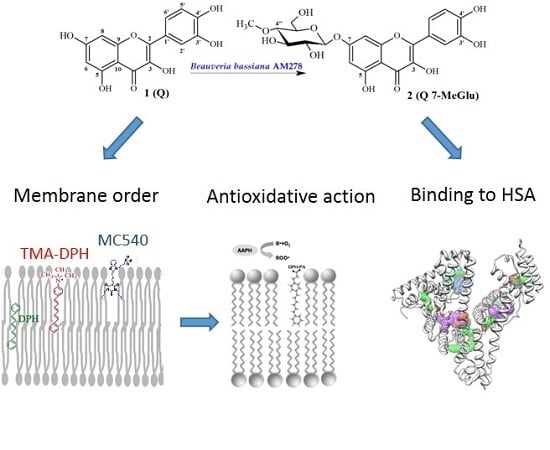
Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
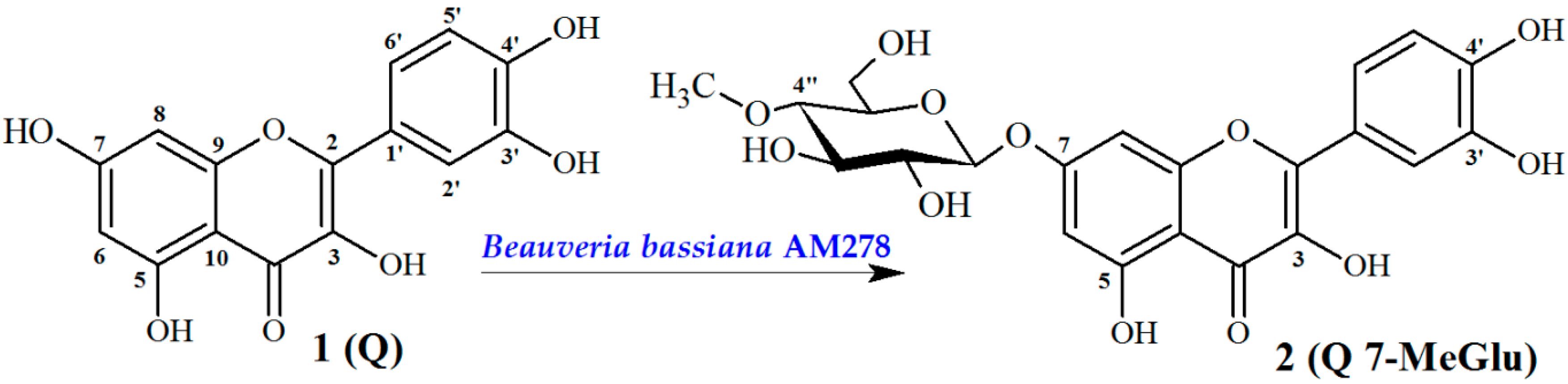
Biotransformation Catalyzed by Beuaveria bassiana AM278
2.2. Interaction with Lipid Membrane
2.2.1. Fluorescence Measurements—MC540, TMA-DPH and DPH Probes
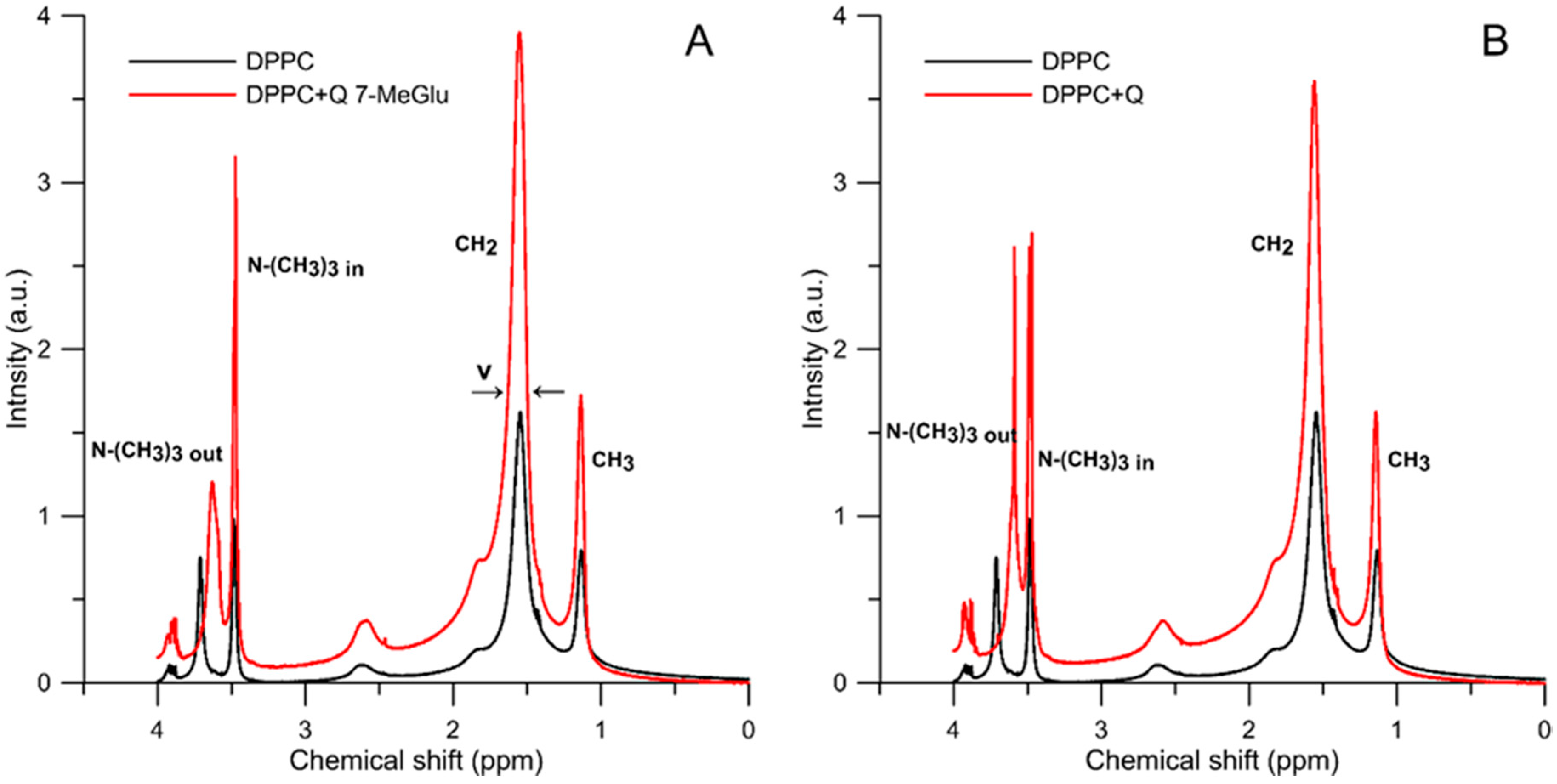
2.2.2. 1H-NMR Study
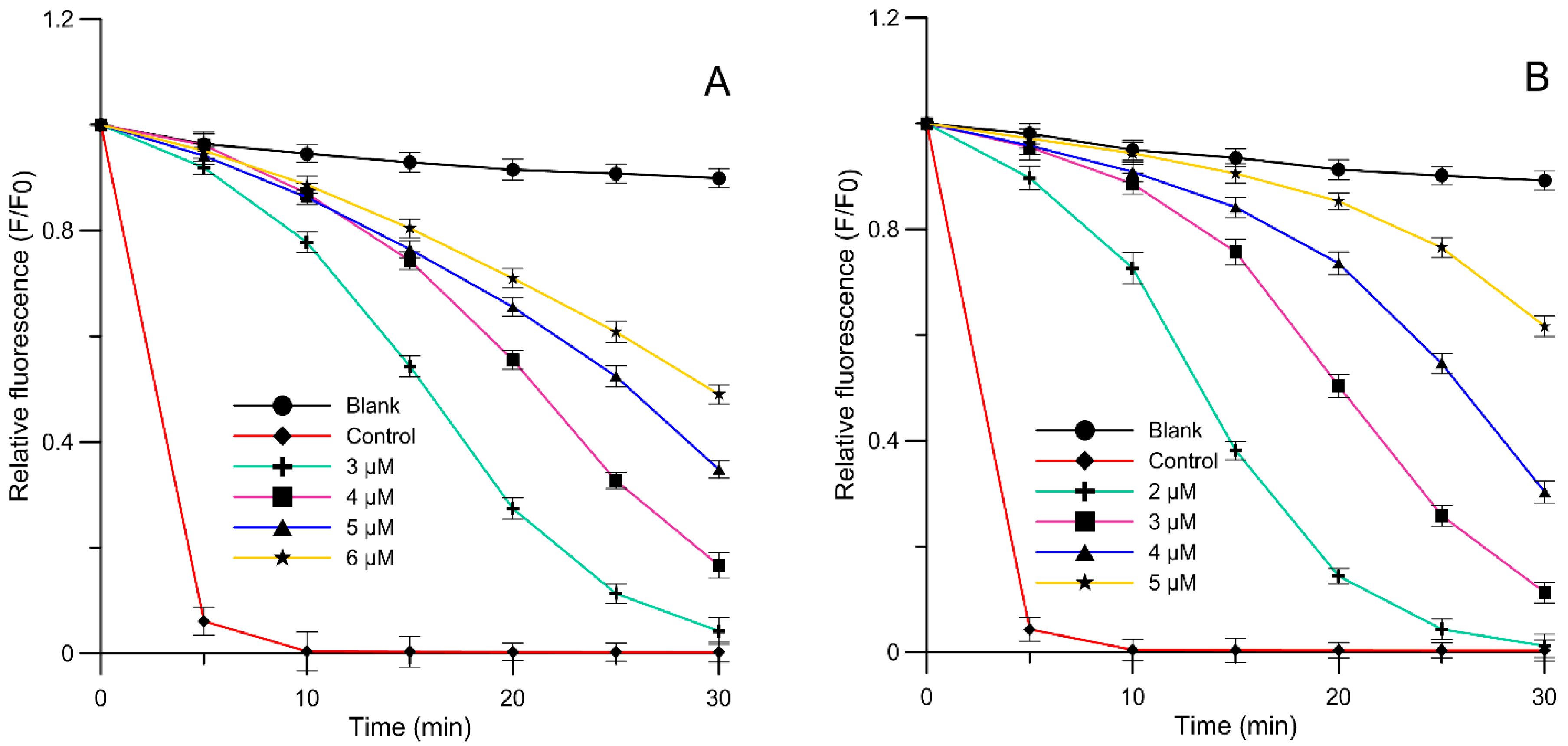
2.3. Liposome Oxidation Assay
2.4. Inhibition of 1 and 2 Cyclooxygenases
2.5. Binding to Human Serum Albumin (HSA)
3. Materials and Methods
3.1. General Information
3.2. Materials
3.3. Microorganism
3.4. Conditions for Biotransformations
3.5. Products Isolation
3.6. Biological Activity Study Methods
3.6.1. Liposome Preparation
3.6.2. Fluorescence Measurements—MC540, TMA-DPH and DPH Probes
3.6.3. 1H-NMR Studies
3.6.4. Liposome Oxidation Assay
3.6.5. Inhibition of Cyclooxygenases 1 and 2
3.6.6. Binding to Human Serum Albumin
3.6.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdelkawy, K.S.; Balyshev, M.E.; Elbarbry, F. A new validated HPLC method for the determination of quercetin: Application to study pharmacokinetics in rats. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Yang, C.; Park, S.; Bazer, F.W.; Song, G. Inhibitory effects of quercetin on progression of human choriocarcinoma cells are mediated through PI3K/AKT and MAPK signal transduction cascades. J. Cell Physiol. 2017, 232, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, L.; Liao, J.; Li, L.; Yao, W.; Xiong, Z.; Zhou, X. Investigation of the anti-cancer effect of quercetin on HepG2 cells in vivo. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 1, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Liu, B.; Yuan, E.; Ma, Y.; Zhu, Y. Preparation and physicochemical properties of the complex of naringenin with hydroxypropyl-beta-cyclodextrin. Molecules 2010, 15, 4401–4407. [Google Scholar] [CrossRef] [PubMed]
- Pulley, G.N. Solubility of Naringin in Water. Ind. Eng. Chem. Anal. Ed. 1936, 8, 360. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suzuki, K.; Yoneyama, M.; Miyake, T. 4G-alpha-d-glucopyranosyl Rutin, and its Preparation and Uses. U.S. Patent 5,026,833, 3 April 1991. [Google Scholar]
- Saija, A.; Tomaino, A.; Trombetta, D.; Pellegrino, M.L.; Tita, B.; Messina, C.; Bonina, F.P.; Rocco, C.; Nicolosi, G.; Castelli, F. ‘In vitro’ antioxidant and photoprotective properties and interactionwith model membranes of three new quercetin esters. Eur. J. Pharm. Biopharm. 2003, 56, 167–174. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Ma, B.; Niu, R.; Zhang, H.; Kun, L. Antitumor activity and safety evaluation of nanaparticle-based delivery of quercetin through intravenous administration in mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 1, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [PubMed]
- Holman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Respective bioavailability of quercetin aglycone and its glycosides in a rat model. Biofactors 2000, 12, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Gunatilaka, A.A.L. Selective 4′-o-methylglycosylation of the pentahydroxy-flavonoid quercetin by Beauveria bassiana atcc 7159. Biocatal. Biotransform. 2006, 24, 396–399. [Google Scholar] [CrossRef]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zeng, J.; Shao, L.; Zhan, J. Efficient bioconversion of quercetin into a novel glycoside by Streptomyces rimosus subsp. rimosus ATCC 10970. J. Biosci. Bioeng. 2013, 115, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Huszcza, E.; Tronina, T. Transformation of isoxanthohumol by fungi. J. Mol. Catal. B Enzym. 2009, 61, 221–224. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and antiproliferative activity of glycosides obtained by biotransformation of xanthohumol. Bioorg. Med. Chem. 2013, 23, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Huszcza, E. Transformation of xanthohumol by Aspergillus ochraceus. J. Basic Microbiol. 2014, 54, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Microbial glycosylation of daidzein, genistein and biochanin A: Two new glucosides of biochanin A. Molecules 2017, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Grogan, G.J.; Holland, H.L. The biocatalytic reactions of Beauveria spp. J. Mol. Catal. B Enzym. 2000, 9, 1–32. [Google Scholar] [CrossRef]
- Huszcza, E.; Bartmańska, A.; Tronina, T. Glycosylation of xanthohumol by fungi. Z. Naturforsch. C 2008, 63, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Strugała, P.; Popłoński, J.; Włoch, P.; Sordon, S.; Bartmańska, A.; Huszcza, E. The influence of glycosylation of natural and synthetic prenylated flavonoids on binding to human serum albumin and inhibition of cyclooxygenases COX-1 and COX-2. Molecules 2017, 22, 1230. [Google Scholar] [CrossRef] [PubMed]
- Alay, M.; Prat, J.; Haro, I.; Rojo, N.; Alsina, M.A.; Busquets, M.A. Spectroscopic analysis of the interaction of a peptide sequence of Hepatitis G virus with bilayers. Talanta 2003, 60, 269–277. [Google Scholar] [CrossRef]
- Włoch, A.; Strugała, P.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Physical effects of buckwheat extract on biological membrane in vitro and its protective properties. J. Membr. Biol. 2016, 249, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Engelke, M.; Bojarski, P.; Bloß, R.; Diehl, H. Tamoxifen perturbs lipid bilayer order and permeability: Comparison of DSC, fluorescence anisotropy, Laurdan generalized polarization and carboxyfluorescein leakage studies. Biophys. Chem. 2001, 90, 157–173. [Google Scholar] [CrossRef]
- Ulrih, N.; Maričić, M.; Ota, A.; Šentjurc, M.; Abram, V. Kaempferol and quercetin interactions with model lipid membranes. Food Res. Inter. 2015, 71, 146–154. [Google Scholar] [CrossRef]
- Arora, A.; Byrem, T.M.; Nair, M.G.; Strasburg, G.M. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch. Biochem. Biophys. 2000, 373, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gabrielska, J.; Soczyńska-Kordala, M.; Hładyszowski, J.; Żyłka, R.; Miśkiewicz, J.; Przestalski, S. Antioxidative effect of quercetin and its equimolar mixtures with phenylthin compounds on liposome membranes. J. Agric. Food Chem. 2006, 54, 7735–7746. [Google Scholar] [CrossRef] [PubMed]
- Tuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Dziubińska, H.; Król, E.; Trębacz, K.; Jarosz-Wilkołazka, A.; Paduch, R.; Gawron, A.; Gruszecki, W.I. Characteristics of quercetin interactions with liposomal and vacuolar membranes. Biochim. Biophys. Acta 2014, 1838, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Košinová, P.; Berka, K.; Wykes, W.; Otyepka, M.; Trouillas, P. Positioning of antioxidant quercetin and its metabolites in lipid bilayer membranes: Implication for their lipid-peroxidation inhibition. J. Phys. Chem. B 2012, 116, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Krishnaswammy, S.; Devashaya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid-membrane interaction: Implication on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bystrov, V.F.; Dubrovina, N.I.; Barsukov, L.I.; Bergelson, L.D. NMR differentiation of the internal and external phospholipid membrane surface using paramagnetic Mn2+ and Eu3+ ions. Chem. Phys. Lipids 1971, 6, 343–350. [Google Scholar] [CrossRef]
- Kaszuba, M.; Hunt, G.R.A. 31P- and 1H-NMR investigations of the effect of n-alcohols on the hydrolysis by phospholipase A2 of phospholipid vesicular membranes. Biochim. Biophys. Acta 1990, 1030, 88–93. [Google Scholar] [CrossRef]
- Movileanu, L.; Neagoe, I.; Flonta, M.L. Interaction of antioxidant quercetin with planar lipid bilayer. Int. J. Pharm. 2000, 205, 135–146. [Google Scholar] [CrossRef]
- Terao, J.; Piskula, M.; Yao, Q. Protective effect of epicatechin, epicatechin gallate and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994, 308, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Gadwhal, M.K.; Joshi, U.J.; Strivastova, S.; Govil, G. Modifying effect of quercetin on model membrane: Studied by molecular dynamic simulation, DSC and NMR. Int. J. Curr. Pharm. Res. 2012, 4, 70–79. [Google Scholar]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Koen, C.L.; Fraga, C.G. Flavonoid membrane interaction: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, H.A.; Pampel, A.; Nissler, L.; Gebhardt, R.; Huster, D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim. Biophys. Acta 2004, 1663, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Gabrielska, J. Interaction between mimic lipid membranes and acylated and nonacylated cyanidin and its bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological activity of Japanese quince extract and its interactions with lipids, erythrocyte membrane, and human albumin. J. Membrane Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.J.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Beretta, C.; Garavaglia, G.; Cavalli, M. COX-1 and COX-2 inhibition in horse blood by phenylbutazone, flunixin, carprofen and meloxicam: An in vitro analysis. Pharmacol. Res. 2005, 52, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, N.; Burns, D.M.; Blau, H.M. DNA demethylation dynamics. Cell 2011, 146, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zou, T.; Wang, L.; Zhang, Y.; Liu, Y. Investigation of the interaction between quercetin and human serum albumin by multiple spectra, electrochemical impedance spectra and molecular modeling. Luminescence 2014, 29, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Quenching of fluorescence. In Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Press: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2006; pp. 234–264. ISBN 0-306-46093-9. [Google Scholar]
- Trnková, L.; Boušová, I.; Staňková, V.; Dršata, J. Study on the interaction of catechins with human serum albumin using spectroscopic and electrophoretic techniques. J. Mol. Struct. 2011, 985, 243–250. [Google Scholar] [CrossRef]
- Islas, M.S.; Naso, L.N.; Lezama, L.; Valcarcel, M.; Salado, C.; Roura-Ferrer, M.; Ferrer, E.G.; Williams, P.A. Insights into the mechanisms underlying the antitumor activity of an oxidovanadium(IV) compound with the antioxidant naringenin. Albumin binding studies. J. Inorg. Biochem. 2015, 149, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Chen, Z.F.; Liu, Z.J.; Li, R.R.; Ouyang, Y.; Hu, Y.J. Study of the structure-activity relationship of flavonoids based on their interaction with human serum albumin. RSC Adv. 2015, 5, 73290–73300. [Google Scholar] [CrossRef]
- Naso, L.G.; Lezama, L.; Valcarcel, M.; Salado, C.; Villacé, P.; Kortazar, D.; Ferrer, E.G.; Williams, P.A. Bovine serum albumin binding, antioxidant and anticancer properties of an oxidovanadium(IV) complex with luteolin. J. Inorg. Biochem. 2016, 157, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Wilgusz, K.; Trynda-Lemiesz, L. Platinum drugs binding to human serum albumin: Effect of non-steroidal anti-inflammatory drugs. J. Photochem. Photobiol. A Chem. 2014, 289, 1–6. [Google Scholar]
- Moradi, N.; Reza, M.; Kooshk, A.; Ghobadi, S.; Shahlaei, M.; Khodarahmi, R. Spectroscopic study of drug-binding characteristics of unmodified and pNPA-based acetylated human serum albumin: Does esterase activity affect microenvironment of drug binding sites on the protein? J. Lumin. 2015, 160, 351–361. [Google Scholar] [CrossRef]
- Xi, J.; Guo, R. Interactions between flavonoids and hemoglobin in lecithin liposomes. Int. J. Biol. Macromol. 2007, 40, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Gabrielska, J.; Oszmiański, J. Antioxidant activity of anthocyanin glycoside derivatives evaluated by the inhibition of liposome oxidation. Z. Naturforsch. C 2005, 60, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Lelkes, P.I.; Miller, I.R. Perturbations of membrane structure by optical probes: I. Location and structural sensitivity of merocyanine 540 bound to phospholipid membranes. J. Membrane Biol. 1980, 52, 1–15. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Fluorescence anisotropy. In Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Press: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2006; pp. 291–314. ISBN 0-306-46093-9. [Google Scholar]
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidifies lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta 1996, 1285, 167–174. [Google Scholar] [CrossRef]
- Arora, A.; Strasburg, G.M. Development and validation of fluorescence spectroscopic assay to evaluate antioxidant efficiency. Application to metal chelators. J. Am. Oil Chem. Soc. 1997, 74, 1031–1040. [Google Scholar] [CrossRef]
- Strugała, P.; Gładkowski, W.; Kucharska, A.Z.; Sokół-Łętowska, A.; Gabrielska, J. Antioxidant activity and anti-inflammatory effect of fruit extracts from blackcurrant, chokeberry, hawthorn, and rosehip, and their mixture with linseed oil on a model lipid membrane. Eur. J. Lipid Sci. Technol. 2016, 118, 461–474. [Google Scholar] [CrossRef]
- Jang, M.S.; Pezzuto, J.M. Assessment of cyclooxygenase inhibitors using in vitro assay systems. Methods Cell Sci. 1997, 19, 25–31. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound quercetin 7-O-β-d-(4″-O-methyl)glucopyranoside is available from the authors. |
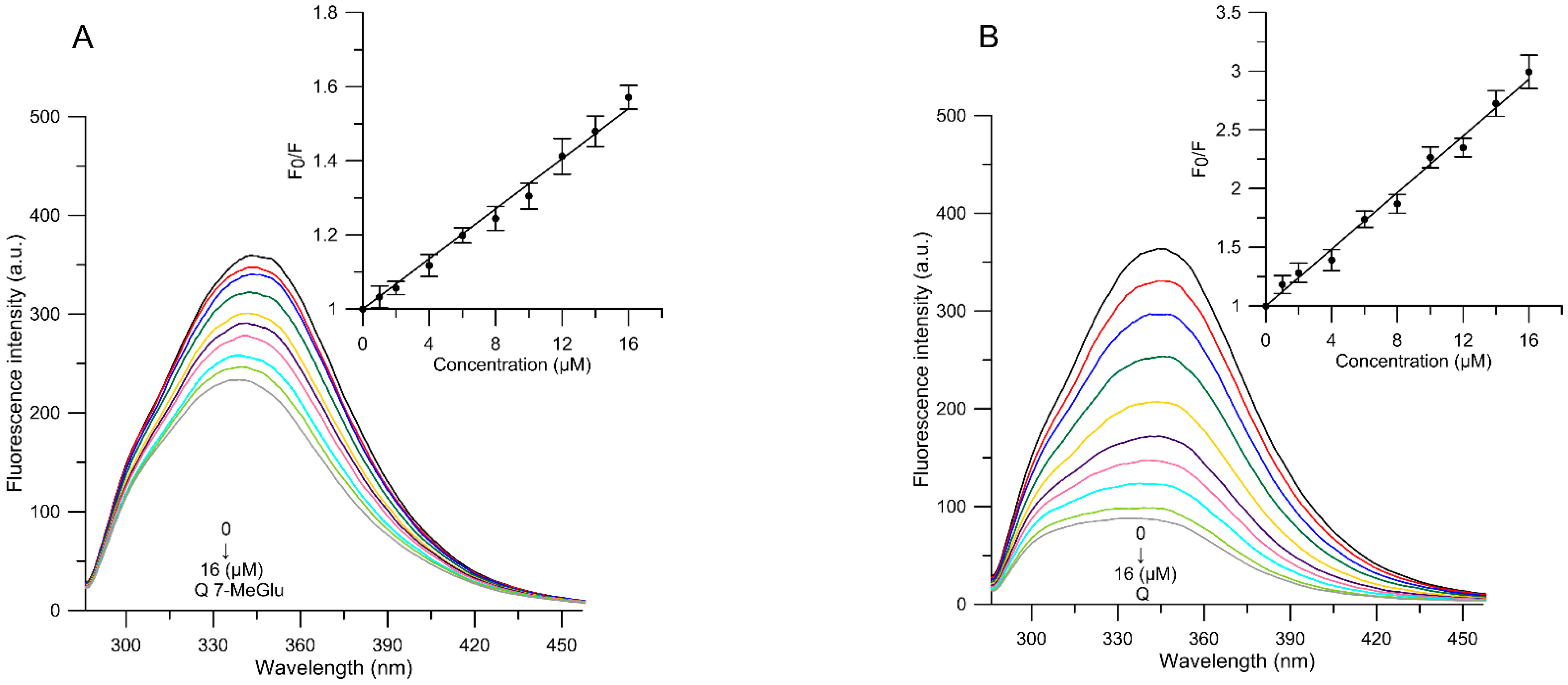
| Compound/Concentration (µM) | MC540 Intensity Change from Control | TMA-DPH Anisotropy Change from Control | DPH Anisotropy Change from Control |
|---|---|---|---|
| Q 7-MeGlu | |||
| 1 | −0.0269 ± 0.0137 * | 0.0518 ± 0.0144 * | 0.0614 ± 0.0359 * |
| 5 | −0.0955 ± 0.0010 * | 0.0845 ± 0.0218 * | 0.1257 ± 0.0193 * |
| 10 | −0.1240 ± 0.0189 * | 0.1005 ± 0.0328 * | 0.2674 ± 0.0584 * |
| Quercetin | |||
| 1 | −0.2890 ± 0.0289 * | 0.1619 ± 0.0133 * | 0.2943 ± 0.0708 * |
| 5 | −0.4585 ± 0.0660 * | 0.2449 ± 0.0122 * | 0.7464 ± 0.0649 * |
| 10 | −0.5580 ± 0.0012 * | 0.3708 ± 0.0674 * | 0.9661 ± 0.0511 * |
| Liposome Composition | Parameter | |||||
|---|---|---|---|---|---|---|
| ν -N+-(CH3)3out (ppm) | ν -N+-(CH3)3in (ppm) | Δδ (ppm) | ν -CH2 (ppm) | ν -CH3 (ppm) | Iout/Iin | |
| DPPC | 0.0365 | 0.0313 | 0.2280 | 0.1121 | 0.0633 | 0.9977 |
| DPPC + Q 7-MeGlu | 0.0882 | 0.0323 | 0.1000 | 0.1179 | 0.0627 | 0.8646 |
| DPPC + Quercetin | 0.0799 | 0.0261 | 0.1560 | 0.1181 | 0.0604 | 1.3305 |
| Compound | IC50 (µM) | ||
|---|---|---|---|
| AAPH PC | COX-1 | COX-2 | |
| Q 7-MeGlu | 5.47 ± 0.47 * | 45.06 ± 2.51 * | 49.23 ± 5.55 * |
| Quercetin | 4.49 ± 0.24 * | 15.72 ± 1.73 * | 16.96 ± 2.43 * |
| L(+) ascorbic acid | 129.46 ± 12.44 *1 | - | - |
| Indomethacin | - | 25.57 ± 0.64 *2 | 21.25 ± 1.90 *2 |
| Compound | T(K) | Ksv (·104 M−1) | Kb (·104 M−1) | n | ∆G (kJ/M) | ∆H (kJ/M) | ∆S (J/(M·K)) |
|---|---|---|---|---|---|---|---|
| Q 7-MeGlu | 300 | 3.837 ± 0.192 * | 9.355 ± 1.442 * | 1.082 ± 0.025 | −12.380 ± 0.286 | −164.46 ± 5.590 | −504.768 ± 18.919 |
| 305 | 3.545 ± 0.025 * | 5.043 ± 1.095 * | 1.024 ± 0.055 | −11.810 ± 0.724 | |||
| 310 | 3.412 ± 0.045 * | 0.071 ± 0.021 * | 0.634 ± 0.026 * | −7.323 ± 0.343 * | |||
| 315 | 3.182 ± 0.013 * | 0.009 ± 0.001 * | 0.437 ± 0.011 * | −5.196 ± 0.151 * | |||
| Quercetin | 300 | 17.510 ± 0.152 * | 88.369 ± 5.236 * | 1.048 ± 0.145 | −13.444 ± 1.962 | −132.616 ± 3.859 | −390.267 ± 12.102 |
| 305 | 16.719 ± 0.135 * | 69.072 ± 2.726 * | 1.014 ± 0.176 | −13.167 ± 2.319 | |||
| 310 | 15.632 ± 0.109 * | 2.486 ± 0.421 * | 0.801 ± 0.055 | −10.755 ± 0.812 * | |||
| 315 | 13.718 ± 0.058 * | 0.345 ± 0.091 * | 0.645 ± 0.023 * | −0.899 ± 0.377 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes. Molecules 2017, 22, 1520. https://doi.org/10.3390/molecules22091520
Strugała P, Tronina T, Huszcza E, Gabrielska J. Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes. Molecules. 2017; 22(9):1520. https://doi.org/10.3390/molecules22091520
Chicago/Turabian StyleStrugała, Paulina, Tomasz Tronina, Ewa Huszcza, and Janina Gabrielska. 2017. "Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes" Molecules 22, no. 9: 1520. https://doi.org/10.3390/molecules22091520