Novel 6- and 7-Substituted Coumarins with Inhibitory Action against Lipoxygenase and Tumor-Associated Carbonic Anhydrase IX
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. Chemistry
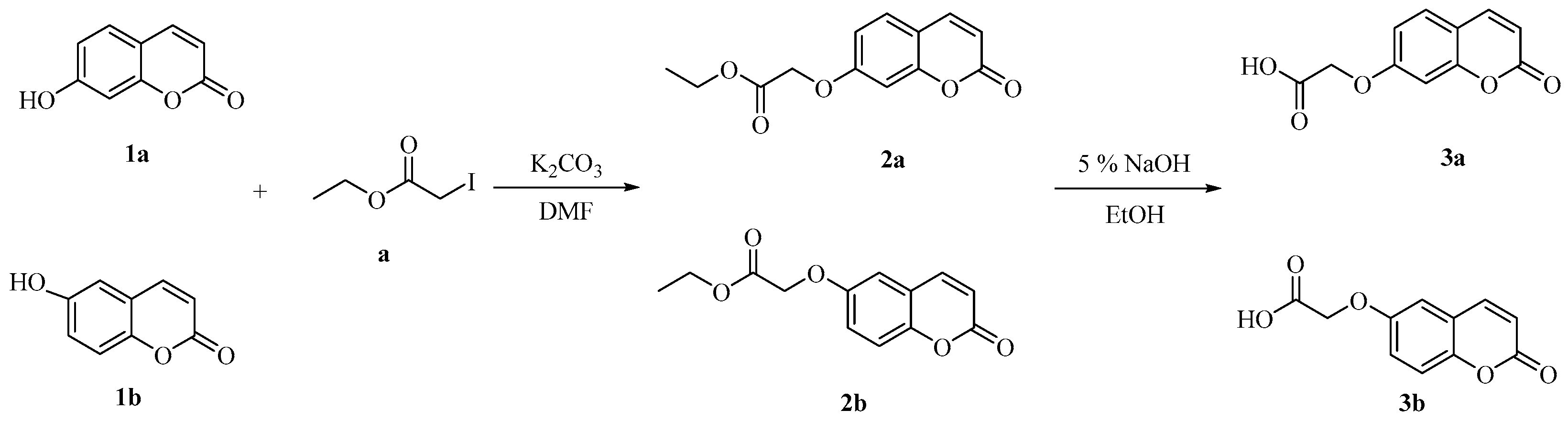
3.2.1. General Procedure for the Synthesis of Compounds 3a–b [39]


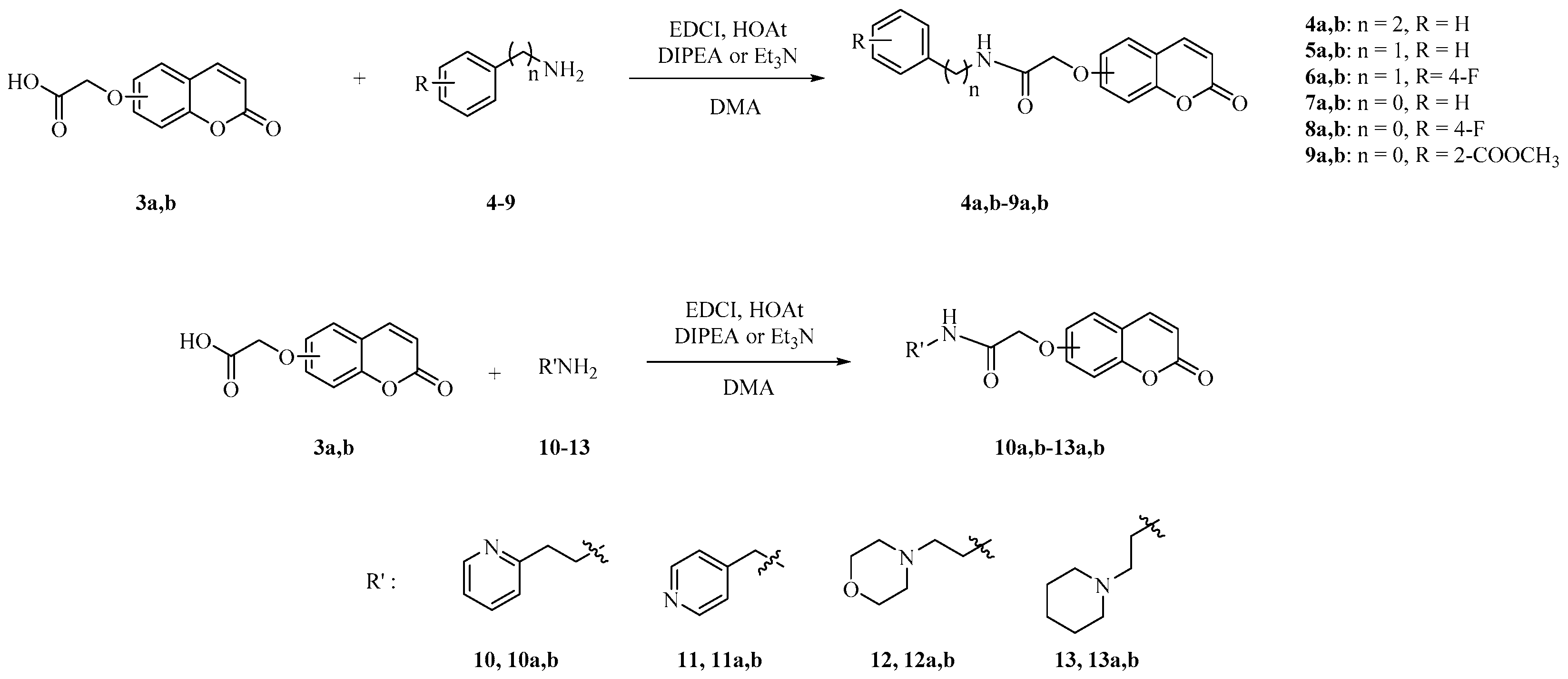
3.2.2. General Procedure for the Synthesis of 4a,b–13a,b




















3.3. Soybean Lipoxygenase Inhibition Studies
3.4. CA Inhibition Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria? J. Enzym. Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.M.; Supuran, C.T.; De Simone, G. Anticancer carbonic anhydrase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2013, 23, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic Anhydrase Inhibition and the Management of Hypoxic Tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Supuran, C.T.; Carta, F. Antiobesity carbonic anhydrase inhibitors: A literature and patent review. Expert Opin. Ther. Pat. 2013, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T. Diuretics with carbonic anhydrase inhibitory action: A patent and literature review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Di Cesare Mannelli, L.; Pinard, M.; Ghelardini, C.; Scozzafava, A.; McKenna, R.; Supuran, C.T. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg. Med. Chem. 2015, 23, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Supuran, C.T. Carbonic Anhydrase Inhibitors. A General Approach for the Preparation of Water-Soluble Sulfonamides Incorporating Polyamino—Polycarboxylate Tails and of Their Metal Complexes Possessing Long-Lasting, Topical Intraocular Pressure-Lowering Properties. J. Med. Chem. 2002, 45, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Del Prete, S.; Di Fonzo, P.; Carginale, V.; Donald, W.A.; Supuran, C.T.; Capasso, C. Comparison of the Sulfonamide Inhibition Profiles of the β- and γ-Carbonic Anhydrases from the Pathogenic Bacterium Burkholderia pseudomallei. Molecules 2017, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Berrino, E.; Bua, S.; Mori, M.; Botta, M.; Murthy, V.S.; Vijayakumar, V.; Tamboli, Y.; Bartolucci, G.; Mugelli, A.; Cerbai, E.; et al. Novel Sulfamide-Containing Compounds as Selective Carbonic Anhydrase I Inhibitors. Molecules 2017, 22, 1049. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Touisni, N.; Maresca, A.; McDonald, P.C.; Lou, Y.; Scozzafava, A.; Dedhar, S.; Winum, J.Y.; Supuran, C.T. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J. Med. Chem. 2011, 54, 8271–8277. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, M.; Ferraroni, M.; Carta, F.; Vullo, D.; Lucarini, L.; Orlandini, E.; Rossello, A.; Nuti, E.; Scozzafava, A.; Masini, E.; et al. Structural insights on carbonic anhydrase inhibitory action, isoform selectivity, and potency of sulfonamides and coumarins incorporating arylsulfonylureido groups. J. Med. Chem. 2014, 57, 9152–9167. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Maresca, A.; Scozzafava, A.; Supuran, C.T. Novel coumarins and 2-thioxo-coumarins as inhibitors of the tumor-associated carbonic anhydrases IX and XII. Bioorg. Med. Chem. 2012, 20, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Vullo, D.; Maresca, A.; Supuran, C.T.; Poulsen, S.A. Natural product coumarins that inhibit human carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; Carta, F.; Scozzafava, A.; Supuran, C.T. Thioxocoumarins Show an Alternative Carbonic Anhydrase Inhibition Mechanism Compared to Coumarins. J. Med. Chem. 2016, 59, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Kucukbay, F.Z.; Kucukbay, H.; Tanc, M.; Supuran, C.T. Synthesis and carbonic anhydrase inhibitory properties of amino acid—Coumarin/quinolinone conjugates incorporating glycine, alanine and phenylalanine moieties. J. Enzym. Inhib. Med. Chem. 2016, 31, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Z.; Kuszynski, C.A.; El-Metwally, T.H.; Adrian, T.E. Lipoxygenase Inhibition Induced Apoptosis, Morphological Changes, and Carbonic Anhydrase Expression in Human Pancreatic Cancer Cells. Biochem. Biophys. Res. Commun. 1999, 266, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef] [PubMed]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands: An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Crooks, S.W.; Stockley, R.A. Leukotriene B4. Int. J. Biochem. Cell. Biol. 1998, 30, 173–178. [Google Scholar] [CrossRef]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef] [PubMed]
- Müller, K. 5-Lipoxygenase and 12-lipoxygenase: Attractive targets for the development of novel antipsoriatic drugs. Arch. Pharm. 1994, 327, 3–19. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Lipoxygenase inhibitors: A comparative QSAR study review and evaluation of new QSARs. Med. Res. Rev. 2008, 28, 39–117. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- BioByte Home Page. Available online: http://www.biobyte.com/ (accessed on 1 June 2012).
- Kavetsou, E.; Gkionis, L.; Galani, G.; Gkolfinopoulou, C.; Argyri, L.; Pontiki, E.; Chroni, A.; Hadjipavlou-Litina, D.; Detsi, A. Synthesis of prenyloxy coumarin analogues and evaluation of their antioxidant, lipoxygenase (LOX) inhibitory and cytotoxic activity. Med. Chem. Res. 2017, 26, 856–866. [Google Scholar] [CrossRef]
- Chang, K.M.; Chen, H.H.; Wang, T.C.; Chen, I.L.; Chen, Y.T.; Yang, S.C.; Chen, Y.L.; Chang, H.H.; Huang, C.H.; Chang, J.Y.; et al. Novel oxime-bearing coumarin derivatives act as potent Nrf2/ARE activators in vitro and in mouse model. Eur. J. Med. Chem. 2015, 106, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Maloney, D.J.; Dedkova, L.M.; Hecht, S.M. Inhibitors of DNA polymerase beta: Activity and mechanism. Bioorg. Med. Chem. 2008, 16, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, M.; Trendafilova, N.; Arfa-Kia, A.F.; Rosair, G.; Kavanagh, K.; Devereux, M.; Walsh, M.; McClean, S.; Creaven, B.S.; Georgieva, I. Novel silver(I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J. Inorg. Biochem. 2016, 163, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Nicolae, A.; Popescu, A. Carbonic anhydrase inhibitors. Part 35. Synthesis of Schiff bases derived from sulfanilamide and aromatic aldehydes: The first inhibitors with equally high affinity towards cytosolic and membrane-bound isozymes. Eur. J. Med. Chem. 1996, 31, 431–438. [Google Scholar] [CrossRef]
- Pacchiano, F.; Aggarwal, M.; Avvaru, B.S.; Robbins, A.H.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Selective hydrophobic pocket binding observed within the carbonic anhydrase II active site accommodate different 4-substituted-ureido-benzenesulfonamides and correlate to inhibitor potency. Chem. Commun. 2010, 46, 8371–8373. [Google Scholar] [CrossRef] [PubMed]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.Y.; Montero, J.L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg. Med. Chem. Lett. 2004, 14, 5427–5433. [Google Scholar] [CrossRef] [PubMed]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.Y.; Montero, J.L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg. Med. Chem. Lett. 2005, 15, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Garaj, V.; Maresca, A.; Wagner, J.; Avvaru, B.S.; Robbins, A.H.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Sulfonamides incorporating 1,3,5-triazine moieties selectively and potently inhibit carbonic anhydrase transmembrane isoforms IX, XII and XIV over cytosolic isoforms I and II: Solution and X-ray crystallographic studies. Bioorg. Med. Chem. 2011, 19, 3105–3119. [Google Scholar] [CrossRef] [PubMed]
- Sentürk, M.; Gülçin, I.; Beydemir, S.; Küfrevioğlu, O.İ.; Supuran, C.T. In Vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem. Biol. Drug Des. 2011, 77, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Mincione, F.; Somma, T.; Scozzafava, G.; Galassi, F.; Masini, E.; Impagnatiello, F.; Supuran, C.T. A new approach to antiglaucoma drugs: Carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J. Enzym. Inhib. Med. Chem. 2012, 27, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Krall, N.; Pretto, F.; Decurtins, W.; Bernardes, G.J.; Supuran, C.T.; Neri, D. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew. Chem. Int. Ed. 2014, 53, 4231–4235. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Hillebrecht, A.; Schulze Wischeler, J.; Innocenti, A.; Heine, A.; Supuran, C.T.; Klebe, G. Saccharin inhibits carbonic anhydrases: Possible explanation for its unpleasant metallic aftertaste. Angew. Chem. Int. Ed. 2007, 46, 7697–7699. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2–13b are available from the authors. |
| Compound | KI (nM) * | |||
|---|---|---|---|---|
| hCA I | hCA II | hCA IV | hCA IX | |
| 2a | >10,000 | >10,000 | 5572 | 247.1 |
| 2b | >10,000 | >10,000 | >10,000 | 2044 |
| 3a | >10,000 | >10,000 | 8480 | 290.2 |
| 3b | >10,000 | >10,000 | >10,000 | 194.9 |
| 4a | >10,000 | >10,000 | >10,000 | 165.7 |
| 4b | >10,000 | >10,000 | >10,000 | 30.5 |
| 5a | >10,000 | >10,000 | >10,000 | 83.7 |
| 5b | >10,000 | >10,000 | >10,000 | 30.2 |
| 6a | >10,000 | >10,000 | >10,000 | 2536 |
| 6b | >10,000 | >10,000 | >10,000 | 2785 |
| 7a | >10,000 | >10,000 | >10,000 | 200.6 |
| 7b | >10,000 | >10,000 | 2649 | 201.9 |
| 8a | >10,000 | >10,000 | 350.4 | 136.1 |
| 8b | >10,000 | >10,000 | 848.3 | 145.6 |
| 9a | >10,000 | >10,000 | >10,000 | 2732 |
| 9b | >10,000 | >10,000 | >10,000 | 2041 |
| 10a | >10,000 | >10,000 | >10,000 | 2377 |
| 10b | >10,000 | >10,000 | >10,000 | 2147 |
| 11a | >10,000 | >10,000 | 766.4 | 122.3 |
| 11b | >10,000 | >10,000 | >10,000 | 1962 |
| 12a | >10,000 | >10,000 | >10,000 | >10,000 |
| 12b | >10,000 | >10,000 | >10,000 | >10,000 |
| 13a | >10,000 | >10,000 | >10,000 | 1969 |
| 13b | >10,000 | >10,000 | >10,000 | 273.7 |
| AAZ | 250 | 12 | 74 | 25 |
| Compounds | ClogP a | % LOX Inhibition at 100 Μμ b | IC50 (μΜ) b |
|---|---|---|---|
| 1a (7-HC) | 1.62 | Reference compound | 43 µM c |
| 1b (6-HC) | 1.62 | 40% | nt e |
| 2a d | 1.89 | 16% | nt e |
| 2b d | 1.89 | 41% | nt e |
| 3a d | 1.03 | 50% | 100 μΜ |
| 3b d | 1.03 | 50% | 100 μΜ |
| 4a | 2.60 | 96% | 10 μΜ |
| 4b | 2.60 | 85% | 10 μΜ |
| 5a | 2.39 | 45% | nt e |
| 5b | 2.39 | 42% | nt e |
| 6a | 2.53 | 63% | 47 μΜ |
| 6b | 2.53 | 26% | nt e |
| 7a | 2.36 | 50% | 100 μΜ |
| 7b | 2.36 | 45% | nt e |
| 8a | 2.76 | 43% | nt e |
| 8b | 2.76 | 11% | nt e |
| 9a | 2.80 | 33% | nt e |
| 9b | 2.80 | 50% | 100 μΜ |
| 10a | 1.11 | 79% | 15 μΜ |
| 10b | 1.11 | 40% | nt e |
| 11a | 0.89 | 77% | 27 μΜ |
| 11b | 0.89 | 37% | nt e |
| 12a | 0.91 | 56% | 42.5 μΜ |
| 12b | 0.91 | 64% | 16.5 μΜ |
| 13a | 2.32 | 50% | 100 μΜ |
| 13b | 2.32 | 37.6% | nt e |
| NDGA | 93% | 0.45 μM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peperidou, A.; Bua, S.; Bozdag, M.; Hadjipavlou-Litina, D.; Supuran, C.T. Novel 6- and 7-Substituted Coumarins with Inhibitory Action against Lipoxygenase and Tumor-Associated Carbonic Anhydrase IX. Molecules 2018, 23, 153. https://doi.org/10.3390/molecules23010153
Peperidou A, Bua S, Bozdag M, Hadjipavlou-Litina D, Supuran CT. Novel 6- and 7-Substituted Coumarins with Inhibitory Action against Lipoxygenase and Tumor-Associated Carbonic Anhydrase IX. Molecules. 2018; 23(1):153. https://doi.org/10.3390/molecules23010153
Chicago/Turabian StylePeperidou, Aikaterini, Silvia Bua, Murat Bozdag, Dimitra Hadjipavlou-Litina, and Claudiu T. Supuran. 2018. "Novel 6- and 7-Substituted Coumarins with Inhibitory Action against Lipoxygenase and Tumor-Associated Carbonic Anhydrase IX" Molecules 23, no. 1: 153. https://doi.org/10.3390/molecules23010153





