Honokiol Improves Liver Steatosis in Ovariectomized Mice
Abstract
:1. Introduction
2. Results
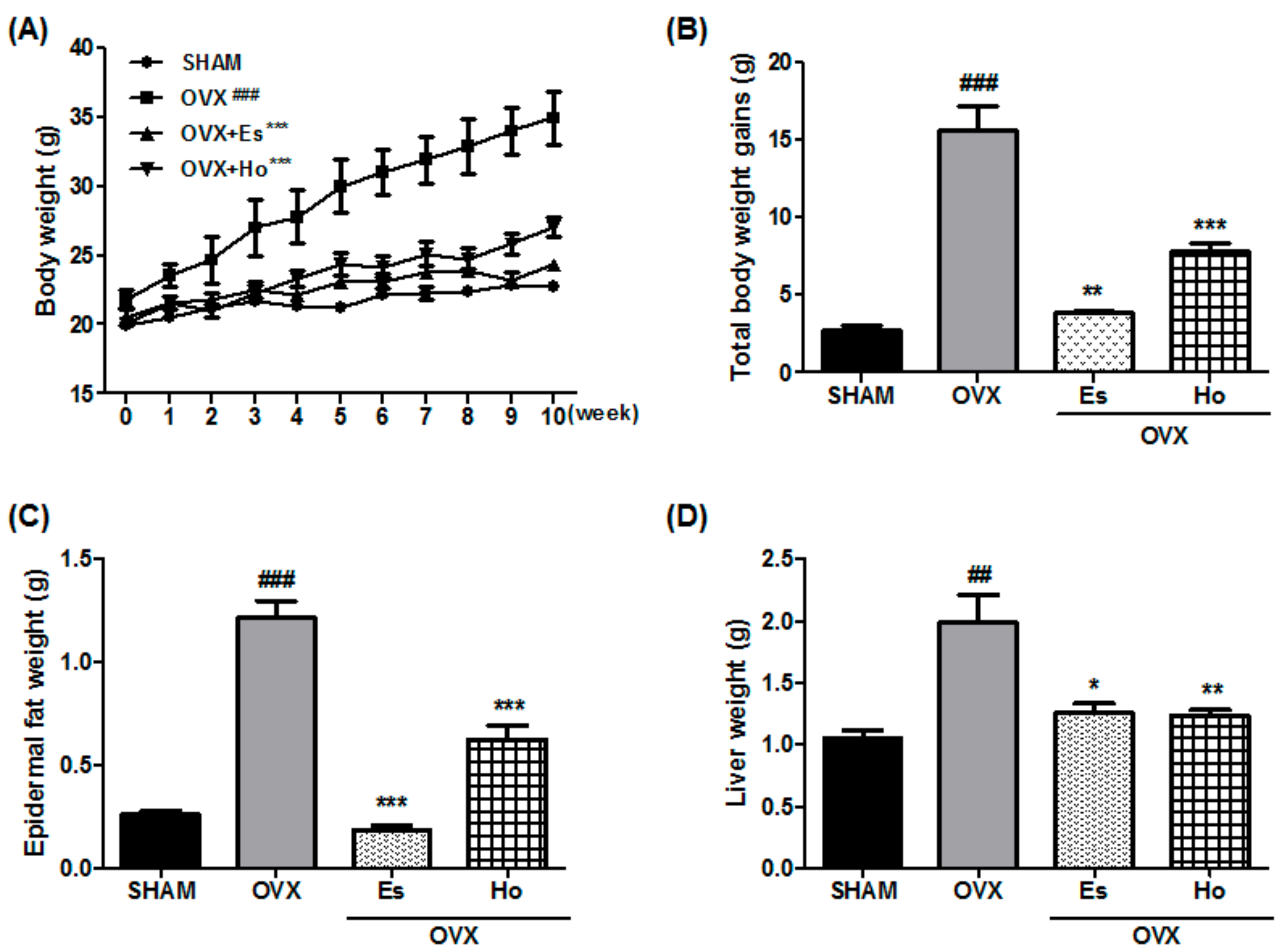
2.1. Ho Reduces Bodyweight, Epidermal Fat, and Liver Weight in OVX Mice
2.2. Ho Regulates Lipid Metabolism and Reduces Liver Injury Markers in OVX Mice
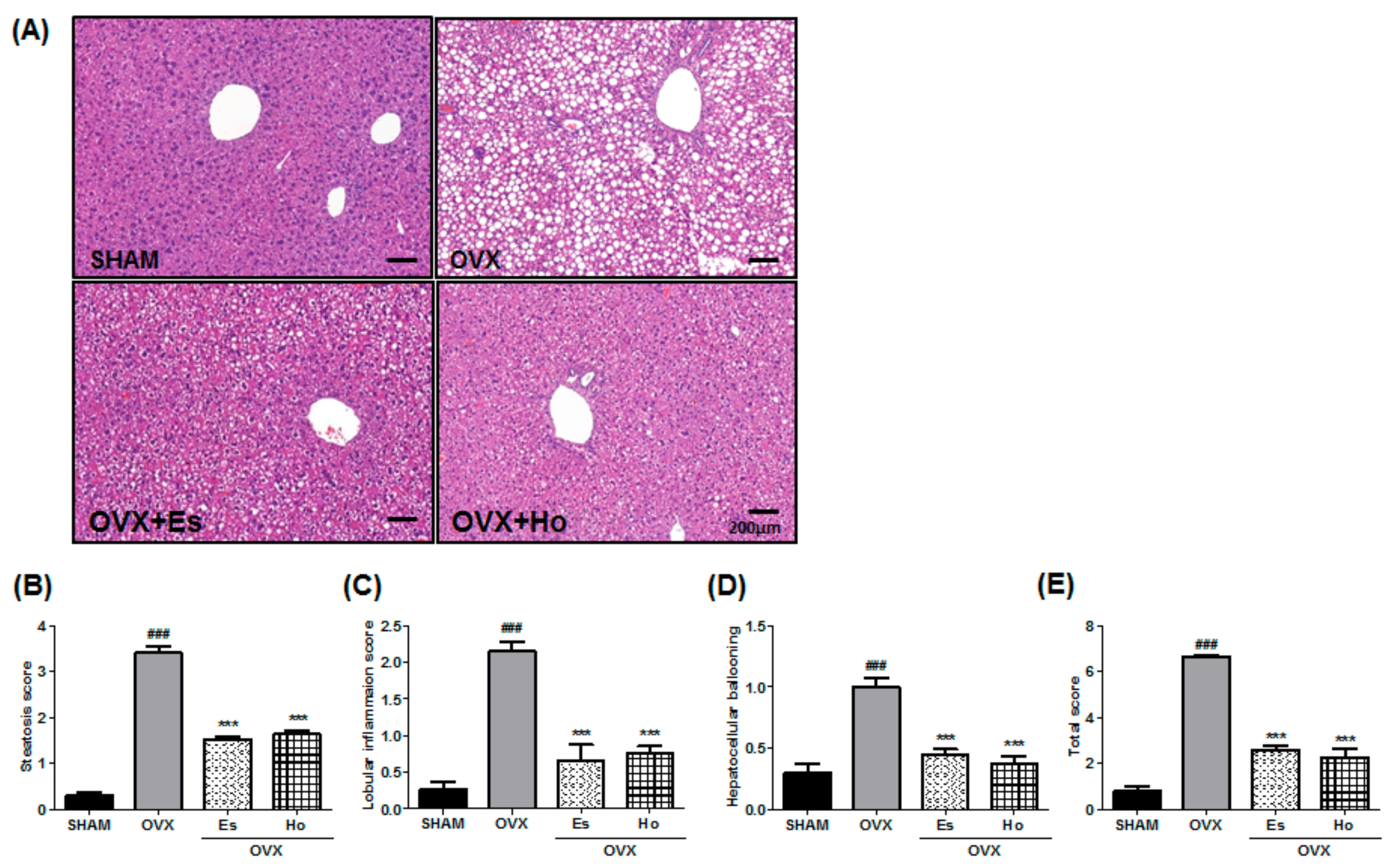
2.3. Ho Inhibits Hepatic Steatosis in OVX Mice
2.4. Ho Inhibits Hepatic Inflammatory Gene Expression in OVX Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Serum Lipid Analysis
4.3. Histological Analysis
4.4. Analysis of Gene Expression in Liver Samples by RT-PCR
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medic-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Kiso, S.; Yoshida, Y.; Chatani, N.; Kizu, T.; Hamano, M.; Tsubakio, M.; Takemura, T.; Ezaki, H.; Hayashi, N.; et al. Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1031–G1043. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M. Nonalcoholic steatohepatitis: Definition and pathology. Semin. Liver Dis. 2001, 21, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Ley, C.J.; Lees, B.; Stevenson, J.C. Sex- and menopause-associated changes in body-fat distribution. Am. J. Clin. Nutr. 1992, 55, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Abdelmalek, M.F. Nonalcoholic fatty liver disease in women. Womens Health (Lond.) 2009, 5, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.H.; Perfield, J.W., 2nd; Strissel, K.J.; Obin, M.S.; Greenberg, A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009, 150, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Kubo, M.; Liu, Z.; Chu, P.; Wang, C.; Yuan, Y.C.; Chen, S. Protective effects of white button mushroom (Agaricus bisporus) against hepatic steatosis in ovariectomized mice as a model of postmenopausal women. PLoS ONE 2011, 6, e26654. [Google Scholar] [CrossRef] [PubMed]
- Esumi, T.; Makado, G.; Zhai, H.; Shimizu, Y.; Mitsumoto, Y.; Fukuyama, Y. Efficient synthesis and structure-activity relationship of honokiol, a neurotrophic biphenyl-type neolignan. Bioorg. Med. Chem. Lett. 2004, 14, 2621–2625. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.S.; Huang, L.S.; Sok, D.E.; Shin, J.; Kwon, B.M.; Youn, U.J.; Bae, K. Protective action of honokiol, administered orally, against oxidative stress in brain of mice challenged with nmda. Phytomedicine 2007, 14, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Liao, P.C.; Ho, C.L.; Wang, E.I.; Chuang, C.C.; Chiu, H.W.; Hung, L.B.; Hua, K.F. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase c, mitogen-activated protein kinase, and the NF-κB pathway to reduce LPS-induced tnfalpha and no expression. J. Agric. Food Chem. 2010, 58, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Arbiser, J.L.; Bishop, G.A. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J. Immunol. 2007, 179, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Honokiol activates the LKB1-AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol. Appl. Pharmacol. 2015, 284, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, H. Honokiol attenuates diet-induced nonalcoholic steatohepatitis by regulating macrophage polarization through activating ppargamma. J. Gastroenterol. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Packirisamy, R.M.; Bobby, Z.; Elizabeth Jacob, S.; Sridhar, M.G. Soy isoflavones (Glycine max) ameliorate hypertriglyceridemia and hepatic steatosis in high fat-fed ovariectomized wistar rats (an experimental model of postmenopausal obesity). J. Nutr. Biochem. 2016, 38, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Toda, K.; Ono, M.; Fujikawa-Adachi, K.; Saibara, T.; Onishi, S.; Enzan, H.; Okada, T.; Shizuta, Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J. Clin. Investig. 2000, 105, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Murata, Y.; Rochira, V.; Tubert, G.; Aranda, C.; Vazquez, M.; Clyne, C.D.; Davis, S.; Simpson, E.R.; Carani, C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: Effects of testosterone, alendronate, and estradiol treatment. J. Clin. Endocrinol. Metab. 2004, 89, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Ishigami, M.; Achiwa, K.; Ishizu, Y.; Kuzuya, T.; Honda, T.; Hayashi, K.; Ishikawa, T.; Katano, Y.; Goto, H. Raloxifene ameliorates liver fibrosis of nonalcoholic steatohepatitis induced by choline-deficient high-fat diet in ovariectomized mice. Dig. Dis. Sci. 2015, 60, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Chiu, Y.S.; Chiu, W.C.; Tung, Y.T.; Chuang, H.L.; Wu, J.H.; Huang, C.C. Proteomics analysis to identify and characterize the molecular signatures of hepatic steatosis in ovariectomized rats as a model of postmenopausal status. Nutrients 2015, 7, 8752–8766. [Google Scholar] [CrossRef] [PubMed]
- Pappachan, J.M.; Babu, S.; Krishnan, B.; Ravindran, N.C. Non-alcoholic fatty liver disease: A clinical update. J. Clin. Transl. Hepatol. 2017, 5, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Guo, M.H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef] [PubMed]
- Elshazly, S.M. Ameliorative effect of nicorandil on high fat diet induced non-alcoholic fatty liver disease in rats. Eur. J. Pharmacol. 2015, 748, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in nash pathogenesis. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.T.; Park, J.H.; Kim, H.J.; Kim, M.S.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Dandelion leaf extract protects against liver injury induced by methionine- and choline-deficient diet in mice. J. Med. Food. 2013, 16, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Benedusi, V.; Meda, C.; Della Torre, S.; Monteleone, G.; Vegeto, E.; Maggi, A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology 2012, 153, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen signaling in metabolic inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.; Halici, Z.; Cadirci, E.; Albayrak, A.; Karakus, E.; Bayir, Y.; Bilen, H.; Sahin, A.; Yuksel, T.N. The effect of alpha-lipoic acid in ovariectomy and inflammation-mediated osteoporosis on the skeletal status of rat bone. Eur. J. Pharmacol. 2013, 718, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H. The role of cytokines in non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. 2012, 18, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, S.; Kandel-Kfir, M.; Shaish, A.; Almog, T.; Shemesh, S.; Barshack, I.; Apte, R.N.; Harats, D.; Kamari, Y. Lack of interleukin-1alpha in kupffer cells attenuates liver inflammation and expression of inflammatory cytokines in hypercholesterolaemic mice. Dig. Liver Dis. 2014, 46, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y.; Huang, J.; Wang, H.Y.; Du, X.W.; Cheng, S.M.; Han, Y.; Wang, L.F.; Li, G.Y.; Wang, J.H. Protective effect of zhuyeqing liquor, a chinese traditional health liquor, on acute alcohol-induced liver injury in mice. J. Inflamm. 2013, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.; Nitta, H.; Takahara, T.; Hasegawa, Y.; Itou, N.; Wakabayashi, G. Effect of preoperative chemotherapy on postoperative liver regeneration following hepatic resection as estimated by liver volume. World J. Surg. Oncol. 2013, 11, 65. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| SHAM | OVX | OVX+Es | OVX+Ho | |
|---|---|---|---|---|
| TC (mg/dL) | 127.4 ± 22.1 | 197.7 ± 16.9 # | 191.5 ± 10.1 | 141.1 ± 14.1 * |
| HDL-C (mg/dL) | 93.3 ± 21.6 | 75.6 ± 4.4 | 95.6 ± 8.9 | 75.8 ± 10.8 |
| LDL-C (mg/dL) | 23.1 ± 2.3 | 103.6 ± 13.4 ## | 80.2 ± 7.7 | 65.2 ± 7.2 * |
| TG (mg/dL) | 64.2 ± 6.3 | 88.0 ± 7.7 | 70.9 ± 6.0 | 56.22 ± 4.3 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.-H.; Hur, H.J.; Jeon, E.-J.; Park, S.-J.; Hwang, J.T.; Lee, A.S.; Lee, K.W.; Sung, M.J. Honokiol Improves Liver Steatosis in Ovariectomized Mice. Molecules 2018, 23, 194. https://doi.org/10.3390/molecules23010194
Jeong Y-H, Hur HJ, Jeon E-J, Park S-J, Hwang JT, Lee AS, Lee KW, Sung MJ. Honokiol Improves Liver Steatosis in Ovariectomized Mice. Molecules. 2018; 23(1):194. https://doi.org/10.3390/molecules23010194
Chicago/Turabian StyleJeong, Yeon-Hui, Haeng Jeon Hur, Eun-Joo Jeon, Su-Jin Park, Jin Taek Hwang, Ae Sin Lee, Kyong Won Lee, and Mi Jeong Sung. 2018. "Honokiol Improves Liver Steatosis in Ovariectomized Mice" Molecules 23, no. 1: 194. https://doi.org/10.3390/molecules23010194
APA StyleJeong, Y.-H., Hur, H. J., Jeon, E.-J., Park, S.-J., Hwang, J. T., Lee, A. S., Lee, K. W., & Sung, M. J. (2018). Honokiol Improves Liver Steatosis in Ovariectomized Mice. Molecules, 23(1), 194. https://doi.org/10.3390/molecules23010194





