Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection
Abstract
:1. Introduction
2. Normal Function of Mitochondria
3. Changes in Mitochondrial Dynamics and Mitophagy in Senescence
3.1. Dynamics
3.2. Mitophagy
4. Peroxysome Proliferator-Activated Receptor-Gamma (PPARγ) Coactivator 1-alpha (PGC-1α)
5. The Role of Reactive Oxygen Species (ROS)
6. Ageing
7. The Role of Free Radicals in Ageing
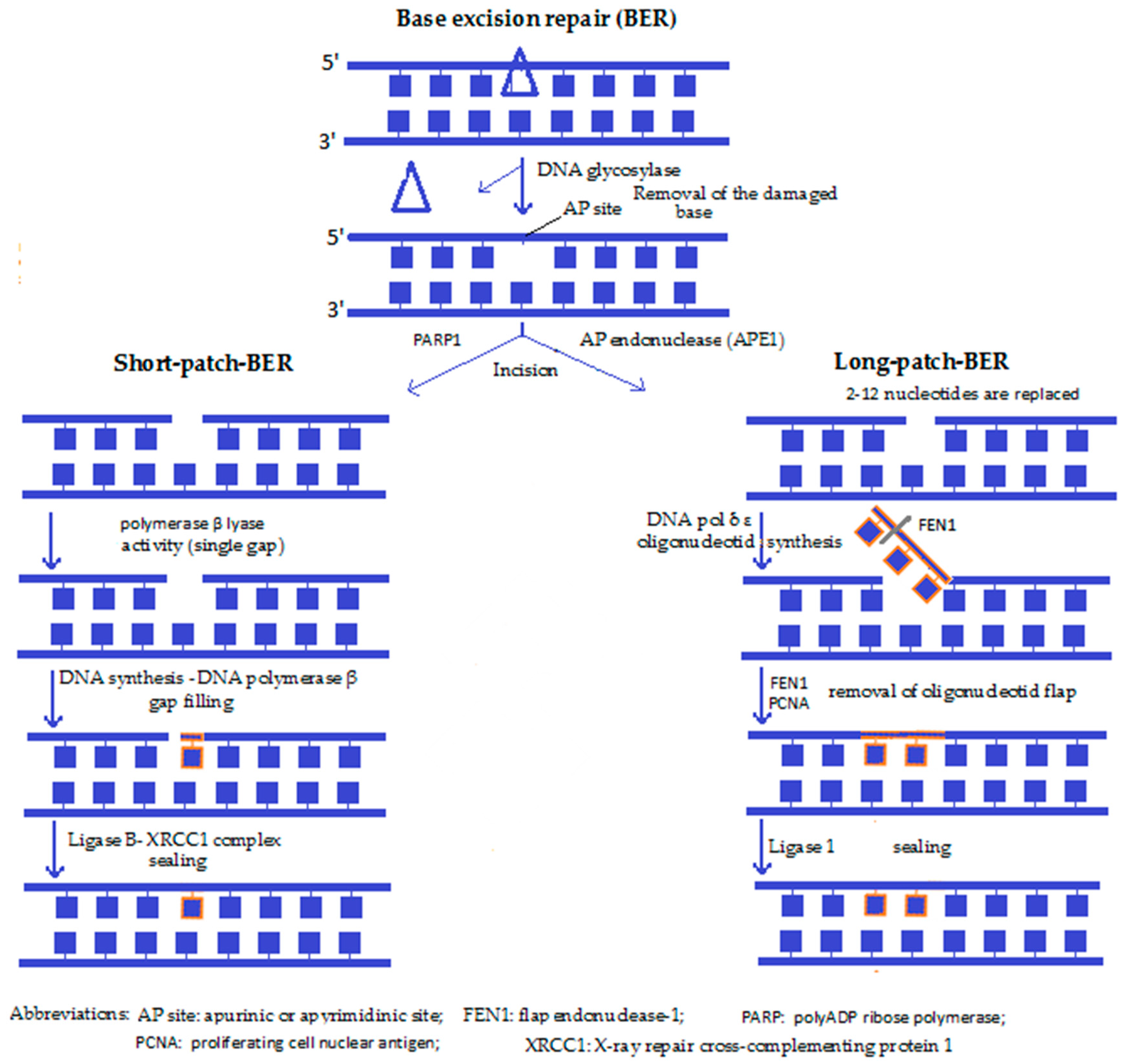
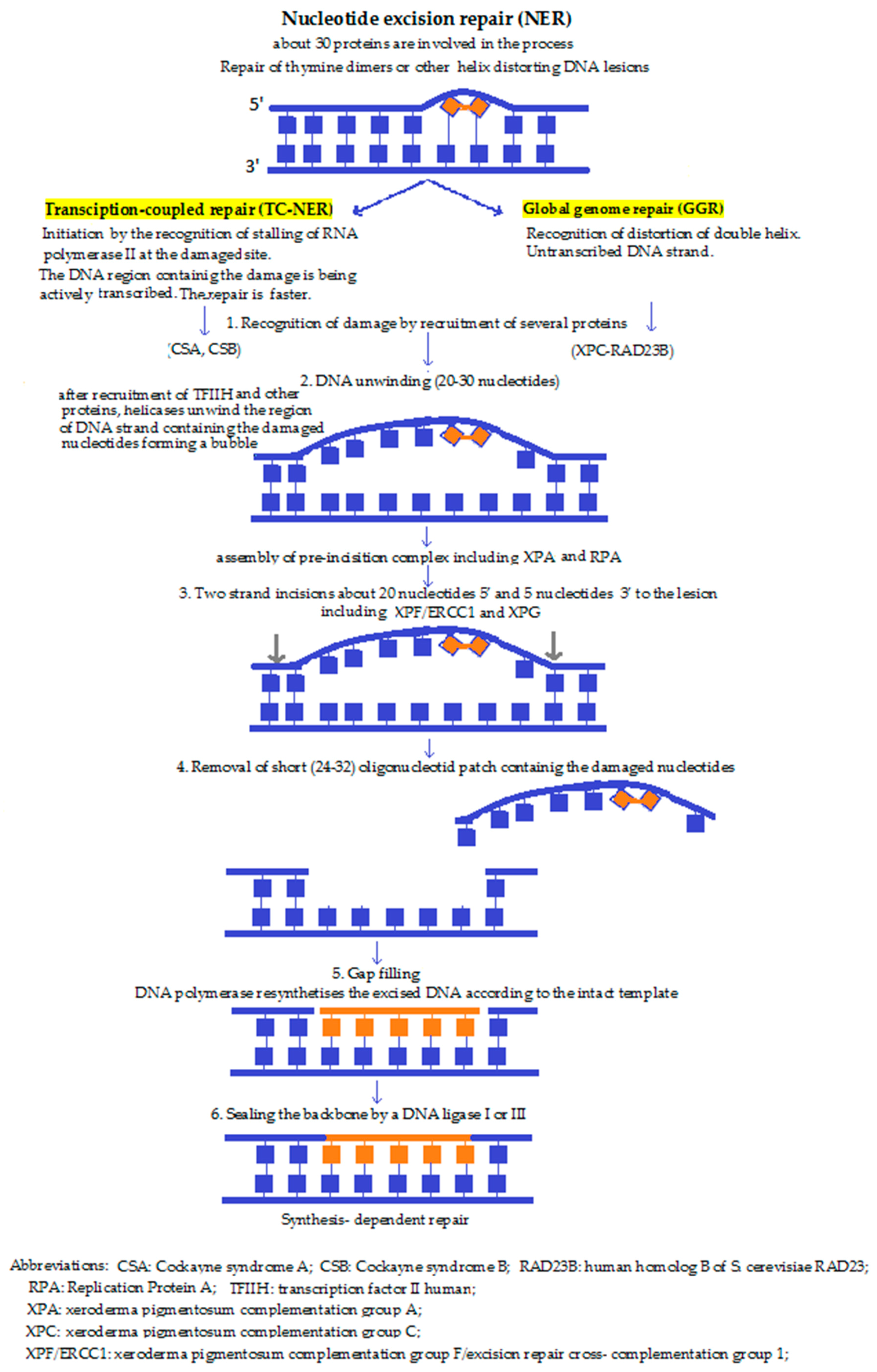
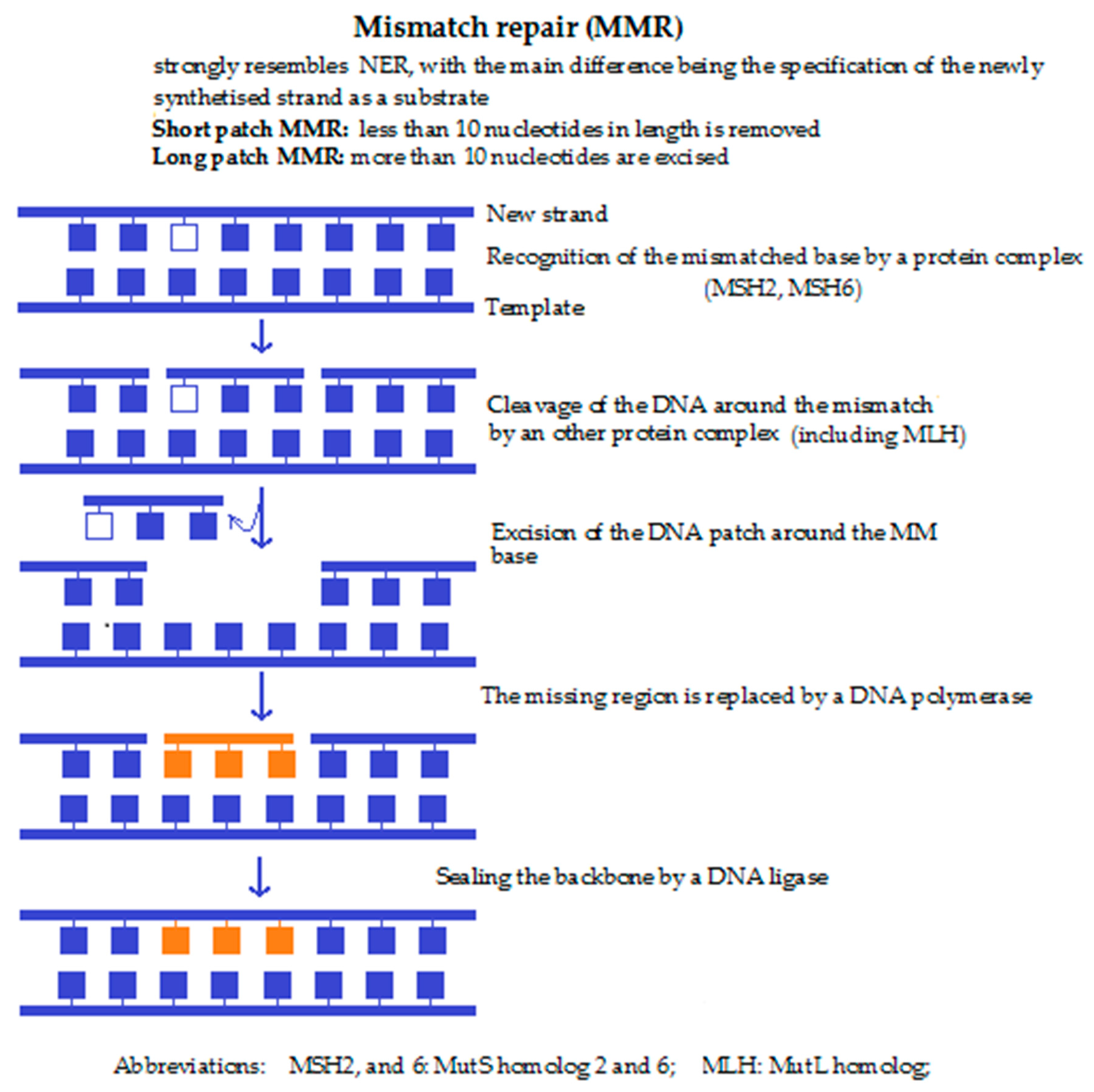
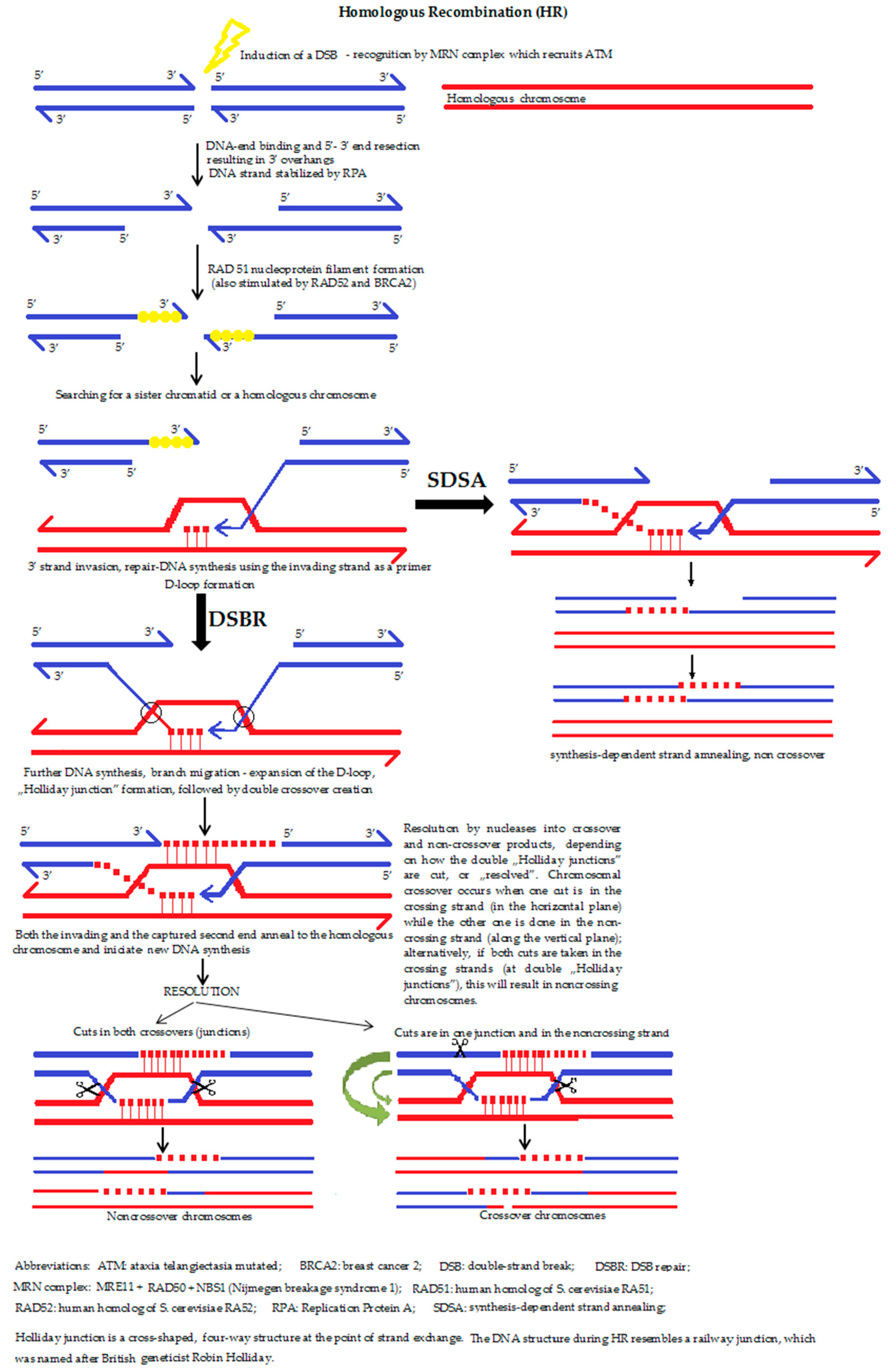
8. Alterations in the Genome of Cells in Senescence
9. PARP
10. Kynurenines in Ageing
11. Kynurenine (KYN) Pathway
12. KYNA
13. 3-OH-KYN and 3-OH-AA
14. QUIN
15. XA and Cinnabarinic Acid
16. The Role of Kynurenine Metabolites in Immunoregulation
17. Kynurenines during Ageing
18. Diabetes
19. Cardiovascular Diseases
20. Cancer
21. Depression
22. Neurodegenerative Diseases
23. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-OH-AA | 3-hydroxyanthranilic acid |
| 3-OH-AAO | 3-hydroxyanthranilic acid oxygenase |
| 3-OH-KYN | 3-hydroxy-l-kynurenine |
| 5-OH-TRP | 5-hydroxy-tryptophan |
| 5-hydroxy tryptamine | serotonin |
| AA | anthranilic acid |
| acetyl-CoA | acetyl-coenzyme A |
| AD | Alzheimer’s disease |
| AhR | aryl hydrocarbon receptor |
| BER | base excision repair |
| CSF | cerebrospinal fluid |
| DSB | double strand break |
| ETC | electron transport chain |
| HD | Huntington’s disease |
| HR | homologous recombination |
| IDO | indolamine-2,3-dioxygenases |
| IFN-α | interferon-α |
| IL1 | interleukin-1 |
| iNKT | invariant natural killer T cell |
| KAT | kynurenine aminotransferase |
| KMO | kynurenine-3-monooxygenase |
| KYNU | kynureninase |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| LPS | lipopolysaccharides |
| L-TRP | l-tryptophan |
| MMR | mismatch repair |
| NAD+ | nicotinamide-adenine dinucleotide |
| NMDA | N-methyl-d-aspartate |
| NMN | nicotinate mononucleotide |
| NER | nucleotide excision repair |
| NHEJ | non-homologous end joining |
| PA | picolinic acid |
| PD | Parkinson’s disease |
| PARG | poly(ADP-ribose) glycohydrolase |
| PARP | poly (ADP-ribose) polymerase |
| PGC-1α | oeroxisome proliferator-activated receptor-gamma (PPARγ) coactivator 1-alpha |
| QPRT | quinolinic acid phosphoribosyl transferase |
| QUIN | quinolinic acid |
| ROS | reactive oxygen species |
| SER | serotonin |
| SSB | single strand break |
| TDO | TRP-2,3-dioxygenase |
| TGFβ | transforming growth factor-β |
| TNF-α | tumour necrosis factor-α |
| TRP | tryptophan |
| XA | xanturenic acid |
References
- Filosto, M.; Scarpelli, M.; Cotelli, M.S.; Vielmi, V.; Todeschini, A.; Gregorelli, V.; Tonin, P.; Tomelleri, G.; Padovani, A. The role of mitochondria in neurodegenerative diseases. J. Neurol. 2011, 258, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E.; Hirschey, M.D.; Finley, L.W.S.; Haigis, M.C. Sirtuin regulation of mitochondria: Energy production, apoptosis, and signaling. Trends Biochem. Sci. 2010, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007, 257, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Szalárdy, L.; Zádori, D.; Klivényi, P.; Toldi, J.; Vécsei, L. Electron Transport Disturbances and Neurodegeneration: From Albert Szent-Györgyi’s Concept (Szeged) till Novel Approaches to Boost Mitochondrial Bioenergetics. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kaguni, L.S. DNA polymerase gamma, the mitochondrial replicase. Annu. Rev. Biochem. 2004, 73, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Browner, W.S.; Kahn, A.J.; Ziv, E.; Reiner, A.P.; Oshima, J.; Cawthon, R.M.; Hsueh, W.C.; Cummings, S.R. The genetics of human longevity. Am. J. Med. 2004, 117, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The maintenance of mitochondrial DNA integrity—Critical analysis and update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Hamann, A.; Osiewacz, H.D. Mitochondrial quality control: An integrated network of pathways. Trends Biochem. Sci. 2012, 37, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Csiszar, A.; Dutta, D.; Balagopal, G.; Calvani, R.; Leeuwenburgh, C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: From mechanisms to therapeutics. AJP Heart Circ. Physiol. 2013, 305, H459–H476. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A.; Suliman, H.B. Redox regulation of mitochondrial biogenesis. Free Radic. Biol. Med. 2012, 53, 2043–2053. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Reddy, T.P. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr. Alzheimer Res. 2011, 8, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, D.; Palacín, M.; Zorzano, A. Mitochondrial Dynamics: Coupling Mitochondrial Fitness with Healthy Aging. Trends Mol. Med. 2017, 23, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Perkins, G.; Schwarzenbacher, R.; Bossy-Wetzel, E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, M. Peroxisome Proliferator-Activated Receptor-gamma in Amyotrophic Lateral Sclerosis and Huntington’s Disease. PPAR Res. 2008, 2008, 418765. [Google Scholar] [CrossRef] [PubMed]
- Blair, I.A. DNA adducts with lipid peroxidation products. J. Biol. Chem. 2008, 283, 15545–15549. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 827, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Kowald, A.; Kirkwood, T.B. Accumulation of defective mitochondria through delayed degradation of damaged organelles and its possible role in the ageing of post-mitotic and dividing cells. J. Theor. Biol. 2000, 202, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Beal, M.F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995, 38, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.T.; Klann, E. Sources and Targets of Reactive Oxygen Species in Synaptic Plasticity and Memory. Antioxid. Redox Singal. 2007, 9, 233–244. [Google Scholar] [CrossRef]
- Liu, J.P. Molecular mechanisms of ageing and related diseases. Clin. Exp. Pharmacol. Physiol. 2014, 41, 445–458. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Scheibye-Knudsen, M. Neurodegeneration in accelerated aging. Dan. Med. J. 2016, 63, B5308. [Google Scholar]
- Vijg, J.; Suh, Y. Genome Instability and Aging. Annu. Rev. Physiol. 2013, 75, 645–668. [Google Scholar] [CrossRef] [PubMed]
- DeBalsi, K.L.; Hoff, K.E.; Copeland, W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017, 33, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radical theory of aging. Mutat. Res. 1992, 275, 257–266. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: An update—Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Linnane, A.W.; Ozawa, T.; Marzuki, S.; Tanaka, M. Mitochondrial dna mutations as an important contributor to ageing and degenerative diseases. Lancet 1989, 333, 642–645. [Google Scholar] [CrossRef]
- Mecocci, P.; MacGarvey, U.; Kaufman, A.E.; Koontz, D.; Shoffner, J.M.; Wallace, D.C.; Beal, M.F. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Cedikova, M.; Pitule, P.; Kripnerova, M.; Markova, M.; Kuncová, J. Multiple roles of mitochondria in aging processes. Physiol. Res. 2016, 65, S519–S531. [Google Scholar] [PubMed]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; D’Adda Di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Murphy, D.L.; Sweasy, J.B. Base excision repair and cancer. Cancer Lett. 2012, 327, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.X.; Correia, S.C.; Zhu, X.; Smith, M.A.; Moreira, P.I.; Castellani, R.J.; Nunomura, A.; Perry, G. Mitochondrial DNA Oxidative Damage and Repair in Aging and Alzheimer’s Disease. Antioxid. Redox Signal. 2013, 18, 2444–2457. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Menck, C.F.M.; Leandro, G.S. Autophagy roles in the modulation of DNA repair pathways. Int. J. Mol. Sci. 2017, 18, 2351. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Carter, K.M.; D’Orazio, J.A. Paracrine regulation of melanocyte genomic stability: A focus on nucleotide excision repair. Pigment Cell Melanoma Res. 2017, 30, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Kamileri, I.; Karakasilioti, I.; Garinis, G.A. Nucleotide excision repair: New tricks with old bricks. Trends Genet. 2012, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Scharer, O.D. Nucleotide Excision Repair in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2013, 5, a012609. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Lan, L.; Zou, L. Regulation of DNA break repair by transcription and RNA. Sci. China Life Sci. 2017, 60, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Symington, L.S. Mechanism and regulation of DNA end resection in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, X. Microhomology-mediated end joining: New players join the team. Cell Biosci. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Vida, A.; Márton, J.; Mikó, E.; Bai, P. Metabolic roles of poly(ADP-ribose) polymerases. Semin. Cell Dev. Biol. 2017, 63, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Al-Enazi, K.A. PARP Enzymes are Potential Targets for Cancer Chemotherapy. Austin J. Pharmacol. Ther. 2014, 2, 4–7. [Google Scholar]
- Virág, L. Structure and function of poly(ADP-ribose) polymerase-1: Role in oxidative stress-related pathologies. Curr. Vasc. Pharmacol. 2005, 3, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Perkins, M.N. Quinolinic acid: A potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981, 72, 411–412. [Google Scholar] [CrossRef]
- Moroni, F. Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites. Eur. J. Pharmacol. 1999, 375, 87–100. [Google Scholar] [CrossRef]
- Ying, W. NAD + /NADH and NADP + /NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.P.; Franco, N.F.; Varney, B.; Sundaram, G.; Brown, D.A.; de Bie, J.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PLoS ONE 2015, 10, e0131389. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W.J. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 2016, 67, 3–19. [Google Scholar] [PubMed]
- Sahm, F.; Oezen, I.; Opitz, C.A.; Radlwimmer, B.; Von Deimling, A.; Ahrendt, T.; Adams, S.; Bode, H.B.; Guillemin, G.J.; Wick, W.; et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 2013, 73, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A Glycine Site Associated with N-Methyl-d-Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Miller, J.; MacGarvey, U.; Flint Beal, M. Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res. Bull. 1992, 28, 233–238. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Hilmas, C.; Pereira, E.F.R.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The Brain Metabolite Kynurenic Acid Inhibits α7 Nicotinic Receptor Activity and Increases Non-α7 Nicotinic Receptor Expression: Physiopathological Implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [PubMed]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. De On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2012, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Moroni, F.; Russi, P.; Carlá, V.; Lombardi, G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci. Lett. 1988, 94, 145–150. [Google Scholar] [CrossRef]
- Zádori, D.; Nyiri, G.; Szonyi, A.; Szatmári, I.; Fülöp, F.; Toldi, J.; Freund, T.F.; Vécsei, L.; Klivényi, P. Neuroprotective effects of a novel kynurenic acid analogue in a transgenic mouse model of Huntington’s disease. J. Neural Transm. 2011, 118, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.W.; et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kepplinger, B.; Baran, H.; Kainz, A.; Ferraz-Leite, H.; Newcombe, J.; Kalina, P. Age-related increase of kynurenic acid in human cerebrospinal fluid—IgG and beta2-microglobulin changes. Neurosignals 2005, 14, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.C.; Ahluwalia, N.; Anderson, T.J.T.; Hardy, G.J.; Sinha, S.; Alvarez-Cardona, J.A.; Pruitt, I.E.; Rhee, E.P.; Colvin, R.A.; Gerszten, R.E. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J. Biol. Chem. 2009, 284, 19189–19195. [Google Scholar] [CrossRef] [PubMed]
- Beamer, C.A.; Seaver, B.P.; Shepherd, D.M. Aryl hydrocarbon receptor (AhR) regulates silica-induced inflammation but not fibrosis. Toxicol. Sci. 2012, 126, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Shoki, O.; Nobuyoshi, N.; Hiroshi, S.; Hiroshi, K.; Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an Endogenous Oxidative Stress Generator, Causes Neuronal Cell Death with Apoptotic Features and Region Selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef]
- Guidetti, P.; Schwarcz, R. 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur. J. Neurosci. 1999, 11, 3857–3863. [Google Scholar] [CrossRef] [PubMed]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Williams, K.R.; Smith, D.G.; Smythe, G.A.; Croitoru-Lamoury, J.; Brew, B.J. Quinolinic acid in the pathogenesis of Alzheimer’s disease. Adv. Exp. Med. Biol. 2003, 527, 167–176. [Google Scholar] [PubMed]
- Brown, J.C., 3rd; Tse, H.W.; Skifter, D.A.; Christie, J.M.; Andaloro, V.J.; Kemp, M.C.; Watkins, J.C.; Jane, D.E.; Monaghan, D.T. [3H]homoquinolinate binds to a subpopulation of NMDA receptors and to a novel binding site. J. Neurochem. 1998, 71, 1464–1470. [Google Scholar] [CrossRef]
- Prado De Carvalho, L.; Bochet, P.; Rossier, J. The endogenous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem. Int. 1996, 28, 445–452. [Google Scholar] [CrossRef]
- Schwarcz, R.; Whetsell, W.O.; Mangano, R.M. Quinolinic acid: An endogenous metabolite that produces axon-sparing lesions in rat brain. Science 1983, 219, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, E.; Camacho, A.; Maldonado, P.D.; Pedraza-Chaverrí, J.; Santamaría, D.; Galván-Arzate, S.; Santamaría, A. Effect of quinolinic acid on endogenous antioxidants in rat corpus striatum. Brain Res. 2000, 858, 436–439. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Croitoru-Lamoury, J.; Dormont, D.; Armati, P.J.; Brew, B.J. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia 2003, 41, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Copeland, C.S.; Neale, S.A.; Bruno, V.; Battaglia, G.; Salt, T.E.; Nicoletti, F. Cinnabarinic acid and xanthurenic acid: Two kynurenine metabolites that interact with metabotropic glutamate receptors. Neuropharmacology 2017, 112, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez De La Cruz, V. Kynurenines with neuroactive and redox properties: Relevance to aging and brain diseases. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural Transm. 2012, 119, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Fallarini, S.; Magliulo, L.; Paoletti, T.; de Lalla, C.; Lombardi, G. Expression of functional GPR35 in human iNKT cells. Biochem. Biophys. Res. Commun. 2010, 398, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Tiszlavicz, Z.; Németh, B.; Fülöp, F.; Vécsei, L.; Tápai, K.; Ocsovszky, I.; Mándi, Y. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-α (TNF-α) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Szalardy, L.; Klivenyi, P.; Zadori, D.; Fulop, F.; Toldi, J.; Vecsei, L. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: Medicinal chemistry aspects. Curr. Med. Chem. 2012, 19, 1899–1920. [Google Scholar] [CrossRef] [PubMed]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer’s disease. J. Neural Transm. 1999, 106, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Stankovic, R.; Cullen, K. M.; Meininger, V.; Garner, B.; Coggan, S.; Grant, R.; Brew, B. J.; Guillemin, G. J. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox. Res. 2010, 18, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Luthi-Carter, R.E.; Augood, S.J.; Schwarcz, R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiol. Dis. 2004, 17, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Pi, L.; Yang, J.; Xiang, Z.; Tang, A. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J. Clin. Neurosci. 2014, 21, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Darlington, L.G.; Mackay, G.M.; Forrest, C.M.; Stoy, N.; George, C.; Stone, T.W. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 2007, 26, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Li, C.-F.; Kuo, C.-C.; Tsai, K.K.; Hou, M.-F.; Hung, W.-C. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 2014, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ovchinnikova, O.; Jönsson, A.; Lundberg, A.M.; Berg, M.; Hansson, G.K.; Ketelhuth, D.F.J. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur. Heart J. 2012, 33, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell. Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Csete, K.; Vecsei, L.; Papp, J.G. Effect of systemic administration of l-kynurenine on corticocerebral blood flow under normal and ischemic conditions of the brain in conscious rabbits. J. Cardiovasc. Pharmacol. 2003, 42, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M.; Schwarz, M.J.; Verkerk, R.; Mueller, H.H.; Zach, J.; Scharpé, S.; Steinbusch, H.W.M.; Leonard, B.E.; Kim, Y.K. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain. Behav. Immun. 2011, 25, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Evidence for an immune response in major depression: A review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 11–38. [Google Scholar] [CrossRef]
- Rogers, K.S.; Evangelista, S.J. 3-Hydroxykynurenine, 3-hydroxyanthranilic acid, and o-aminophenol inhibit leucine-stimulated insulin release from rat pancreatic islets. Proc. Soc. Exp. Biol. Med. 1985, 178, 275–278. [Google Scholar] [CrossRef] [PubMed]
- De Bie, J.; Guest, J.; Guillemin, G.J.; Grant, R. Central kynurenine pathway shift with age in women. J. Neurochem. 2016, 136, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. Effect of quinaldic acid and its relatives on insulin-release from isolated Langerhans islets. Acta Vitaminol. Enzymol. 1975, 29, 227–231. [Google Scholar] [PubMed]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Sathyasaikumar, K.V.; Stachowski, E.K.; Amori, L.; Guidetti, P.; Muchowski, P.J.; Schwarcz, R. Dysfunctional kynurenine pathway metabolism in the R6/2 mouse model of Huntington’s disease. J. Neurochem. 2010, 113, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Levy, C.; Heyes, D.J.; Lafite, P.; Outeiro, T.F.; Giorgini, F.; Leys, D.; Scrutton, N.S. Structural basis of kynurenine 3-monooxygenase inhibition. Nature 2013, 496, 382–385. [Google Scholar] [CrossRef] [PubMed]

| Diseases | Mutations | Abnormal Mitochondrial Dynamics | |
|---|---|---|---|
| Neurodegenerative disorders | Familial forms of Parkinson’s diseases | PARK2 and PARK6 (PINK1) genes | Abnormal Parkin and PINK1 proteins impair mitophagy |
| Alzheimer’s diseases | increased mitochondrial fission | ||
| Huntington’s disease | Huntingtin gene | mutant huntingtin impair mitochondrial trafficking | |
| Amyotrophic lateral sclerosis | Superoxide dismutase 1 gene | Drp1-mediated mitochondrial fission | |
| Cardiovascular diseases | Cardiac hypertrophy | ||
| Heart failure | excessive fission and/or decreased fusion | ||
| Myocardial infarction | |||
| Myocardial ischemia–reperfusion injury | excessive fission and/or decreased fusion | ||
| Metabolic Diseases | Type 2 diabetes mellitus | excessive fission and/or decreased fusion | |
| Glucose intolerance | excessive fission and/or decreased fusion | ||
| Insulin resistance | |||
| Obesity | |||
| Others | Charcot-Marie-Tooth syndrome | Mitofusin-2 gene | altered axonal mitochondrial transport |
| Dominant Optic Atrophy | OPA1 and OPA3 genes | mitochondrial fission/fusion defect | |
| Sarcopenia | aberrant mitochondrial fission/fusion |
| IDO or Kynurenine Metabolites | Effect on the Immune System (Mostly Mediated by AhR Activation) |
|---|---|
| IDO | role in immunoregulation during infection, autoimmunity, pregnancy, transplantation and neoplasia |
| IDO activation [70,89] |
|
| L-KYN/KYNA | blockage of antigens-driven specific T-cell proliferation, induction of T-cell death the reduction of IL-4 release (inducer of Th2 type reaction) from iNKT (invariant natural killer T cell) cells via GPR35 activation [90] the inhibition of LPS-induced TNF-α secretion in peripheral blood mononuclear cells via GPR35 receptor activation [72]; and also on CD14+ peripheral blood monocytes [57,91]);
|
| 3-OH-AA and QUIN | the selective apoptosis of Th1 helper cells, promoting the proliferation of Th2 cells |
| QUIN | increase in MCP-1 expression (a strong chemoattractant for monocytes in the brain). |
| Diseases | Alteration | Refs. | |
|---|---|---|---|
| Neurodegenerative diseases | Parkinson’s disease | ↓ KYNA ↑ 3-OH-KYN in brain | [93] |
| Alzheimer disease | upregulation of IDO1; ↑ QUIN in the brain; ↑ KYNA in putamen and caudate nuclei | [80,94] | |
| Amyotrophic lateral sclerosis | in serum, CSF: ↑ TRP, ↑ L-KYN, ↑ QUIN; in serum: ↓ PA | [95] | |
| Huntington’s disease | early phase: ↑ QUIN, 3-OH-KYN, 3-OH-AO activity; ↓ KYNA, KATs activities in the brain | [70,96] | |
| Neurovascular diseases | Ischemic stroke | ↓ TRP, ↑ KYN/TRP ratio in serum | [97,98] |
| Cancer | ↑ KYN levels can increase the proliferation and invasion of cancer cells ↑ QPRT activity in some cancer → longer survival of tumour cells → worse prognosis | [59,99] | |
| Cardiovascular diseases | Atherogenesis | Contradictory, further investigations are needed to elucidate the role of IDO in atherogenesis | [100] |
| Cardiac arrest | ↑ kynurenines predicted the risk of acute myocardial infarction in patients with stable angina pectoris | [101] | |
| Hypertension | TRP catabolites might have a role in the regulation of blood pressure | [102,103] | |
| Psychiatric diseases | Schizophrenia | ↑ KYNA in CSF, post-mortem tissues; ↓ KYNA, KYNA/KYN, KYNA/3-OH-KYN and 3-OH-KYN in blood | [104,105] |
| Depression | ↑ KYN and QUIN in CSF; ↓ SER synthesis ↑KYNA in the brain | [88,106] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. https://doi.org/10.3390/molecules23010191
Sas K, Szabó E, Vécsei L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules. 2018; 23(1):191. https://doi.org/10.3390/molecules23010191
Chicago/Turabian StyleSas, Katalin, Elza Szabó, and László Vécsei. 2018. "Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection" Molecules 23, no. 1: 191. https://doi.org/10.3390/molecules23010191