Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion
Abstract
:1. Introduction
2. Results and Discussion
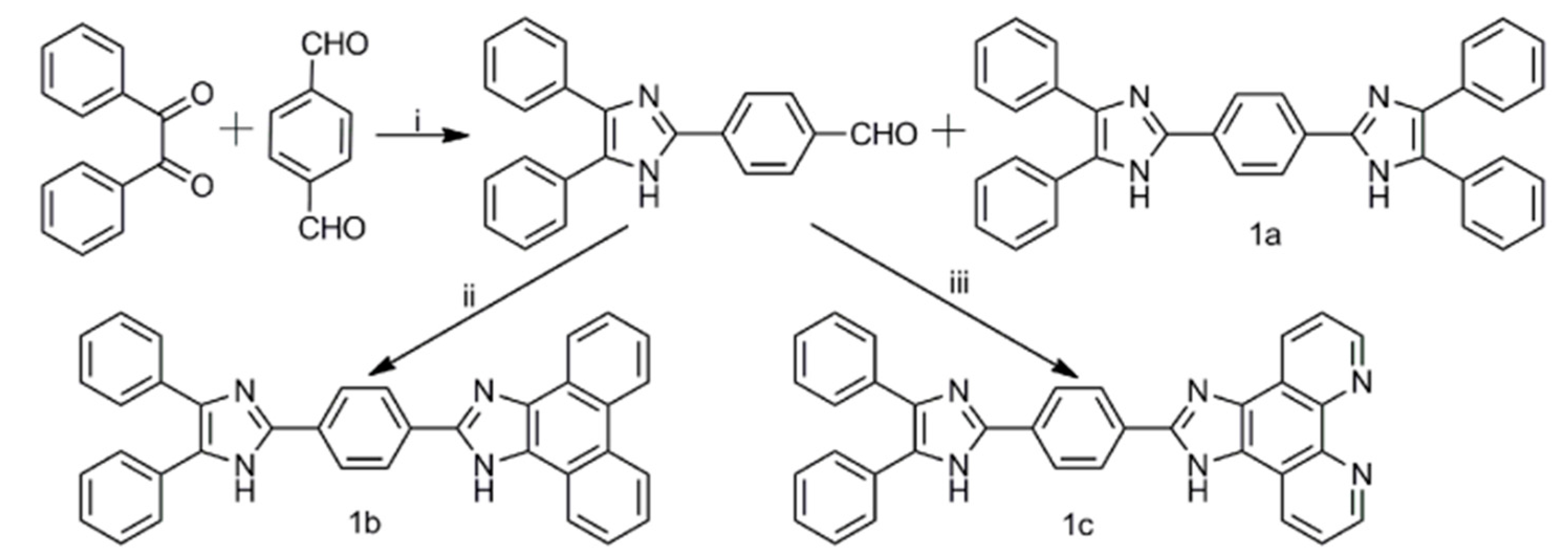
2.1. Synthesis of Compounds 1a–1c
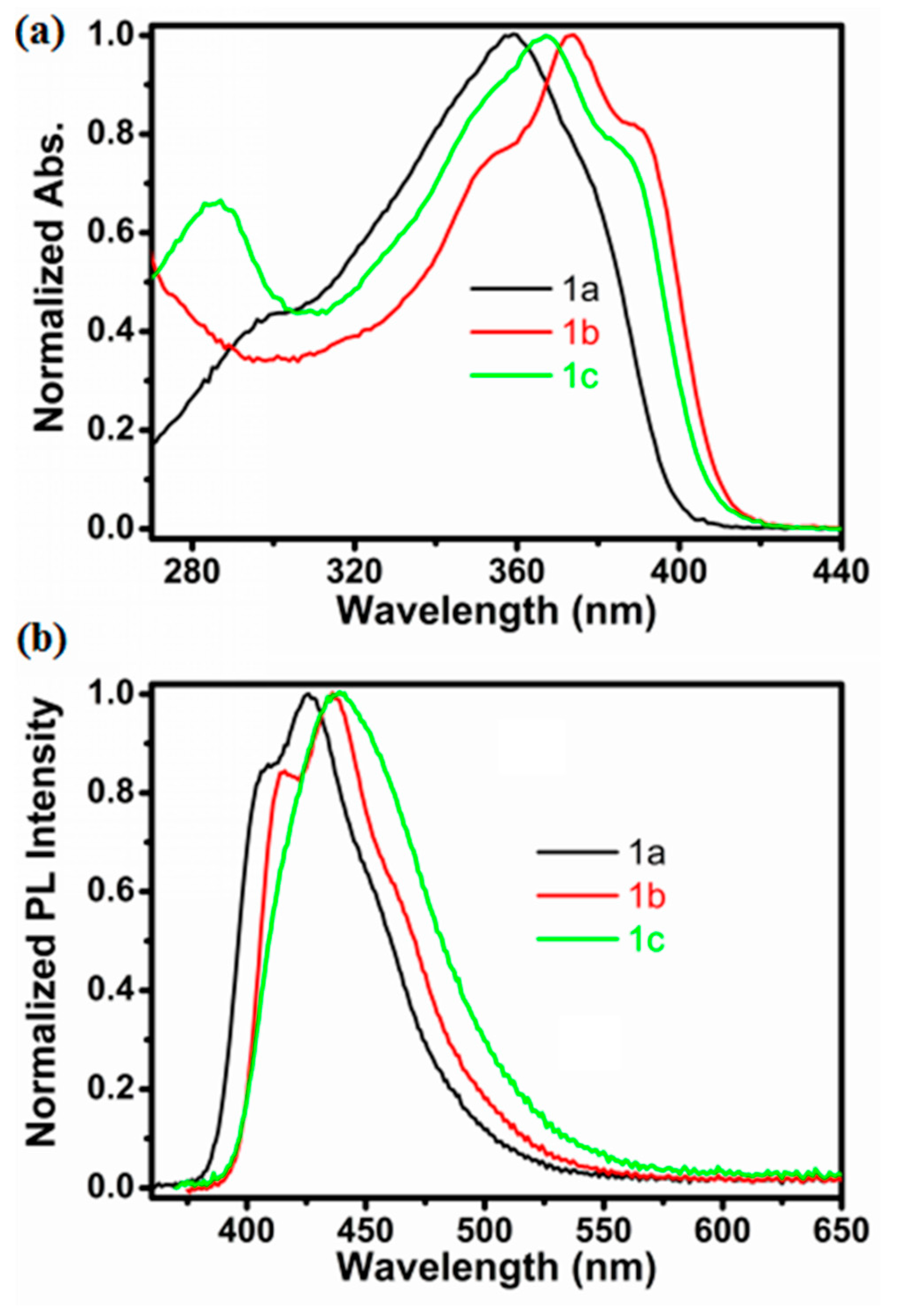
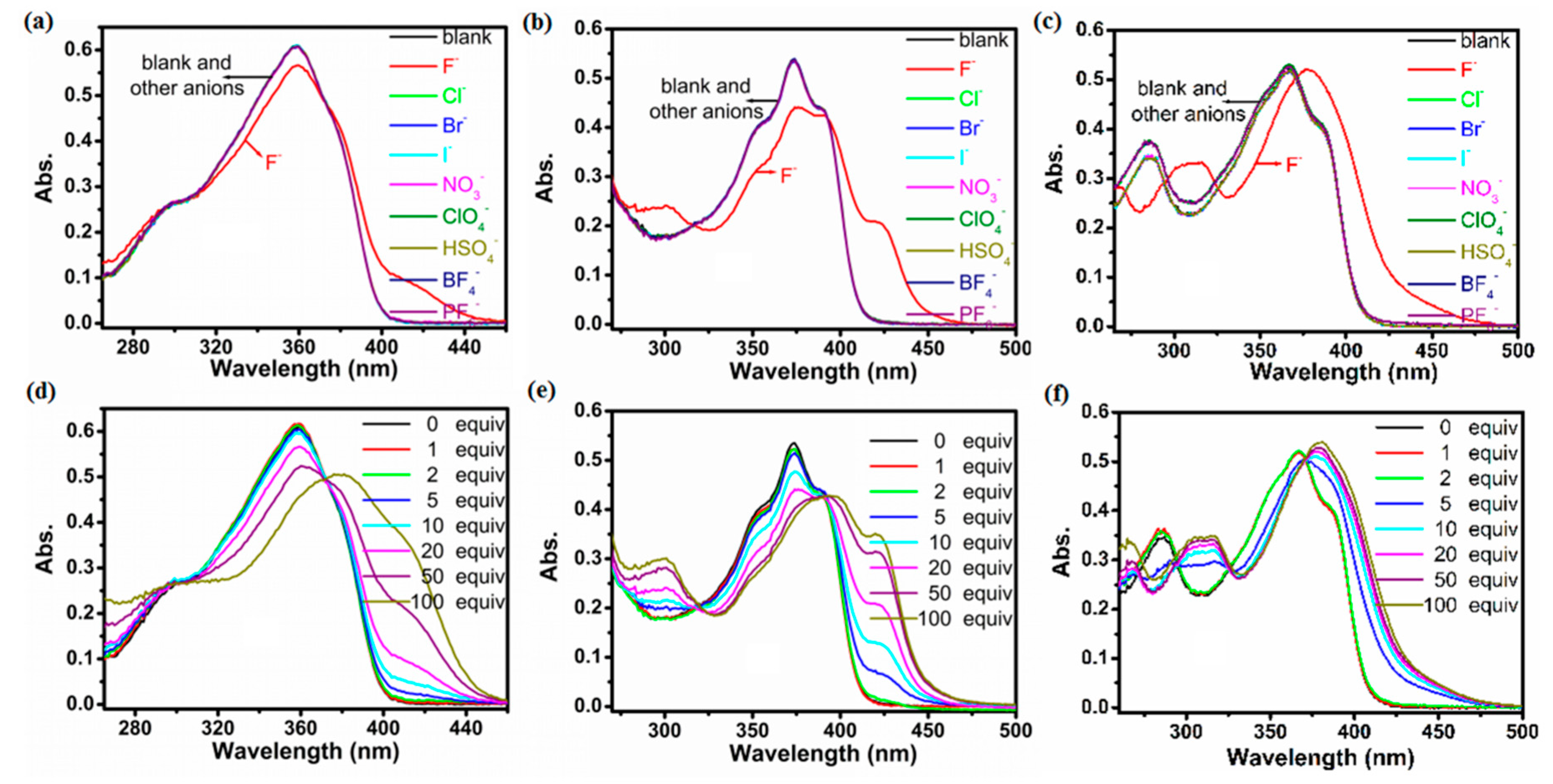
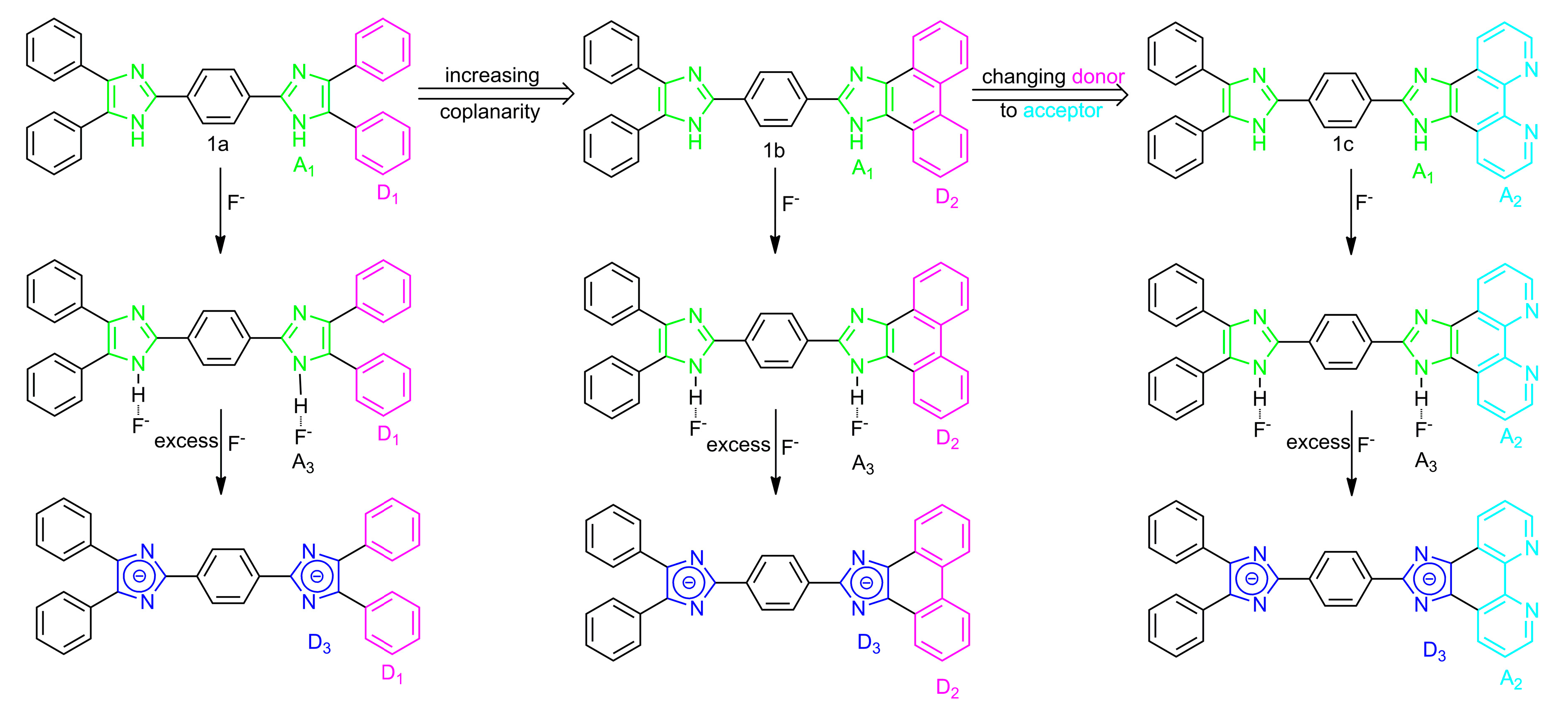
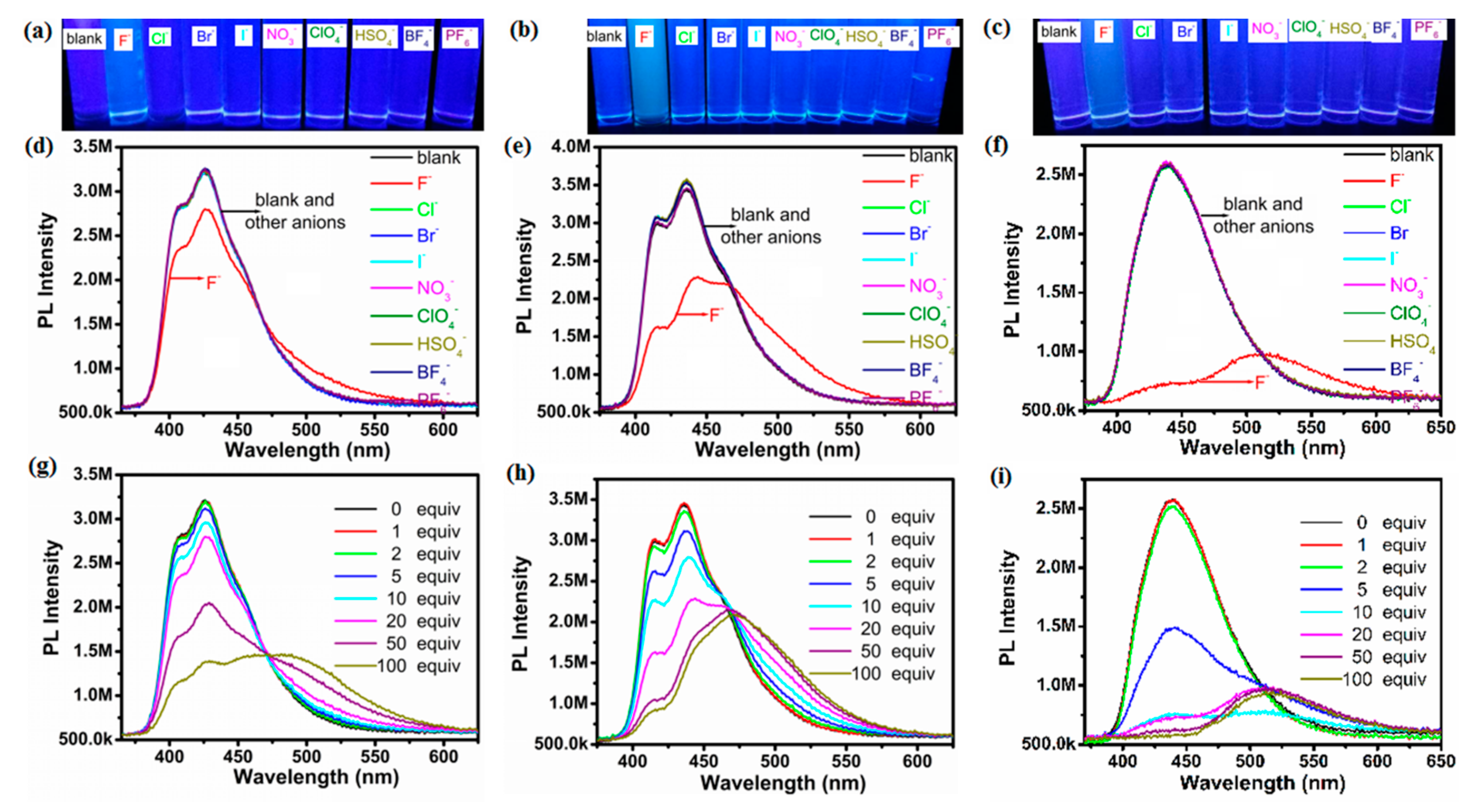
2.2. Optical Properties of Compounds 1a–1c
3. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Descalzo, A.B.; Rurack, K.; Weisshoff, H.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P.; Hoffmann, K.; Soto, J. Rational design of a chromo- and fluorogenic hybrid chemosensor material for the detection of long-chain carboxylates. J. Am. Chem. Soc. 2005, 127, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Vetrichelvan, M.; Nagarajan, R.; Valiyaveettil, S. Carbazole-containing conjugated copolymers as colorimetric/fluorimetric sensor for iodide anion. Macromolecules 2006, 39, 8303–8310. [Google Scholar] [CrossRef]
- Lin, T.-P.; Chen, C.-Y.; Wen, Y.-S.; Sun, S.-S. Synthesis, photophysical, and anion-sensing properties of quinoxalinebis (sulfonamide) functionalized receptors and their metal complexes. Inorg. Chem. 2007, 46, 9201–9212. [Google Scholar] [CrossRef] [PubMed]
- Ajayakumar, M.; Mukhopadhyay, P.; Yadav, S.; Ghosh, S. Single-electron transfer driven cyanide sensing: A new multimodal approach. Org. Lett. 2010, 12, 2646–2649. [Google Scholar] [CrossRef] [PubMed]
- Eun Jun, M.; Roy, B.; Han Ahn, K. “Turn-on” fluorescent sensing with “reactive” probes. Chem. Commun. 2011, 47, 7583–7601. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef] [PubMed]
- Ros-Lis, J.V.; Martinez-Manez, R.; Soto, J. Subphthalocyanines as fluoro-chromogenic probes for anions and their application to the highly selective and sensitive cyanide detection. Chem. Commun. 2005, 5260–5262. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Müller, A.M.; Bardeen, C.J.; Cheng, Q. Self-assembly combined with photopolymerization for the fabrication of fluorescence “turn-on” vesicle sensors with reversible “on-off” switching properties. Adv. Mater. 2006, 18, 55–60. [Google Scholar] [CrossRef]
- Garcia, F.; Garcia, J.M.; Garcia-Acosta, B.; Martinez-Manez, R.; Sancenon, F.; Soto, J. Pyrylium-containing polymers as sensory materials for the colorimetric sensing of cyanide in water. Chem. Commun. 2005, 2790–2792. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, J.; Ma, J.; Zhu, D.; Zhang, Y.; Liang, L.; Lu, M. An efficient long fluorescence lifetime polymer-based sensor based on europium complex as chromophore for the specific detection of F−, CH3COO−, and H2PO4−. Polym. Chem. 2012, 3, 2640–2648. [Google Scholar] [CrossRef]
- Isaad, J.; Perwuelz, A. New color chemosensors for cyanide based on water soluble azo dyes. Tetrahedron Lett. 2010, 51, 5810–5814. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.F.; Yoon, J. Fluorescence and colorimetric chemosensors for fluoride-ion detection. Chem. Rev. 2014, 114, 5511–5571. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Feng, J.; Hu, D.; Wang, S.; Li, S.; Li, Y.; Yang, G. A rapid aqueous fluoride ion sensor with dual output modes. Angew. Chem. Int. Ed. 2010, 49, 4915–4918. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Swager, T.M. A fluorescent self-amplifying wavelength-responsive sensory polymer for fluoride ions. Angew. Chem. Int. Ed. 2003, 42, 4803–4806. [Google Scholar] [CrossRef] [PubMed]
- Ke, B.; Chen, W.; Ni, N.; Cheng, Y.; Dai, C.; Dinh, H.; Wang, B. A fluorescent probe for rapid aqueous fluoride detection and cell imaging. Chem. Commun. 2013, 49, 2494–2496. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Piersanti, G.; Retini, M.; Varrese, M.A.; Zappia, G. New coumarin-urea based receptor for anions: A selective off–on fluorescence response to fluoride. Tetrahedron 2012, 68, 3768–3775. [Google Scholar] [CrossRef]
- Martinez-Manez, R.; Sancenon, F. New advances in fluorogenic anion chemosensors. J. Fluoresc. 2005, 15, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Qi, H.; Wang, L.; Su, Z.; Wang, G. A ratiometric fluorescent probe for fluoride ion employing the excited-state intramolecular proton transfer. Talanta 2009, 80, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Liu, B.; Wang, H.; Tian, J.; Bai, R. A “naked eye” and ratiometric fluorescent chemosensor for rapid detection of F− based on combination of desilylation reaction and excited-state proton transfer. Chem. Commun. 2011, 47, 3957–3959. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, G.; Chellappa, D. Rhodamine based sensor for naked-eye detection and live cell imaging of fluoride ions. J. Mater. Chem. B 2013, 1, 5768–5772. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.; Koh, M.; Park, S.B.; Hong, J.I. Fluorescent probe for detection of fluoride in water and bioimaging in A549 human lung carcinoma cells. Chem. Commun. 2009, 21, 4735–4737. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Mosca, L. The interaction of fluoride with fluorogenic ureas: An ON1-OFF-ON2 response. J. Am. Chem. Soc. 2013, 135, 6345–6355. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Singha, S.; Wang, T.; Seo, E.; Lee, J.H.; Lee, S.J.; Kim, K.H.; Ahn, K.H. In vivo two-photon fluorescent imaging of fluoride with a desilylation-based reactive probe. Chem. Commun. 2012, 48, 10243–10245. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Zhao, Y.G.; Duan, C.Y.; Zhang, B.G.; Bai, Z.P. A highly selective chromo- and fluorogenic dual responding fluoride sensor: Naked-eye detection of F- ion in natural water via a test paper. Dalton Trans. 2006, 30, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wu, Z.; Zhou, Y.; Li, Y.; Xu, Z. Colorimetric fluoride sensor based on 1,8-naphthalimide derivatives. Dyes Pigments 2011, 91, 442–445. [Google Scholar] [CrossRef]
- Rao, M.R.; Mobin, S.M.; Ravikanth, M. Boron–dipyrromethene based specific chemodosimeter for fluoride ion. Tetrahedron 2010, 66, 1728–1734. [Google Scholar] [CrossRef]
- Hou, P.; Chen, S.; Wang, H.; Wang, J.; Voitchovsky, K.; Song, X. An aqueous red emitting fluorescent fluoride sensing probe exhibiting a large Stokes shift and its application in cell imaging. Chem. Commun. 2014, 50, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, Q.; Li, Z.; Lai, G.; Jiang, J.; Shen, Z. A specific chemodosimeter for fluoride ion based on a pyrene derivative with trimethylsilylethynyl groups. Org. Biomol. Chem. 2011, 9, 4558–4562. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Das, A.K.; Manna, A.; Maity, A.K.; Fun, H.-K.; Quah, C.K.; Saha, P. A colorimetric and ratiometric fluorescent turn-on fluoride chemodosimeter and application in live cell imaging: High selectivity via specific SiO cleavage in semi aqueous media and prompt recovery of ESIPT along with the X-ray structures. Tetrahedron Lett. 2014, 55, 2633–2638. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Wang, Z.; Zhang, Q. Azaacenes as active elements for sensing and bio applications. J. Mater. Chem. B 2016, 4, 7060–7074. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Wang, Z.; Liu, G.; Nie, L.; Ganguly, R.; Li, Y.; Zhang, Q. Synthesis, physical properties, and sensing behaviour of a novel naphthalenediimide derivative. Dyes Pigments 2016, 131, 224–230. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Karmakar, P.; Roy, J.; Manna, S.; Maiti, K.; Sahoo, P.; Mandal, D. Colorimetric and ratiometric fluorescent chemosensor for fluoride ions based on phenanthroimidazole (PI): Spectroscopic, NMR and density functional studies. RSC Adv. 2015, 5, 37935–37942. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Cyanide and fluoride colorimetric sensing by novel imidazo-anthraquinones functionalised with indole and carbazole. Supramol. Chem. 2013, 26, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Thomas, K.R.J. 2-Hydroxyarylimidazole-based colorimetric and ratiometric fluoride ion sensors. RSC Adv. 2014, 4, 56466–56474. [Google Scholar] [CrossRef]
- Gwon, S.Y.; Kim, S.H. Anion sensing and F(-)-induced reversible photoreaction of D-pi-A type dye containing imidazole moiety as donor. Spectrochim. Acta A 2014, 117, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Li, Q.; Lan, H.; Xiao, S.; Li, Y.; Tan, R.; Yi, T. A fluorescent non-conventional organogelator with gelation-assisted piezochromic and fluoride-sensing properties. Dyes Pigments 2017, 137, 111–116. [Google Scholar] [CrossRef]
- Manivannan, R.; Satheshkumar, A.; Elango, K.P. Tuning of the H-bonding ability of imidazole N–H towards the colorimetric sensing of fluoride and cyanide ions as their sodium salts in water. New J. Chem. 2013, 37, 3152–3160. [Google Scholar] [CrossRef]
- Jayasudha, P.; Manivannan, R.; Elango, K.P. Highly selective colorimetric receptors for detection of fluoride ion in aqueous solution based on quinone-imidazole ensemble—Influence of hydroxyl group. Sens. Actuator B Chem. 2016, 237, 230–238. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, R.; Agarwal, M. Rationally designed tri-armed imidazole–indole hybrids as naked eye receptors for fluoride ion sensing. Synth. Commun. 2017, 47, 1307–1318. [Google Scholar] [CrossRef]
- Gu, P.-Y.; Gao, J.; Zhang, Q.; Liu, G.; Zhou, F.; Xu, Q.-F.; Lu, J.-M. Tuning optical properties of phenanthroline derivatives through varying excitation wavelength and pH values. J. Mater. Chem. C 2014, 2, 1539–1544. [Google Scholar] [CrossRef]
- Tian, M.; Wang, C.; Wang, L.; Luo, K.; Zhao, A.; Guo, C. Study on the synthesis and structure-effect relationship of multi-aryl imidazoles with their fluorescence properties. Luminescence 2014, 29, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, X.; Zhou, F.; Weng, L.-P.; Guo, L.-T.; Chen, M.; Chao, H.; Ji, L.-N. pH responsive luminescent switches of ruthenium(II) complexes containing two imidazole groups: Synthesis, spectroscopy, electrochemistry and theoretical calculations. Inorg. Chim. Acta 2009, 362, 4960–4966. [Google Scholar] [CrossRef]
- Xie, N.; Chen, Y. Synthesis and photophysical properties of 1,4-bis(4,5-diarylimidazol) benzene dyes. J. Photochem. Photobiol. A 2007, 189, 253–257. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Liu, R.; Zhu, X.-L.; Cai, X.; Zhu, H.-J. A highly efficient and selective probe for F− detection based on 1H-imidazo[4,5-b]phenazine derivative. Chin. Chem. Lett. 2015, 26, 339–342. [Google Scholar] [CrossRef]
- Niu, H.T.; Su, D.; Jiang, X.; Yang, W.; Yin, Z.; He, J.; Cheng, J.P. A simple yet highly selective colorimetric sensor for cyanide anion in an aqueous environment. Org. Biomol. Chem. 2008, 6, 3038–3040. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a–1c are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, F. Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion. Molecules 2017, 22, 1519. https://doi.org/10.3390/molecules22091519
Zhang L, Liu F. Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion. Molecules. 2017; 22(9):1519. https://doi.org/10.3390/molecules22091519
Chicago/Turabian StyleZhang, Liang, and Fang Liu. 2017. "Synthesis of Bisimidazole Derivatives for Selective Sensing of Fluoride Ion" Molecules 22, no. 9: 1519. https://doi.org/10.3390/molecules22091519





