1. Introduction
People in China and other Asian countries have used Traditional Chinese Medicine (TCM) to treat various diseases for centuries.
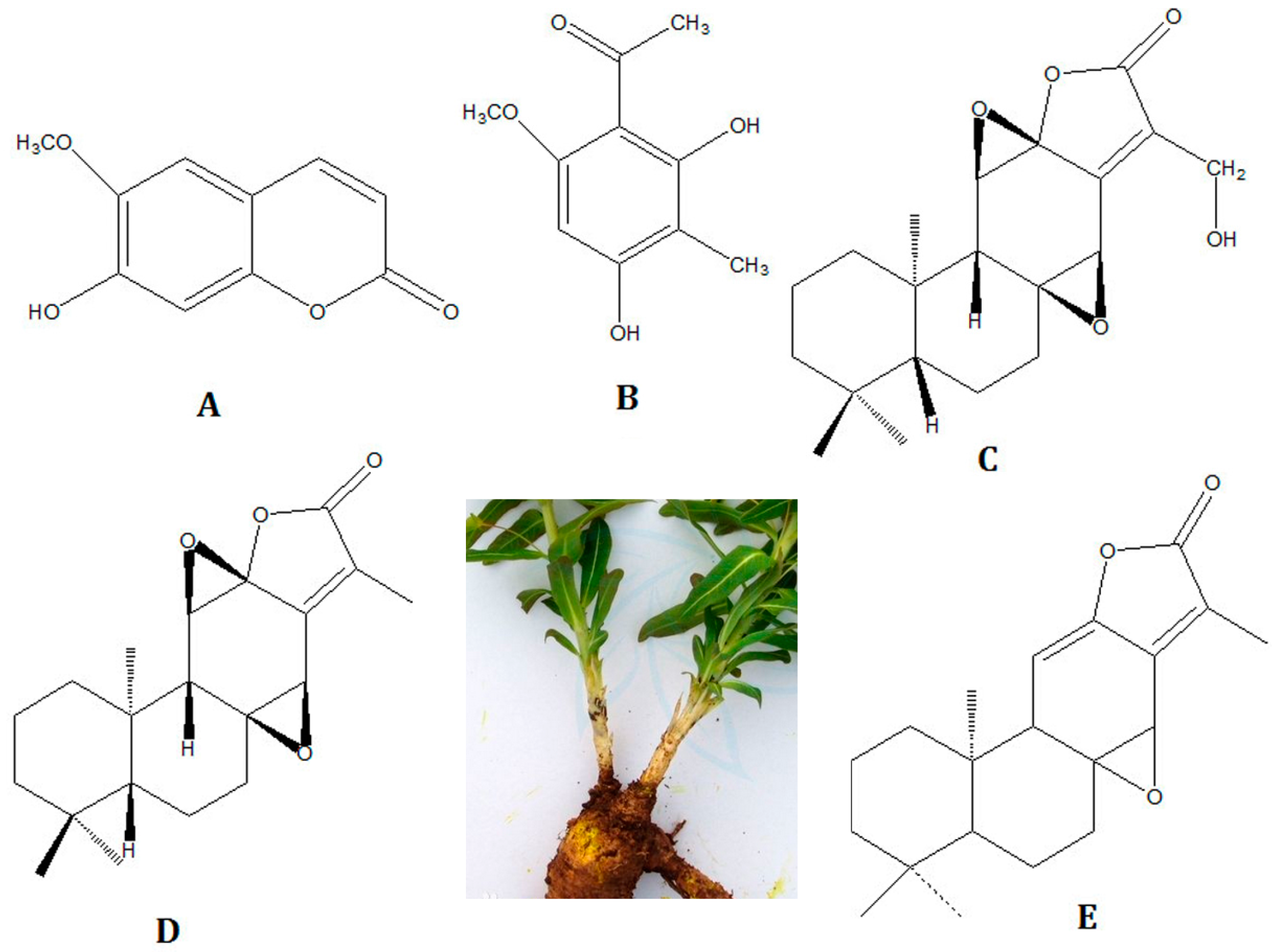
Euphorbia fischeriana also known as “Langdudaji” in China, the root of
E. fischeriana Steud., is one of the most famous TCM herbs. It has been used in many TCM formulations for thousands of years. It has been used for the treatment of edema, phlegm accumulation, inflammation, ascites and cancer in clinical practice for many years and has shown great efficacy [
1,
2,
3]. Modern medical research showed that the extracts of
E. fischeriana were found to inhibit the growth of Lewis lung carcinoma and ascetic hepatoma in mice [
4]. Hot AcOEt extracts and cold Et
2O extracts of
E. fischeriana showed most effective inhibition rates on tuberculosis bacillus in vitro [
5]. The crude extracts of
E. fischeriana can increase survival rate of the mice inoculated with L
615 leukemia. Liu et al. obtained jolkinolide A, jolkinolide B and 17-hydroxyjolkinolide B from ethanol extracts of
Euphorbia fischeriana which exhibited nematicidal activity [
6]. Previous studies of this plant have shown that it mainly contains diterpenoids [
7,
8], triterpenoids [
9] and steroids [
10]. Terpenoids, which have an isoprene or isopentane type skeleton, are considered the major constituents and the main bioactive ingredients in the
E. fischeriana. Up to now, more than 40 of these terpenoids have been isolated from various parts of
E. fischeriana [
11]. Usually, Scopoletin, 2,4-Dihydroxy-6-methoxy-3-methylacetophenone, 17-Hydroxyjolkinolide B, Jolkinolide B and Jolkinolide A are the most abundant among these founded terpenoids. The main terpenoids extract have been demonstrated to possess similar pharmacological bioactivity, including strong antitumor activity against several tumor lines such as human prostate, hepatic carcinoma, and leukemia cancer [
12,
13], anti-tuberculosis effect [
14], and antibacterial effect [
15]. Scopoletin possess enhancing effects on lymphocyte mitogen responsiveness, it could be a potential anti-tumoral compound to use for cancer treatment [
16]; 2,4-Dihydroxy-6-methoxy-3-methylacetophenone has inhibitive activity against mycobacterium tuberculosis [
17]; 17-Hydroxyjolkinolide B can inhibit growth and induce apoptosis of tumor cells, which is a promising anticancer drug candidate as a potent signal transducer and activator of transcription signaling inhibitor [
18]; Jolkinolide B has the anti-proliferation effect on human chronic myeloid leukemia cells [
19,
20]; and Jolkinolide A has a significant inhibition activity of the growth of cells of S-180 and Ehrlich’s ascites carcinoma [
21].
Due to the wide range of biological activities, the use of
E. fischeriana has increased vigorously and gained popularity. However, the content and distribution of terpenoids in
E. fischeriana are affected by different plant origins and harvest seasons. Therefore, a simple and rapid method to extract and determine major terpenoids, especially the two phenolics and three terpenoids in
E. fischeriana is extremely desirable to quality control of
E. fischeriana. The herb and the five compounds structures are shown in
Figure 1. Up to now, high performance liquid chromatography (HPLC) is the most common methods to analyze the major terpenoids in
E. fischeriana [
22,
23]. Liquid chromatography tandem mass spectrometry (LC-MS/MS) [
24] and high performance liquid chromatography combined with evaporative light scattering detection (HPLC-ELSD) [
25] are also applied to analyze the major terpenoids in
E. fischeriana as well. During these analytical processes, the sample extraction methods including heat reflux, soxhlet or sonication were usually used to extract terpenoids from
E. fischeriana. However, these conventional methods were high solvent and time consuming, and requiring additional cleanup, filtration and concentration steps. Until now, there is no effective standardized extraction method for analyzing terpenoids in
E. fischeriana. Therefore, an alternatively simple and effective extraction method is of great necessary in the recent years.
Barker firstly introduced matrix solid phase dispersion (MSPD) technology in 1989 for the extraction of drug residues from animal tissue [
26]. Since then, the technique has induced considerable interests because of the unique properties of MSPD providing the simple, low-cost and convenient benefits. As one of the most promising techniques, MSPD has been successfully applied to solve many difficult analytical problems. Recently, MSPD has been applied more and more frequently as a potential and effective alternative to conventional extraction methods in the extraction of active ingredients from medicinal plants [
27,
28]. However, to the best of our knowledge, there is no report on MSPD as an extraction method for the simultaneous extraction of active compounds, mainly terpenoids, from
E. fischeriana in the literature.
In this study, to achieve the maximum extraction yield, MSPD as alternative sample preparation method followed by ultra-performance liquid chromatography coupled with the quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS) separation was applied to extract and determine the main two phenolics and three terpenoids and other components in E. fischeriana. The effects of MSPD extraction for terpenoids were evaluated and optimized by various operating parameters, including dispersing sorbent, elution solvent and volume, and the ratio of dispersing sorbent to sample. Then, as validation for HPLC method, linearity, precision, accuracy, etc. were evaluated. MSPD-UPLC/Q-TOF-MS, as a powerful technique, was used for characterization of the main components in E. fischeriana. In addition, we compare the extraction yield obtained by the MSPD developed with those obtained by conventional extraction methods. It was expected that this research would be helpful for control the quality and make sure clinical therapeutic efficacy of E. fischeriana.
3. Materials and Methods
3.1. Chemicals and Reagents
All chemicals and reagents used were of the highest grade available. Scopoletin, 2,4-Dihydroxy-6-methoxy-3-methylacetophenone, and Jolkinolide B were purchased from the National Institute for the Pharmaceutical and Biological Products (Beijing, China). The standards of 17-hydroxyjolkinolide B and Jolkinolide A were available from Qiqihar Medical University. The purity of all standards was above 98.0%. Formic acid was purchased from Kangkede Science and Technology Co., Ltd. (Tianjin, China). Methanol and acetonitrile (HPLC grade) were purchased from Dima technology Inc. (Richmond, VA, USA). Analytical grade methanol and ethanol were purchased from Tianjin Fuchen Chemical Factory (Tianjin, China). Ultra-pure water (18.2 MΩ) was prepared with a PALL Purelab plus water purification system (Ann Arbor, MI, USA). Silica gel (200–300 mesh), Florisil (100–200 mesh) and neutral alumina (200 mesh) were obtained from Qingdao Haiyang Chemical Subsidiary Factory (Qingdao, Shandong Province, China). C18-bonded silica (200–300 mesh) was obtained from National Institute for the control of Pharmaceutical and Biological Products (Beijing, China). All solvents were filtered through a 0.22 μm membrane and were then degassed by sonication in an ultrasonic bath before use.
3.2. Plant Materials
The Euphorbia fischeriana herbs were collected in Qiqihar (Qiqihar, Heilongjiang Province, China), Harbin (Harbin, Heilongjiang Province, China), Mudanjiang (Mudanjiang, Heilongjiang Province, China), Changchun (Changchun, Jilin Province, China) and Baoding (Baoding, Hebei Province, China) and verified as the genuine medicinal herbs by professor Lina Guo of Qiqihar Medical University. The voucher specimens are kept in the reference library for the medicinal herbs in Qiqihar Medical University.
3.3. Standard Solution
The individual standard stock solutions (1 mg/mL) of Scopoletin, 2,4-Dihydroxy-6-methoxy-3-methylacetophenone, 17-Hydroxyjolkinolide B, Jolkinolide B, and Jolkinolide A were prepared by dissolving accurate amounts of pure standards in methonal. Mixed stock solution at a final concentration ranging from 50 to 200 μg/mL was dissolved in methanol. Series of working standard solutions were obtained by further dilution from the mixed stock solutions with methanol to prepare calibration curve. All solutions were stored at 4 °C before analysis.
3.4. Sample Preparation for LC-Q-TOF-MS
3.4.1. MSPD Extraction
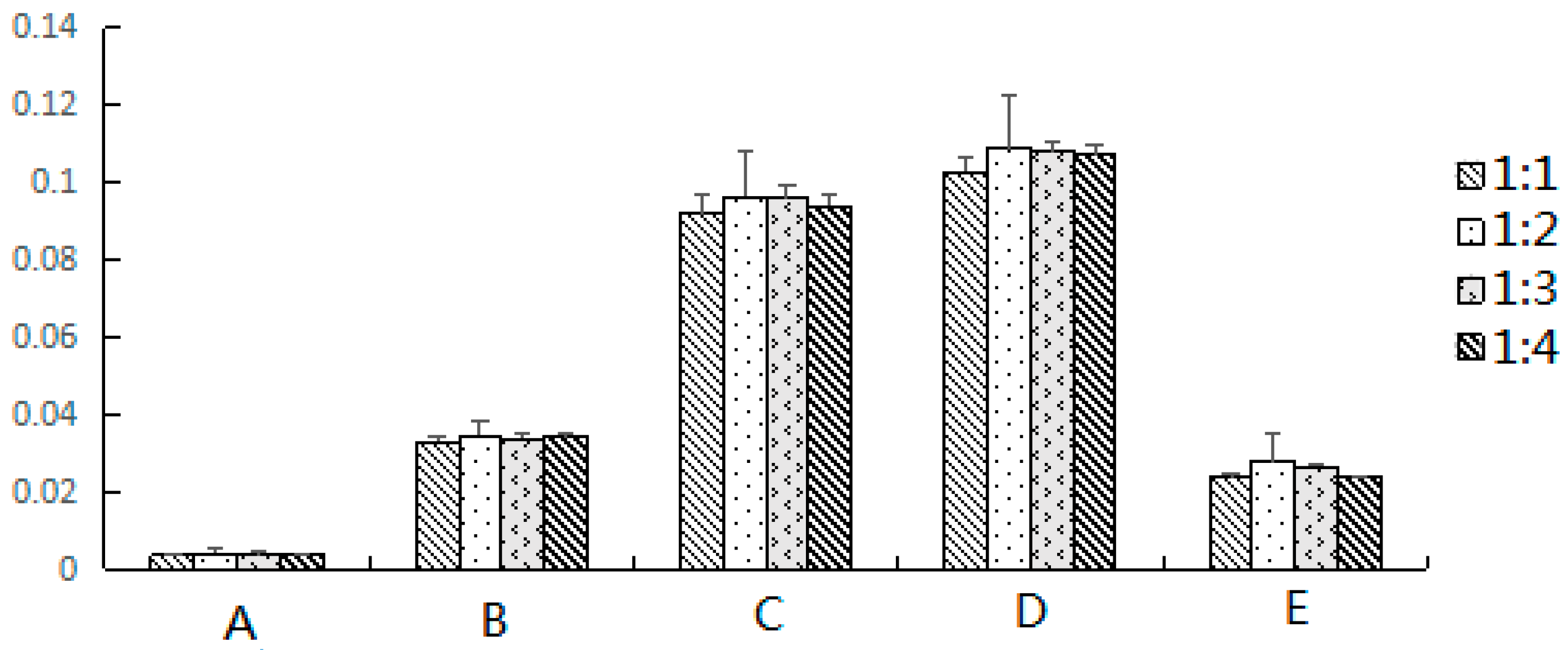
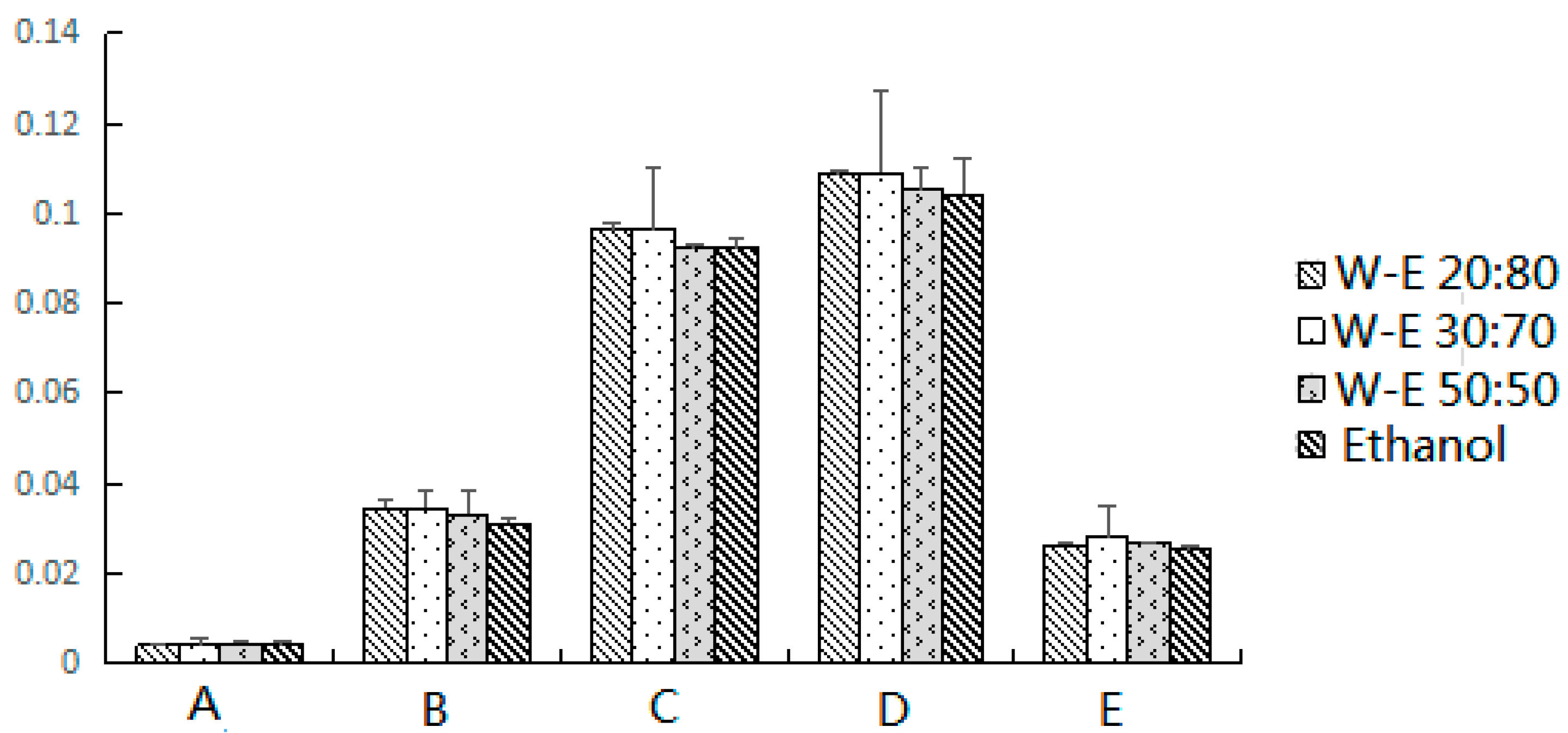
First, 0.1 g of sample and 0.2 g of dispersion adsorbent were placed in the agate mortar and blended using an agate pestle until a visually homogeneous dispersed mixture was obtained. The air-dried E. fischeriana samples used in the present study were pulverized to powder (has been pulverized through 200 mesh sieve). The complete dispersed mixture was transferred into the column (volume 5 mL, and diameter 8 mm) with a layer of absorbent cotton on the bottom (a thin layer to make sure the sample does not leak out). After fill, a thin layer of absorbent cotton was added at the top of the sample. Then the column was eluted with 10 mL of water:ethanol (30:70, v:v) by gravity flow. The target analytes were eluted out and collected in a 25 mL brown volumetric flask, and filtered through a 0.45 μm filter membrane before analysis. Five microliters of the sample solution was injected to the instrument and separated under the chromatographic conditions.
3.4.2. Ultrasonic Extraction
Finely ground powder (1.0 g) was accurately weighed and extracted with 50 mL of 70% ethanol-water solution in ultrasonic bath (power: 400 W, frequency: 37 kHz) for 30 min in 40 °C and filtered. This extraction was repeated once more. The combined filtrate was evaporated to dryness. The residue was then dissolved and diluted using methanol to 50 mL volumetric flask and filtered through a 0.45 μm filter membrane before analysis.
3.4.3. Reflux Extraction
One gram of sample and 50 mL of 70% ethanol-water solution were put into a 500 mL distilling flask. The mixture was heated at 90 °C and refluxed for 2 h. The extract was transferred into a 50 mL of volumetric flask and diluted to the mark with ethanol. Before analysis, samples underwent filtration with a 0.45 μm filter membrane.
3.5. Analytical Method
LC-DAD analysis was performed on a Waters Alliance UPLC system (Waters, Milford, MA, USA), equipped with a binary solvent delivery system and Empower™ 3 software, 2489 ultraviolet detector and 2707 automatic sampler. Separations were performed on a waters ACQUITY BEH C18 column (2.1 mm × 100 mm, 1.8 μm) operating at 30 °C. Different mobile phase components, for example acetonitrile, methanol and aqueous, were evaluated. The proportions of organic and aqueous components of the mobile phase, the pH and the flow rate were systematically varied to optimize the method. The mobile phase eventually selected was a gradient prepared from acetonitrile (component A) and 0.1% (v/v) formic acid in water (component B). The UPLC elution condition was optimized as follows: 4% A (0–5 min), 4–35% A (5–10 min), 35–70% A (10–25 min), 70–80% A (25–35 min), and 80–100% A (35–40 min). The original composition was then used for 5 min to restore the initial conditions. The flow rate was set at 0.8 mL/min and the injection volume of reference compounds and samples was 5 μL. The analytes were monitored at 210 nm.
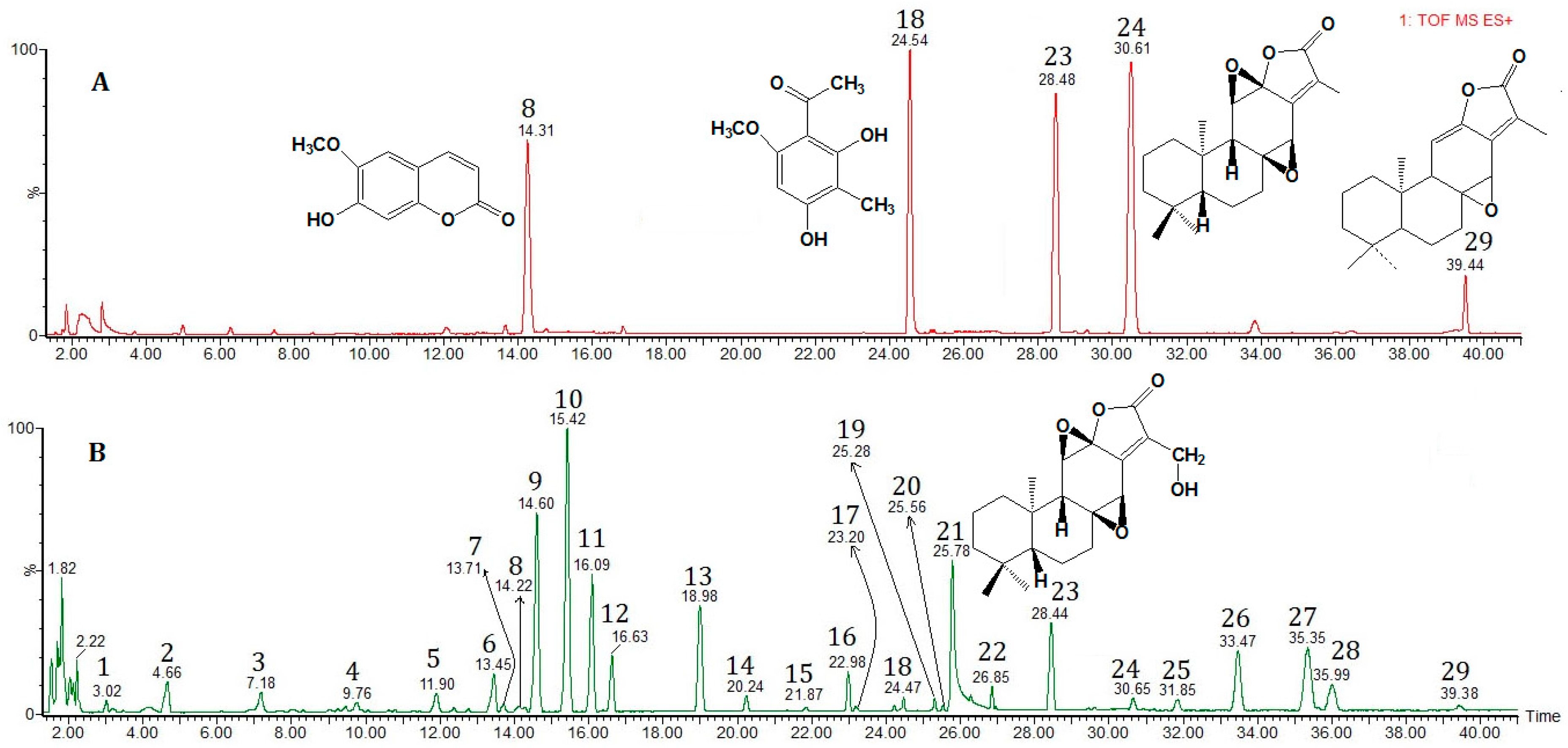
Identification of compounds in Euphorbia fischeriana by UPLC/Q-TOF-MS was performed with a Waters (USA) Xevo Q-TOF-MS equipped with an electrospray ionization (ESI) source. The electrospray source included one nebulizer used for the LC eluent and a second used for the internal reference solution, which consisted of solution formate introduced into the TOF-MS by means of an automated calibrant delivery system to obtain accurate mass measurement. Post-column sample introduction was achieved by use of a split value. UPLC/Q-TOF-MS analysis was performed in positive (ESI+) ion mode under the operating condition: Capillary voltage, 10 kV; cone voltage, 15 V; the flow rate of nebulizer gas and cone gas: 800 L/h and 50 L/h; gas temperature, 230 °C. Full scan spectra were acquired in the mass range of m/z 50–1000. The accurate mass and molecular formula assignments were obtained with the MassLynx 4.1 software (Waters MS Technologies, Milford, MA, USA).
3.6. Method Validation for Quantification
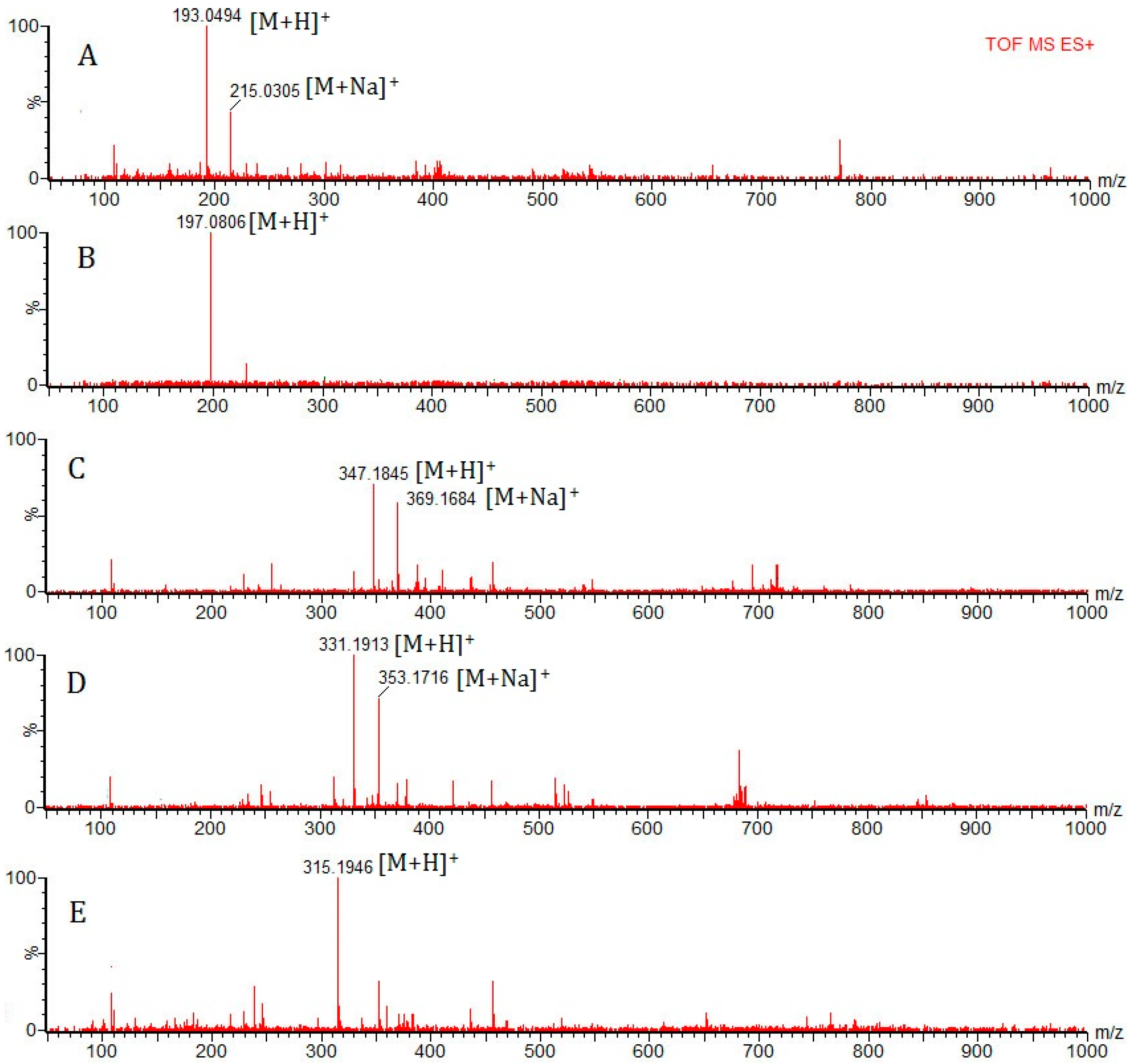
Among the 25 identified compounds, 5 compounds were quantified by UPLC/Q-TOF-MS. Peak area was integrated at the expected retention times under full scan MS conditions.
Calibration curves (seven points) were obtained using external standard calibrations for 5 analytes injecting the mixed standard solution in the wide concentration range: Scopoletin (A): 0.625–50 μg/mL; 2,4-Dihydroxy-6-methoxy-3-methylacetophenone (B): 2.5–200 μg/mL; 17-Hydroxyjolkinolide B (C): 1.25–100 μg/mL; Jolkinolide B (D): 2.5–200 μg/mL; and Jolkinolide A (E): 0.625–50 μg/mL. Calibration curves were established by plotting the peak area versus the concentration of each analyte.
The limits of detection (LODs) were estimated from the injection of a standard solution, successively diluted until reaching a concentration level corresponding to a signal-to-noise (S/N) ratio of 3. The limits of quantification (LOQs) were defined and determined as the minimum quantified amount of the analytes at a S/N ratio of about 10.
Precision of the method was checked for intraday and interday variability. The intraday variability study was carried out by the injection of the middle concentration standard solution six consecutive times in the same day. The interday variability study was carried out for three successive days using the same solution. The stability was tested with the sample at room temperature and analyzed at 0, 6, 12, 24 and 48 h within 2 days. To confirm the repeatability, six different samples solutions prepared from the same sample were analyzed. Variations were expressed by RSD.
The accuracy of the analytical method was determined by spiking into the Euphorbia fischeriana powder with different amounts of authentic standards with known contents of the five analytes. Then, the samples were treated according to the sample extraction procedure. Three replicates were performed for the test.
4. Discussion
To the best of our knowledge, this is the first time to determine the five active compounds in Euphorbia fischeriana using MSPD method for extraction. Comparing with traditional methods, such as Ultrasonic extraction and Reflux extraction, MSPD method has the advantages of time saving, less solvent consumption, no emulsification, etc. Importantly, MSPD will improve the recovery and enhance the detection capability of analytes through greatly enhancing the ability of separation and enrichment the target compounds. The results obtained in this paper show that the extraction yields of the two phenolics and three terpenoids obtained by the MSPD are higher than those of traditional reflux and sonication extraction methods. Identification of the traditional Chinese medicine is important to control the quality of the herbs and differentiate positive or negative herbs and to ensure efficacy and safely use in clinic. UPLC/Q-TOF-MS as a more and more important method in the study of traditional Chinese medicine combines high resolution, high selectivity and high separation advantages. With the development of traditional Chinese medicine, studies on the pharmacodynamic basic substances have become the focus of the whole academic community. Thus, the MSPD and UPLC/Q-TOF-MS techniques were introduced to analyze the material basis of Euphorbia fischeriana. This analysis method may facilitate the scientific extraction and quality control, as well as the elucidation of the action mechanism of traditional Chinese medicine.
Compounds of 2,4-Dihydroxy-6-methoxy-3-methylacetophenone and Jolkinolide B were also listed as the quality standard of
Euphorbia fischeriana in the “Common and important standard for drug safety (2006BAI14B01)” [
22]. Although it was contained in 2015 version of Chinese Pharmacopoeia, the detection methods are obsolete and insensitive; and there is no determination of the active compounds. At the same time, it is necessary to develop a method for chemical profiling to supplement the quality control of
Euphorbia fischeriana. The research on effective substance basis is momentous for the modern study of the Chinese herbs. To elute the target compounds completely with the minimum volume of elution solvent, 8 mL, 10 mL and 12 mL were studied. Finally, 10 mL was chosen considering the extraction efficiency and solvent consumption.
Among the four columns (Dikma–C
18, Agilent–C
18, Waters–ACQUITY BEH C
18 and Phenomenex–C
18) that were tested for the separation of the sample, Waters ACQUITY BEH C
18 column gave the best chromatographic resolution. Among the identified compounds, Compounds
1,
2,
3 and
17 listed in
Table 2 cannot be identified through the exact mass data according to the literature.