations caused by the rotation of the magnetic moments [101]. Most of the employed designed strategies consist in the encapsulation of SPIONs of ca. 5–10 nm within MSNs, which can be accomplished by using aerosol techniques [102] or sol-gel process [103,104,105,106] etc. The incorporation of SPIONs within MSNs permits the employ of AM fields, which triggers temperature increase. MSNs can incorporate temperature-responsive moieties acting as gatekeepers able to undergo physicochemical changes that provoke pore opening and drug release. Figure 5 displays a representative example based on drug-loaded magnetic MSNs whose pore outlets have been grafted with single-DNA strands that hybridize with Fe3O4 SPIONs functionalized with the complementary DNA strand, acting as capping agents. The application of an AM field provokes heat that trigger the dehybridization of the DNA, allowing the cargo release in a reversible fashion [107].
- Ultrasound-triggered MSNs. Ultrasounds (US) constitute an efficient method to attain spatiotemporal control of drug delivery at the target site and preventing the damage of healthy tissues. Other of the advantages of the use of US regards its non-invasiveness, absence of ionizing radiations and the easily regulation of tissue penetration depth by tuning US parameters (frequency, duty cycles and exposure times) [76,108]. US waves can trigger drug release from MSNs through thermal effect. Mechanophores, i.e., chemical bonds that cleave under US radiation, can be used to design of US-triggered MSNs. Thus, 2-tetrahydropyranyl methacrylate, a hydrophobic monomer with a US-sensitive group, can transform to hydrophilic methacrylic acid [109,110]. This phase transformation under US stimulus has been used to develop US-responsive drug delivery MSNs by using such moieties as mesopore gatekeepers (Figure 5) [111,112].
- Light-triggered MSNs. Light constitutes another useful alternative with non-invasive and spatiotemporal control to design stimuli-responsive MSNs able to achieve on-demand drug release triggered by illumination with a specific wavelength (ultraviolet, UV, visible, Vis, or near-infrared, NIR, regions) [113,114]. The advantages of the use of light relies on its easy application, low toxicity and precise focalization in the desired place. Nonetheless, the main constrain is its low tissue penetration capability, which can be solved by using auxiliary medical devices such as those use in laparoscopy surgeries. Up to date UV stimulus has been by far the most widely used radiation to trigger drug release from MSNs [36,113] because this light has the highest power and can break bonds with ease. However, UV light present several drawbacks for current biomedical applications, such as its toxicity and low tissue penetrability [115,116,117,118]. Thus, recently Vis light is receiving growing attention since it offers a less harmful and higher penetrability rate than UV radiation. Figure 5 displays a representative example of a Vis light-triggered MSNs-based drug delivery system [119]. In this case, MSNs are decorated with porphyrin nanocaps anchored via reactive oxygen species (ROS)-cleavable linkages. When Vis light stimulus is applied, the porphyrin blocking caps provoke singlet oxygen molecules that break the sensitive linker and trigger the opening of mesopores and allowing drug release.
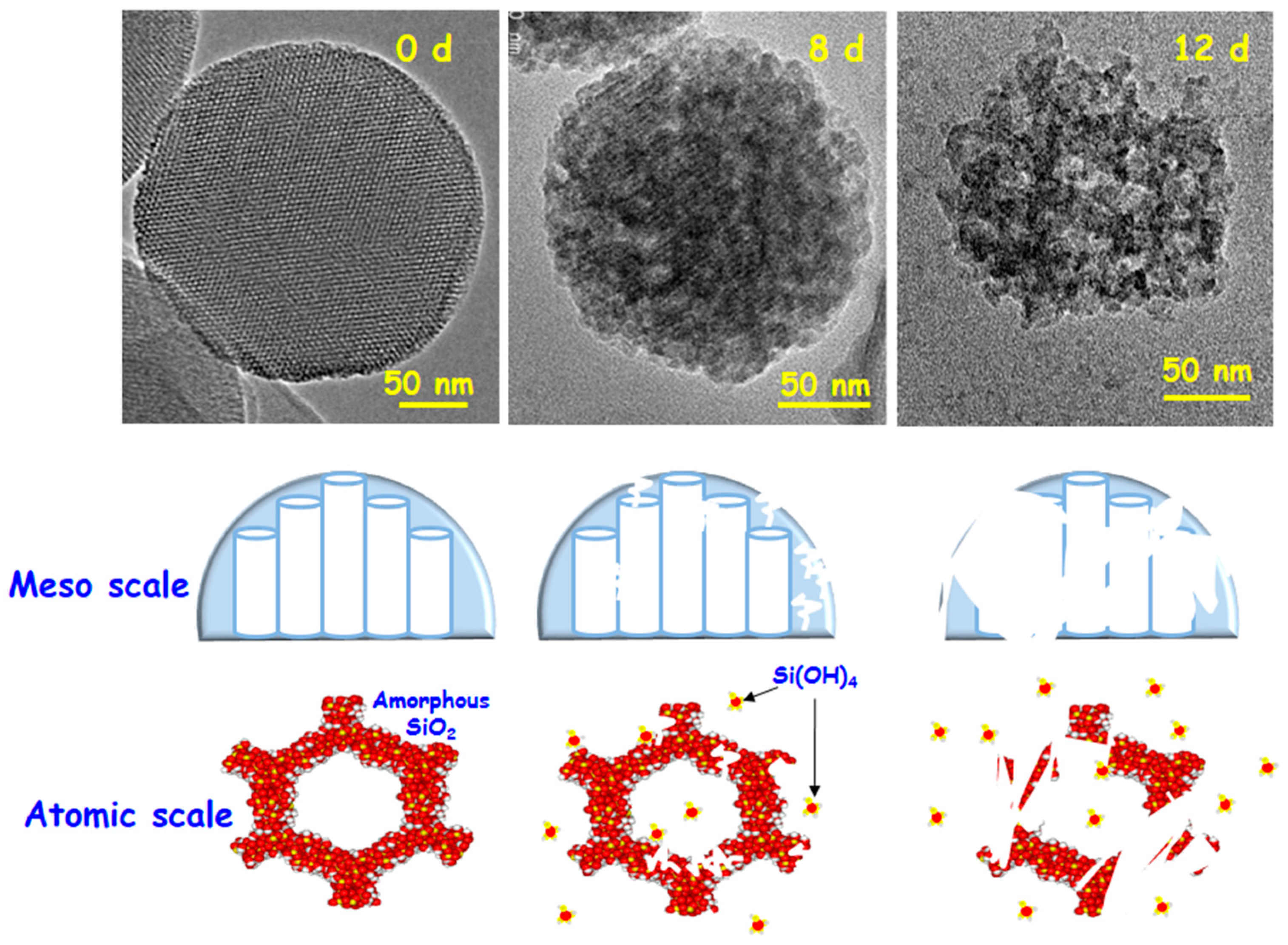
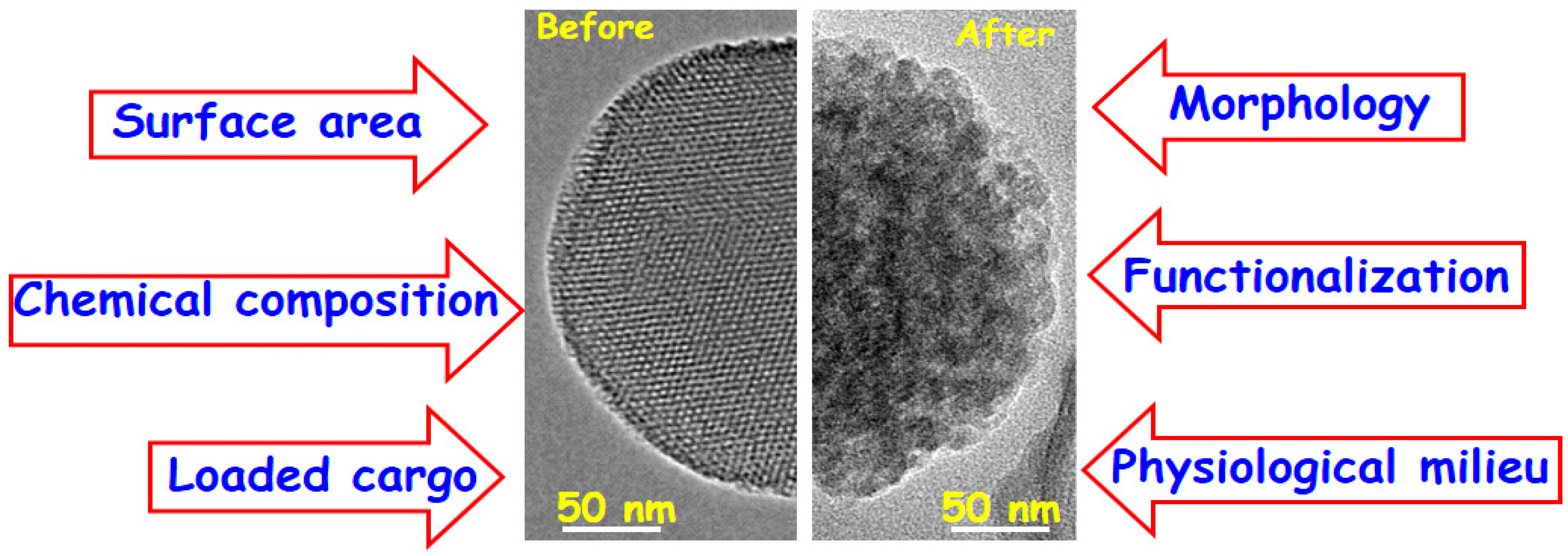
3. Performance in Physiological Fluids
4. Benefits and Downsides of MSNs for Drug Delivery
4.1. Benefits of MSNs for Drug Delivery
4.2. Downsides of MSNs for Drug Delivery
Acknowledgments
Conflicts of Interest
References
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, A.; Manchanda, R.; McGoron, A. Theranostic applications of nanomaterials in cancer: Drug delivery, image-guided therapy, and multifunctional platforms. Appl. Biochem. Biotechnol. 2011, 165, 1628–1651. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Sahlgren, C.; Linden, M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Curr. Drug Targets 2011, 12, 1166–1186. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Argyo, C.; Weiss, V.; Braeuchle, C.; Bein, T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Butler, K.S.; Durfee, P.N.; Theron, C.; Ashley, C.E.; Carnes, E.C.; Brinker, C.J. Protocells: Modular Mesoporous Silica Nanoparticle-Supported Lipid Bilayers for Drug Delivery. Small 2016, 12, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine 2016, 12, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Singh, R.K.; Khanal, D.; Patel, K.D.; Lee, E.-J.; Leong, K.W.; Chrzanowski, W.; Kim, H.-W. Smart multifunctional drug delivery towards anticancer therapy harmonized in mesoporous nanoparticles. Nanoscale 2015, 7, 14191–14216. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Soler-Illia, G.J.A.A.; Azzaroni, O. Gated supramolecular chemistry in hybrid mesoporous silica nanoarchitectures: Controlled delivery and molecular transport in response to chemical, physical and biological stimuli. Chem. Commun. 2015, 51, 6050–6075. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Durand, J.-O. Targeted treatment of cancer with nanotherapeutics based on mesoporous silica nanoparticles. ChemPlusChem 2015, 80, 26–36. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-N.; Zhang, C.-Q.; Wang, W.; Wang, P.C.; Zhou, J.-P.; Liang, X.-J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [PubMed]
- He, Q.; Shi, J. MSN Anti-Cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Adv. Mater. 2014, 26, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Hurley, K.R.; Haynes, C.L. Critical Considerations in the Biomedical Use of Mesoporous Silica Nanoparticles. J. Phys. Chem. Lett. 2012, 3, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2011, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Colilla, M.; Vallet-Regí, M. Smart mesoporous nanomaterials for antitumor therapy. Nanomaterials 2015, 5, 1906–1937. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; González, B. Medical applications of organic–inorganic hybrid materials within the field of silica-based bioceramics. Chem. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Morrow, M.P.; Asefa, T.; Sharma, K.K.; Duncan, C.; Anan, A.; Penefsky, H.S.; Goodisman, J.; Souid, A.-K. Mesoporous silica nanoparticles inhibit cellular respiration. Nano Lett. 2008, 8, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-M.; Chung, T.-H.; Hung, Y.; Lu, F.; Wu, S.-H.; Mou, C.-Y.; Yao, M.; Chen, Y.-C. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2008, 231, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.; Roggers, R.A.; Zhao, Y.; Trewyn, B.G. Interaction effects of mesoporous silica nanoparticles with different morphologies on human red blood cells. RSC Adv. 2013, 3, 2454–2461. [Google Scholar] [CrossRef]
- Villegas, M.R.; Baeza, A.; Noureddine, A.; Durfee, P.; Butler, K.; Agola, J.; Brinker, J.C.; Vallet Regí, M. Multifunctional protocells for enhanced penetration in 3D extracellular tumoral matrices. Chem. Mater. 2017. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2012, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies forengineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as Organs: Complex Tissues That Interface with the Entire Organism. Dev. Cell 2010, 18, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.-C.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Zhong, Y.; Cui, Y. pH-controllable drug carrier with SERS activity for targeting cancer cells. Biosens. Bioelectron. 2014, 57, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Baeza, A.; Rodríguez-Milla, M.A.; García-Castro, J.; Vallet-Regí, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef]
- Mickler, F.M.; Moeckl, L.; Ruthardt, N.; Ogris, M.; Wagner, E.; Braeuchle, C. Tuning nanoparticle uptake: Live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012, 12, 3417–3423. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Targeting of porous hybrid silica nanoparticles to Cancer Cells. ACS Nano 2009, 3, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Z.; Zink, J.I.; Tamanoi, F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: Enhanced efficacy by folate modification. Nanomedicine 2012, 8, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Wu, L.-C.; Lu, S.-Y.; Chang, L.-L.; Teng, I.T.; Yang, C.-M.; Ho, J.-A.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Lamers, G.E.M.; Morrhayim, J.; Chatzopoulou, A.; Schaaf, M.; den Dulk, H.; Backendorf, C.; Zink, J.I.; Kros, A. Folic acid-modified mesoporous silica nanoparticles for cellular and nuclear targeted drug delivery. Adv. Healthc. Mater. 2013, 2, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Taylor-Pashow, K.M.L.; Huxford, R.C.; Della Rocca, J.; Okoruwa, C.; An, H.; Lin, W.; Lin, W. Multifunctional mesoporous silica nanospheres with cleavable Gd(III) chelates as MRI contrast agents: Synthesis, characterization, target-specificity, and renal clearance. Small 2011, 7, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Selective topotecan delivery to cancer cells by targeted pH-sensitive mesoporous silica nanoparticles. RSC Adv. 2016, 6, 50923–50932. [Google Scholar] [CrossRef]
- López, V.; Villegas, M.R.; Rodríguez, V.; Villaverde, G.; Lozano, D.; Baeza, A.; Vallet-Regí, M. Janus Mesoporous Silica Nanoparticles for Dual Targeting of Tumor Cells and Mitochondria. ACS Appl. Mater. Interfaces 2017, 9, 26697–26706. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, K.; Huang, S.; Ju, E.; Liu, Z.; Yin, M.; Ren, J.; Qu, X. A smart nanoassembly for multistage targeted drug delivery and magnetic resonance imaging. Adv. Funct. Mater. 2014, 24, 3612–3620. [Google Scholar] [CrossRef]
- Pan, L.; Liu, J.; He, Q.; Wang, L.; Shi, J. Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials 2013, 34, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Milgroom, A.; Intrator, M.; Madhavan, K.; Mazzaro, L.; Shandas, R.; Liu, B.; Park, D. Mesoporous silica nanoparticles as a breast-cancer targeting ultrasound contrast agent. Colloids Surf. B 2014, 116, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-P.; Chen, C.-Y.; Hung, Y.; Chang, F.-H.; Mou, C.-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Deng, Z.; Zhen, Z.; Hu, X.; Wu, S.; Xu, Z.; Chu, P.K. Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials 2011, 32, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Blumen, S.R.; MacPherson, M.B.; Steinbacher, J.L.; Mossman, B.T.; Landry, C.C. Enhanced uptake of porous silica microparticles by bifunctional surface modification with a targeting antibody and a biocompatible polymer. ACS Appl. Mater. Interfaces 2010, 2, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radio labeled mesoporous silica nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Baeza, A.; Melen, G.J.; Alfranca, A.; Ramírez, M.; Vallet-Regí, M. A new targeting agent for the selective drug delivery of nanocarriers for treating neuroblastoma. J. Mater. Chem. B 2015, 3, 4831–4842. [Google Scholar] [CrossRef]
- Villaverde, G.; Nairi, V.; Baeza, A.; Vallet-Regí, M. Double sequential encrypted targeting sequence: A new concept for bone cancer treatment. Chem. Eur. J. 2017, 23, 7174–7179. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2017, 65, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Lee, C.-H.; Chen, M.-C.; Souris, J.S.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Chen, C.-T.; Lo, L.-W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics—The trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157. [Google Scholar] [CrossRef]
- Fang, I.J.; Slowing, I.I.; Wu, K.C.W.; Lin, V.S.Y.; Trewyn, B.G. Ligand conformation dictates membrane and endosomal trafficking of arginine-glycine-aspartate (RGD)-functionalized mesoporous silica nanoparticles. Chemistry 2012, 18, 7787–7792. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.-F.; Chen, W.-H.; Liu, Y.; Zhang, J.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Charge-reversal plug gate nanovalves on peptide-functionalized mesoporous silica nanoparticles for targeted drug delivery. J. Mater. Chem. B 2013, 1, 5723–5732. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Z.-F.; Wang, Y.; Chen, W.-H.; Luo, G.-F.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Multifunctional envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J. Am. Chem. Soc. 2013, 135, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Jia, H.-Z.; Zhang, J.; Liu, C.-W.; Zhuo, R.-X.; Zhang, X.-Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small 2014, 10, 591–598. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, Y.; Zhu, H.; Pang, G.; Zheng, W.; Wong, Y.-S.; Chen, T. Cancer-targeted monodisperse mesoporous silica nanoparticles as carrier of ruthenium polypyridyl complexes to enhance theranostic effects. Adv. Funct. Mater. 2014, 24, 2754–2763. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, F.; Li, Y.; Xu, M.; Li, L.; Wu, C.; Miyoshi, H.; Liu, Y. VCAM-1-targeted core/shell nanoparticles for selective adhesion and delivery to endothelial cells with lipopolysaccharide-induced inflammation under shear flow and cellular magnetic resonance imaging in vitro. Int. J. Nanomed. 2013, 8, 1897–1906. [Google Scholar]
- Goel, S.; Chen, F.; Hong, H.; Valdovinos, H.F.; Hernandez, R.; Shi, S.; Barnhart, T.E.; Cai, W. VEGF(121)-Conjugated Mesoporous Silica Nanoparticle: A Tumor Targeted Drug Delivery System. ACS Appl. Mater. Interfaces 2014, 6, 21677–21685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Y.; Lv, W.; Wang, Z.; Lv, L.; Wang, B.; Liu, X.; Liu, Y.; Hu, Q.; Sun, W.; et al. Dual Targeted Nanocarrier for Brain Ischemic Stroke Treatment. J. Control. Release 2016, 233, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, H.; Jia, X.; Lu, W.-L.; Lou, J.; Wei, Y. A Dual targeting Nanocarrier Based on Poly(amidoamine) Dendrimers Conjugated with Transferrin and Tamoxifen for Treating Brain Gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yang, H.; Zhang, T.; Li, Y.; Li, N.; Tang, B. Dual targeted Nanocarrier Based on Cell Surface Receptor and Intracellular mRNA: An Effective Strategy for Cancer Cell Imaging and Therapy. Anal. Chem. 2013, 85, 6930–6935. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.-S.; Ding, H.-M.; Ma, Y.-Q. Can Dual Targeting Enhance Cellular Uptake of Nanoparticles? Nanoscale 2017, 9, 8982–8989. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Hybrid Collagenase Nanocapsules for Enhanced Nanocarrier Penetration in Tumoral Tissues. ACS Appl. Mater. Interfaces 2015, 7, 24075–24081. [Google Scholar] [CrossRef] [PubMed]
- Vegh, I.; Grau, M.; Gracia, M.; Grande, J.; de la Torre, P.; Flores, A.I. Decidua mesenchymal stem cells migrated toward mammary tumors in vitro and in vivo affecting tumor growth and tumor development. Cancer Gene Ther. 2013, 20, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; de la Torre, P.; Manzano, M.; Cabañas, M.V.; Flores, A.I.; Vallet-Regí, M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Lu, X.; Yuan, Y.; Qian, J.; Zhou, H.; Lu, X.; Shi, J.; Liu, C. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011, 32, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lin, Y.; Wang, J.; Wu, L.; Wei, W.; Ren, J.; Qu, X. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host-guest interactions and switchable enzymatic activity and cellular effects of CeO2. Adv. Healthc. Mater. 2013, 2, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhou, X.; He, C.; Qiu, K.; Nie, W.; Chen, L.; Wang, H.; Mo, X.; Zhang, Y. Polyelectrolyte multilayer functionalized mesoporous silica nanoparticles for pH-responsive drug delivery: Layer thickness-dependent release profiles and biocompatibility. J. Mater. Chem. B 2013, 1, 5886–5898. [Google Scholar] [CrossRef]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.-L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, C.; Liu, X.; Ma, R.; Kong, D.; Shi, L. A multifunctional nanocarrier based on nanogated mesoporous silica for enhanced tumor-specific uptake and intracellular delivery. Macromol. Biosci. 2012, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Rim, H.P.; Min, K.H.; Lee, H.J.; Jeong, S.Y.; Lee, S.C. pH-tunable calcium phosphate covered mesoporous silica nanocontainers for intracellular controlled release of guest drugs. Angew. Chem. Int. Ed. 2011, 50, 8853–8857. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Lozano, D.; Vallet-Regí, M.; Manzano, M. Self-immolative polymers as novel pH-responsive gatekeepers for drug delivery. RSC Adv. 2017, 7, 132–136. [Google Scholar] [CrossRef]
- Zou, Z.; He, D.; He, X.; Wang, K.; Yang, X.; Qing, Z.; Zhou, Q. Natural Gelatin Capped Mesoporous Silica Nanoparticles for Intracellular Acid-Triggered Drug Delivery. Langmuir 2013, 29, 12804–12810. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nguyen, K.T.; Borah, P.; Ang, C.Y.; Zhao, Y. Functional silica nanoparticles for redox-triggered drug/ssDNA co-delivery. Adv. Healthc. Mater. 2012, 1, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niemela, M.; Westermarck, J.; Rosenholm, J.M. Mesoporous silica nanoparticles with redox-responsive surface linkers for charge-reversible loading and release of short oligonucleotides. Dalton Trans. 2014, 43, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.-Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Porta, F.; Planinsek, O.; Kros, A.; Gaberscek, M. Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: Smaller is better. Phys. Chem. Chem. Phys. 2013, 15, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Xua, J.-H.; Gao, F.-P.; Li, L.-L.; Ma, H.L.; Fan, Y.-S.; Liu, W.; Guo, S.-S.; Zhao, X.-Z.; Wang, H. Gelatin–mesoporous silica nanoparticles as matrix metalloproteinases-degradable drug delivery systems in vivo. Microporous Mesoporous Mater. 2015, 204, 226–234. [Google Scholar] [CrossRef]
- Agostini, A.; Mondragón, L.; Coll, C.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Pérez-Payá, E.; Amorós, P. Dual enzyme-triggered controlled release on capped nanometric silica mesoporous supports. ChemistryOpen 2012, 1, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, H.; Kim, S.; Kim, C. Enzyme responsive nanocontainers with cyclodextrin gatekeepers and synergistic effects in release of guests. J. Am. Chem. Soc. 2009, 131, 16614–16615. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Guisasola, E.; Torres-Pardo, A.; González-Calbet, J.M.; Melen, G.J.; Ramirez, M.; Vallet-Regí, M. Hybrid enzyme-polymeric capsules/mesoporous silica nanodevice for in situ cytotoxic agent generation. Adv. Funct. Mater. 2014, 24, 4625–4633. [Google Scholar] [CrossRef]
- Mas, N.; Arcos, D.; Polo, L.; Aznar, E.; Sánchez-Salcedo, S.; Sancenón, F.; García, A.; Marcos, M.D.; Baeza, A.; Vallet-Regí, M.; et al. Towards the development of smart 3D “gated scaffolds” for on-command delivery. Small 2014, 10, 4859–4864. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Villalonga, R.; Gimenez, C.; Sancenon, F.; Marcos, M.D.; Martinez-Mañez, R.; Diez, P.; Pingarrón, J.M.; Amoros, P. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Bernardos, A.; Martínez-Máñez, R.; Maquieira, A.; Marcos, M.D.; Pastor-Navarro, N.; Puchades, R.; Sancenón, F.; Soto, J.; Amorós, P. Controlled delivery systems using antibody-capped mesoporous nanocontainers. J. Am. Chem. Soc. 2009, 131, 14075–14080. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhao, Y.; He, D.; Wang, K.; Xu, F.; Tang, J. ATP-responsive controlled release system using aptamer-functionalized mesoporous silica nanoparticles. Langmuir 2012, 28, 12909–12915. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Haefeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Boissiere, C.; Grosso, D.; Chaumonnot, A.; Nicole, L.; Sanchez, C. Aerosol route to functional nanostructured inorganic and hybrid porous materials. Adv. Mater. 2011, 23, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Fal-Miyar, V.; Ruiz-Hernández, E.; García-Hernández, M.; Ruiz-González, M.L.; González-Calbet, J.; Vallet-Regí, M. Supramolecular mechanisms in the synthesis of mesoporous magnetic nanospheres for hyperthermia. J. Mater. Chem. 2012, 22, 64–72. [Google Scholar] [CrossRef]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; de la Fuente, J.M.; Vallet-Regí, M. Magnetic-Responsive Release Controlled by Hot Spot Effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef] [PubMed]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Vallet-Regí, M. Design of thermoresponsive polymeric gates with opposite controlled release behaviors. RSC Adv. 2016, 6, 42510–42516. [Google Scholar] [CrossRef]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pelletier, M.; Zhang, H.J.; Xia, H.S.; Zhao, Y. High-frequency ultrasound-responsive block copolymer micelle. Langmuir 2009, 25, 13201–13205. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Boissière, O.; Zhao, Y.; Yan, B.; Tremblay, L.; Lacelle, S.; Xia, H.; Zhao, Y. Advanced stimuli-responsive polymer nanocapsules with enhanced capabilities for payloads delivery. Langmuir 2012, 28, 16463–16468. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; de la Torre, P.; Cabañas, M.V.; Manzano, M.; Grau, M.; Flores, A.I.; Vallet-Regí, M. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 2017, 4, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature 2003, 421, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Zhao, Y.-L.; Khashab, N.M.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. Light-operated mechanized nanoparticles. J. Am. Chem. Soc. 2009, 131, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Boudjelal, M.; Kang, S. Ultraviolet irradiation of human skin causes functional vitamin A deficiency, preventable by all-trans retinoic acid pre-treatment. Nat. Med. 1999, 5, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Traber, M.G.; Weber, C.; Yan, L.J.; Packer, L. UV-irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic. Biol. Med. 1998, 24, 55–65. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Packer, L. Antioxidant defense mechanisms in murine epidermis and dermis and their responses to ultraviolet light. J. Investig. Dermatol. 1993, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, J.; Carling, C.J.; Almutairi, A. Photocontrolled release using one-photon absorption of visible or NIR light. J. Control. Release 2015, 219, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Lozano, D.; Baeza, A.; Colilla, M.; Vallet-Regí, M. A novel visible light responsive nanosystem for cancer treatment. Nanoscale 2017, 9, 15967–15973. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M.; Vallet-Regí, M. Tuning mesoporous silica dissolution in physiological environments: A review. J. Mater. Sci. 2017, 52, 8761–8771. [Google Scholar] [CrossRef]
- Choi, E.; Kim, S. How Can Doxorubicin Loading Orchestrate in Vitro Degradation Behaviors of Mesoporous Silica Nanoparticles under a Physiological Condition? Langmuir 2017, 33, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L. Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed.; HarperCollins College Publishers: New York, NY, USA, 1993; Appendix E. [Google Scholar]
- Bunker, B.C. Molecular Mechanisms for Corrosion of Silica and Silicate Glasses. J. Non-Cryst. Solids 1994, 179, 300–308. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry; Wiley-Interscience: Hoboken, NJ, USA, 1979. [Google Scholar]
- Popplewell, J.F.; King, S.J.; Day, J.P.; Ackrill, P.; Fifield, L.K.; Cresswell, R.G.; Di Tada, M.L.; Liu, K. Kinetics of Uptake and Elimination of Silicic Acid by a Human Subject: A Novel Application of Si-32 and Accelerator Mass Spectrometry. J. Inorg. Biochem. 1998, 69, 177–180. [Google Scholar] [CrossRef]
- Braun, K.; Pochert, A.; Beck, M.; Fiedler, R.; Gruber, J.; Lindén, M. Dissolution kinetics of mesoporous silica nanoparticles in different simulated body fluids. J. Sol-Gel Sci. Technol. 2016, 79, 319–327. [Google Scholar] [CrossRef]
- Yamada, H.; Urata, C.; Aoyama, Y.; Osada, S.; Yamauchi, Y.; Kuroda, K. Preparation of colloidal mesoporous silica nanoparticles with different diameters and their unique degradation behavior in static aqueous systems. Chem. Mater. 2012, 24, 1462–1471. [Google Scholar] [CrossRef]
- Hao, N.; Liu, H.; Li, L.; Chen, D.; Li, L.; Tang, F. In vitro degradation behavior of silica nanoparticles under physiological conditions. J. Nanosci. Nanotechnol. 2012, 12, 6346–6354. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Young, N.P.; Townley, H.E. Characterization and comparison of mesoporous silica particles for optimized drug delivery. Nanomater. Nanotechnol. 2014, 4, 21. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Dong, X.; Liang, J.; Shi, J. Preparation of mesoporous calcium doped silica spheres with narrow size dispersion and their drug loading and degradation behavior. Microporous Mesoporous Mater. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- Mitchell, K.K.P.; Liberman, A.; Kummel, A.C.; Trogler, W.C. Iron(III)-Doped, Silica Nanoshells: A Biodegradable Form of Silica. J. Am. Chem. Soc. 2012, 134, 13997–14003. [Google Scholar] [CrossRef] [PubMed]
- Fontecave, T.; Sanchez, C.; Azaïs, T.; Boissière, C. Chemical modification as a versatile tool for tuning stability of silica based mesoporous carriers in biologically relevant conditions. Chem. Mater. 2012, 24, 4326–4336. [Google Scholar] [CrossRef]
- Cauda, V.; Argyo, C.; Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 8693–8699. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo Biodistribution and Urinary Excretion of Mesoporous Silica Nanoparticles: Effects of Particle Size and PEGylation. Small 2011, 17, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Löffler, B. CLINAM summit May 7–10, 2017. Eur. J. Nanomed. 2017, 9, 45. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- BCC Research. Nanotechnology in Medical Applications: The Global Market; BCC Research: Wellesley, MA, USA, 2015. [Google Scholar]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Frangioni, J.V. Nanoparticles for Biomedical Imaging: Fundamentals of Clinical Translation. Mol. Imaging 2010, 9, 291–310. [Google Scholar] [PubMed]

| Targeting Ligand a | Tumor Cell Receptor b | Target Cell Line c | Ref. |
| Tf | TfR | PANC-1, BT-549 | [34] |
| Tf | TfR | HeLa | [35] |
| Tf | TfR | HT1080 | [36] |
| EGF | EGFR | HuH-7 | [37] |
| FA | FAR (FR-α) | Hela, PANC, U2Os, MDA-MB-231, SK-BR-3, MiaPaca-2, LnCAP | [38,39,40,41,42,43,44,45,46] |
| Methotrexate | FR-α | HeLa | [47] |
| Anisamide | Sigma receptor | ASPC-1 | [44] |
| TAT peptides | Importing α and β receptors | Hela; MCF-7/ADR | [48,49,50] |
| IL-13 peptide | IL-13Rα2 | U251 | [51] |
| Anti-herceptin | HER2 | SK-BR3 | [52] |
| Anti-HER2/neu | HER2/neu | BT474 | [53] |
| Anti-ErbB2 | ErbB2 | MCF-7 | [54] |
| Anti-ME1 | Mesothelin | MM | [55] |
| Anti-TRC105 | CD105/endoglin | HUVECs | [56] |
| MABG | NET | NB1691-luc | [57] |
| RGD-type peptide (RDGRC) | NRP-1 | HOS | [58] |
| ConA | SA | HOS | [59] |
| HA | CD44 | MCF-7, MDA-MB-231, 4T1 | [60] |
| Targeting Ligand a | Tumor Blood Vessel Receptor b | Target Cell Line c | Ref. |
| c(RGDyK) | ανβ3 integrins | U87-MG | [61] |
| cRGD | ανβ3 integrins | MDA-MB 435 | [34] |
| K7RGD; c-RGDFK | ανβ3 integrins | HeLa | [62] |
| K8(RGD)2 | ανβ3 integrins | U87-MG | [63] |
| N3GPLGRGRGDK-Ad | ανβ3 integrins | SCC-7, HT-29 | [61] |
| N3RGDFFFFC | ανβ3 integrins | U87-MG | [64] |
| Thiolated-RGD | ανβ3 integrins | A375, HepG2, MCF-7, Neuro-2a | [66] |
| Anti-(VCAM-1) | (VCAM-1)R | HUVEC-CS | [67] |
| VEGF | VEGFR | U87-MG | [68] |
| Stimulus | Responsive Linker | Blocking Cap | Ref. |
|---|---|---|---|
| pH | Acetal linker | Au NPs | [78] |
| pH | Boronate ester | Fe3O4 NPs | [79] |
| pH | Ferrocenyl moieties | β-CD-modified CeO2 NPs | [80] |
| pH | PAH-PSS PEM | PAH-PSS PEM | [81] |
| pH | Aromatic amines | CDs | [82] |
| pH | Benzoic-imine bonds | Polypseudorotaxanes | [83] |
| pH | CaP soluble at acid pH | CaP coating | [84] |
| pH | Self-immolative polymer | Self-immolative polymer | [85] |
| pH | Gelatin | Gelatin coating | [45,86] |
| pH | 3,9-Bis(3-aminopropyl)-2,4,8,10-tetraoxaspiro [5.5] undecane (ATU) | Poly(acrylic acid) PAA | [59] |
| Redox potential | —S—S— | ssDNA | [87] |
| Redox potential | —S—S— | PEG | [88] |
| Redox potential | —S—S— | CdS NPs | [89] |
| Redox potential | —S—S— | PPI dendrimer | [90] |
| Enzymes | MMP-degradable gelatin | Gelatin coating | [91] |
| Enzymes | β-galactosidase-cleavable oligosaccharide | β-galacto-oligosaccharide | [92] |
| Enzymes | MMP9-sensitive peptide sequence (RSWMGLP) | Avidin | [93] |
| Enzymes | Protease-sensitive peptide sequences (CGPQGIWGQGCR) | PNIPAm-PEGDA shell | [94] |
| Enzymes | α-amylase and lipase cleavable stalks | CDs | [95] |
| Enzymes | HRP-polymer nanocapsule | - | [96] |
| Enzymes | Phosphate-phosphate APasa-hydrolizable bonds | ATP | [97] |
| Small molecules | Ionizable benzimidazole group | CD-modified glucose oxidase | [98] |
| Small molecules | pAb | pAb | [99] |
| Small molecules | ATP aptamer | ATP aptamer | [100] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. https://doi.org/10.3390/molecules23010047
Vallet-Regí M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules. 2018; 23(1):47. https://doi.org/10.3390/molecules23010047
Chicago/Turabian StyleVallet-Regí, María, Montserrat Colilla, Isabel Izquierdo-Barba, and Miguel Manzano. 2018. "Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights" Molecules 23, no. 1: 47. https://doi.org/10.3390/molecules23010047
APA StyleVallet-Regí, M., Colilla, M., Izquierdo-Barba, I., & Manzano, M. (2018). Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules, 23(1), 47. https://doi.org/10.3390/molecules23010047








