Fenton Discoloration of Ultrasonicated Purple Cactus Pear Juice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Color and Browning Index
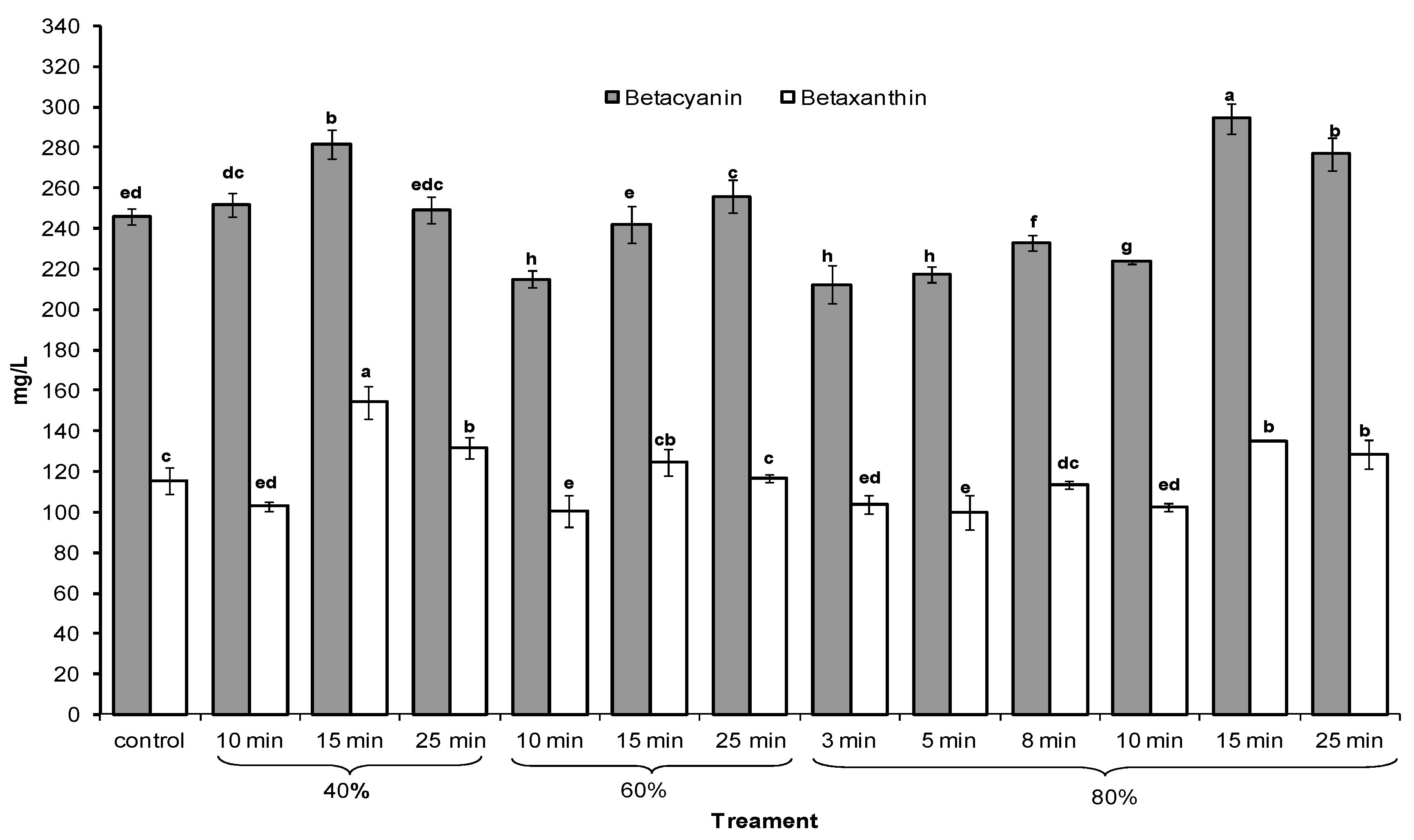
2.2. Betalains
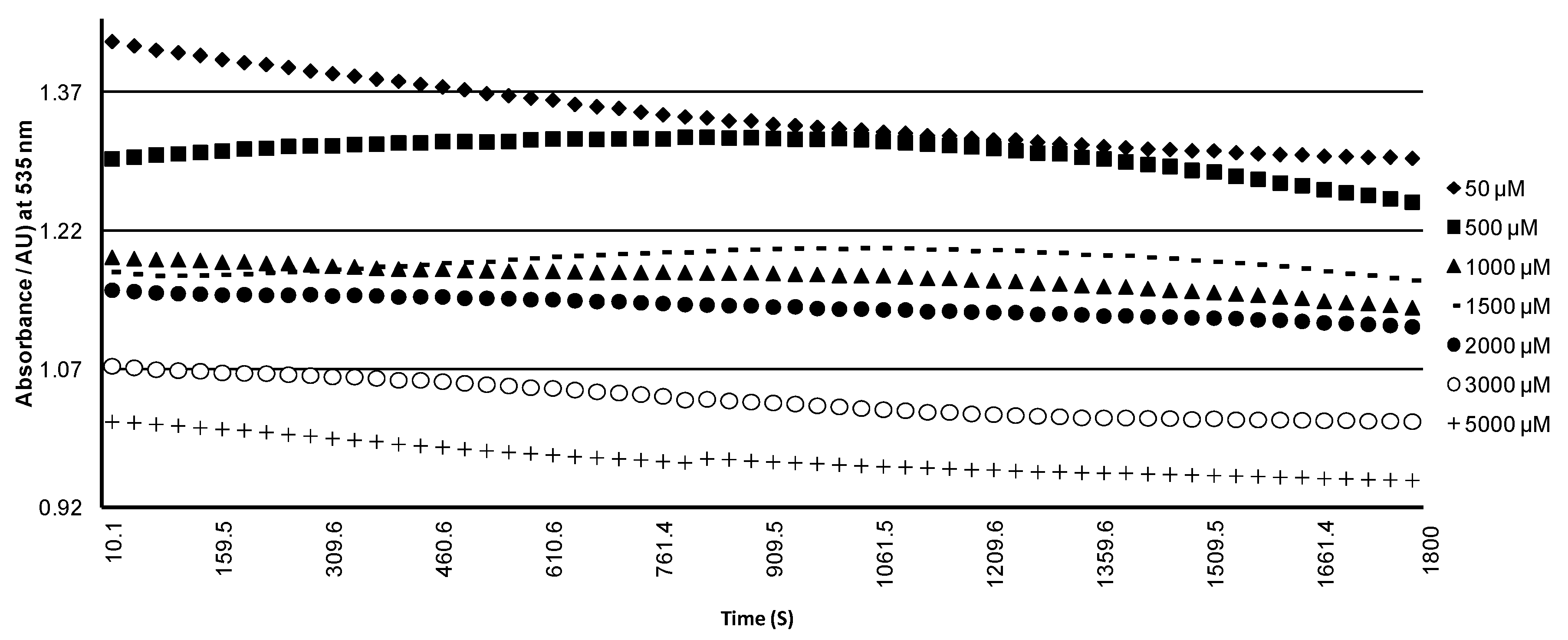
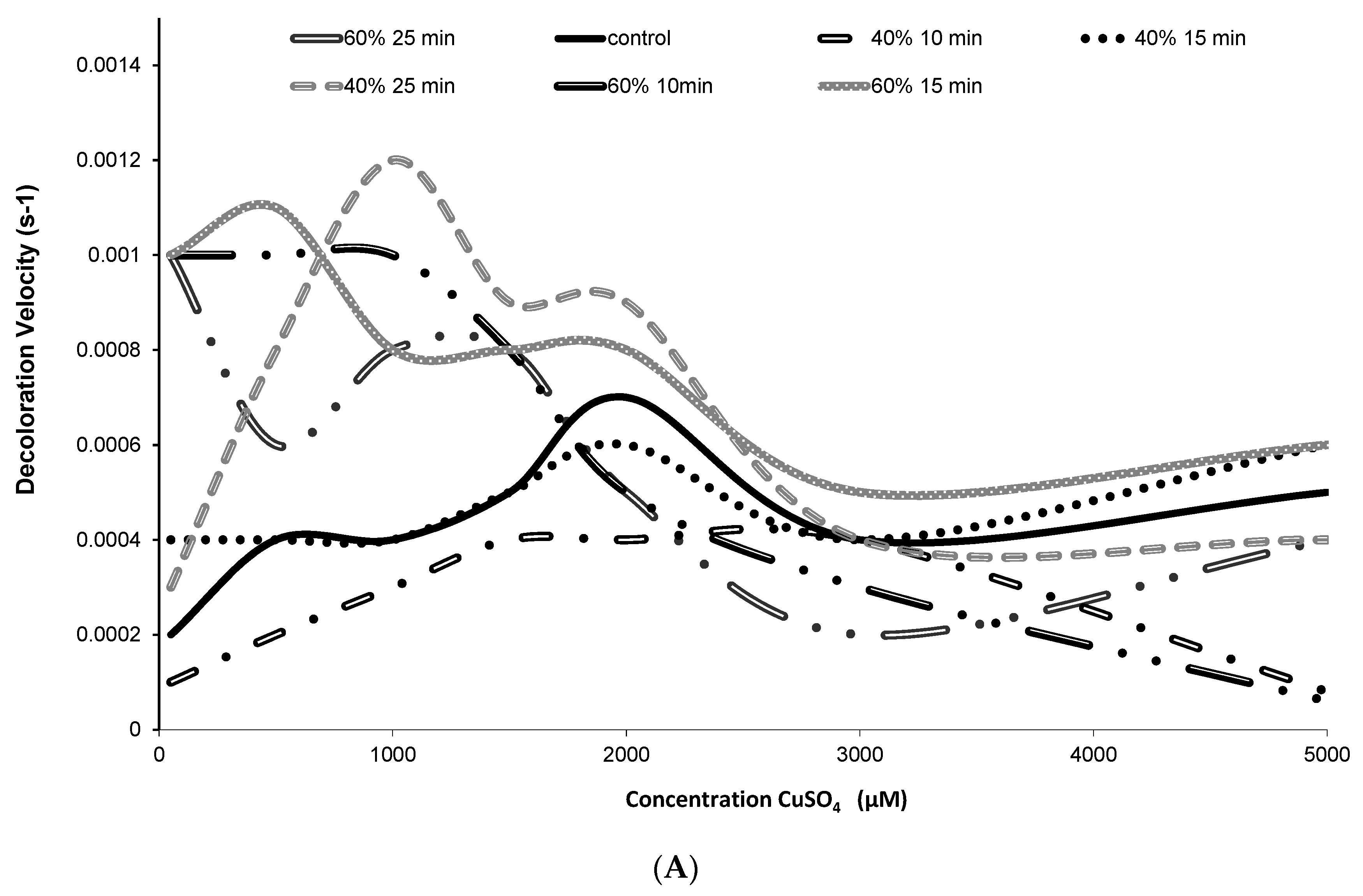
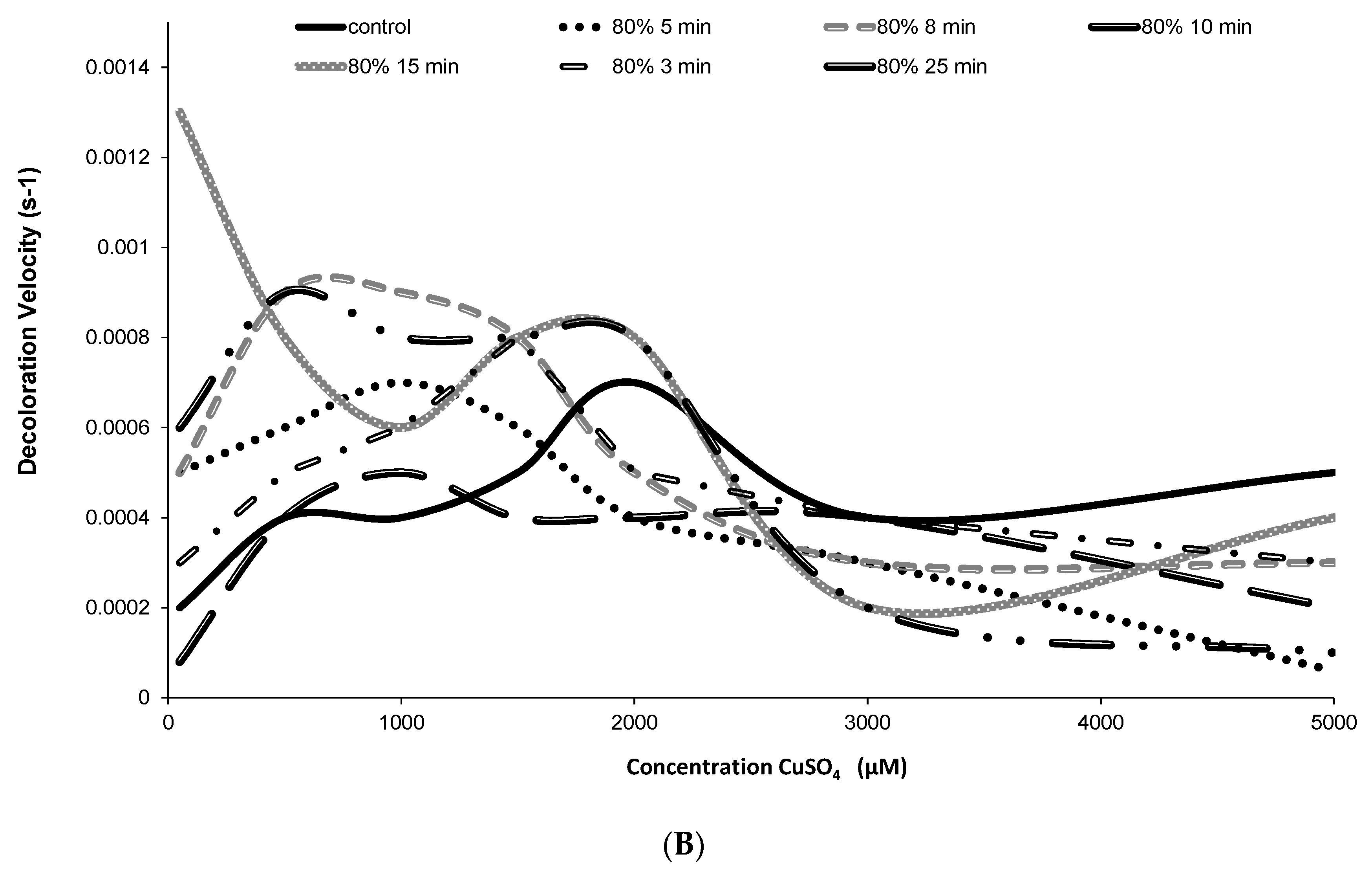
2.3. Release and Stability of Betacyanins in the Presence of Hydroxyl Radicals (HO•)
3. Materials and Methods
3.1. Preparation of Juice and Ultrasound Treatment
3.2. Determination of Color
3.3. Browning Index (BI)
3.4. Determination of Betalain Content
3.5. Stability of Betacyanin Pigments in the Presence of Hydroxyl Radicals (HO•)
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sala, F.J.; Burgos, J.; Condon, S.; Lopez, O.; Raso, J. Effect of heat and ultrasound on microorganisms and enzymes. In New Methods of Food Preservation; Gould, G.W., Ed.; Springer Science Business Media: Gaithersburg, MA, USA, 1995; pp. 176–204. [Google Scholar]
- Valdramaris, V.P.; Cullen, P.J.; Tiwari, B.K.; O’Donnell, C.P. Quantitative modeling approaches for ascorbic acid degradation and non-enzymatic browning of orange juice during ultrasound processing. J. Food Eng. 2010, 96, 449–454. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Delgado-Olivares, L.; Villanueva-Sánchez, J.; Alanís-García, E. Effects of ultrasoundtreatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013, 20, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cansino, N.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; Manríquez-Torres, J.D.J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochem. 2015, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Jolalpa-Barrera, J.L.; Aguilar-Zamora, A.; Ortiz-Barreto, O.; García-López, L. Production and commercialization of fresh prickly pear under different modalities in Hidalgo, Mexico. Rev. Mex. Agronegocios 2011, 28, 605–614. [Google Scholar]
- Cruz-Cansino, N.; Carrera, G.P.; Rojas, Q.Z.; Olivares, L.D.; García, E.A. Ultrasound Processing on Green Cactus Pear (Opuntia ficus Indica) Juice: Physical, Microbiological and Antioxidant Properties. J. Food Process. Technol. 2013, 4, 1–6. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, G.H.; Lee, H.S. Effect of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002, 35, 735–759. [Google Scholar] [CrossRef]
- Von Elbe, J.H.; Schwatz, S.J. Absence of mutagenic activity and a short-term toxity study of beet pigments as food colorants. Arch. Toxicol. 1981, 49, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aparicio, A.; Gutierrez-Lopez, G. Production of food related colorants by culture of plant cells. In Chemicals via Higher Plant Bioengineering; Shahidi, F., Kolodziejczyk, P., Whitaker, J.R., Lopez-Munguia, A., Fuller, G., Eds.; Springer Science Business Media: New York, NY, USA, 1999; Volume 464, pp. 195–210. [Google Scholar]
- Rodríguez-Sánchez, J.A.; Victoria, M.T.C.; Barragán-Huerta, B.E. Betaxanthins and antioxidant capacity in Stenocereuspruinosus: Stability and use in food. Food Res. Int. 2017, 91, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.K.; Hakimuddin, F.; Paliyath, G.; Fisher, H. Antioxidant and antiproliferative activity of polyphenols in novel high-polyphenol grape lines. Food Res. Int. 2008, 4, 419–428. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C1–3. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [PubMed]
- Wolfram, R.; Budinsky, A.; Efthimiou, Y.; Stomatopoulos, J.; Oguogho, A.; Sinzinger, H. Daily prickly pear consumption improves platelet function. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 61–66. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Shahidi, F.; Jeon, Y.J. Potential antioxidative effects of cactus pear fruit (Opuntiaficus-indica) extract on radical scavenging and DNA damage reduction in human peripheral lymphocytes. J. Food Lipids 2006, 13, 445–458. [Google Scholar] [CrossRef]
- Meydav, S.; Saguy, I.; Kopelman, I.J. Browning determination in citrus products. J. Agric. Food Chem. 1977, 25, 602–604. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Anthocyanins, characterization and measurement with UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Willey & Sons: Corvallis, OR, USA, 2001; pp. 1–13. [Google Scholar]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Castellar, R.; Obón, J.M.; Almela, L. Monitoring by liquid chromatography coupled to mass spectrometry the impact of pH and temperature on the pigment pattern of cactus pear fruit extracts. J. Chromatogr. Sci. 2007, 45, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Czapski, G. [24] Reaction of ·OH. Methods Enzymol. 1984, 105, 209–215. [Google Scholar] [PubMed]
- Friedrich, L.C.; de-Paiva e Silva Zanta, C.L.; Machulek, A., Jr.; de Oliveira Silva, V.; Herbert Quina, F. Interference of inorganic ions on phenol degradation by the Fenton reaction. Sci. Agric. 2012, 69, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, A.; Ferraz, A.; Contreras, D.; Rodríguez, J. Mechanism and applications of the Fenton reaction assisted by iron-reducing phenolic compounds. Quím. Nova 2007, 30, 623–628. [Google Scholar] [CrossRef]
- Sumaya-Martínez, M.T.; Cruz-Jaime, S.; Madrigal-Santillán, E.; García-Paredes, J.D.; Cariño-Cortés, R.; Cruz-Cansino, N.; Valadez-Vega, C.; Martinez-Cardenas, L.; Alanís-García, E. Betalain, acid ascorbic, phenolic contents and antioxidant properties of purple, red, yellow and white cactus pears. Int. J. Mol. Sci. 2011, 12, 6452–6468. [Google Scholar] [CrossRef]
- Gnanasekharan, V.; Shewfelt, R.L.; Chinnan, M.S. Detection of colour changes in green vegetables. J. Food Sci. 1992, 57, 149–154. [Google Scholar] [CrossRef]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color properties and stability of betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Inactivation kinetics of pectin methylesterase, cloud retention in sonicated orange juice. Innov. Food Sci. Emerg. Technol. 2009, 10, 166–171. [Google Scholar] [CrossRef]
- Morales, J.F.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and flurescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Fonteles, V.T.; Garcia-Maia-Costa, M.; Tiberio-de-Jesús, A.L.; Alcântara-de-Miranda, R.A.; Narciso-Fernandes, F.A.; Rodrigues, S. Power ultrasound processing of cantaloupe melón juice: Effects on quality parameters. Food Res. Int. 2012, 48, 41–48. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, Y.; Yue, T.; Chen, T.; Lo, Y.M. Effect of ultrasound treatments on thermoacidophilic Alicyclobacillus acidoterrestris in apple juice. J. Food Process. Preserv. 2009, 33, 370–383. [Google Scholar] [CrossRef]
- Piatelli, M. The Betalains: Structure, biosynthesis and chemical taxonomy. In The Biochemistry of Plants: A Comprensivetretise; Stumpf, P.K., Conn, E.E., Eds.; Academic Press: Davis, CA, USA, 1981; Volume 6, pp. 557–575. [Google Scholar]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidant. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Butera, D.; D’Arpa, D.; Di Gaudio, F.; Allegra, M.; Gentile, C.; Livrea, M.A. Increase resistance to oxidant of betalain-enriched human low density lipoproteins. Free Radic. Res. 2003, 37, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Butera, D.; Allegra, M.; Fazzari, M.; Livrea, M.A. Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells ex vivo-induced oxidative hemolysis in humans. J. Agric. Food Chem. 2005, 53, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- El-Samahy, S.K.; Abd El-Hady, E.A.; Habiba, R.A.; Moussa, T.A. Chemical and rheological characteristics of orange-yellow cactus pear pulp from Egypt. J. Prof. Assoc. Cactus Dev. 2006, 8, 39–51. [Google Scholar]
- Livrea, M.A.; Tesoriere, L. Antioxidative Effects of cactus pear (Opuntia ficus-indica (L) Mill) fruits from Sicily and biovailability of betalain components in healthy humans. Acta Hortic. 2009, 811, 197–204. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation-structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Sapers, G.M.; Hornstein, J.S. Varietal differences in colorant properties and stability of red beet pigments. J. Food Sci. 1979, 44, 1245–1248. [Google Scholar] [CrossRef]
- Aronoff, S.; Aronoff, E.M. Thermal degradation of dehydrated beets II. Chromatographic separation of red beet-root pigments. J. Food Sci. 1948, 13, 59–65. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, C491–C498. [Google Scholar] [CrossRef]
- Huang, A.S.; Von-Elbe, J.H. Stability comparison of two betacyanine pigments-amaranthine and betanine. J. Food Sci. 1986, 51, 670–674. [Google Scholar] [CrossRef]
- Fang, C.; Yangzhao, S.; Guanghua, Z.; Xiaojun, L.; Xiaosong, H.; Jihong, W.; Zhengfu, W. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-perfomance liquid chromatography-mass spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar]
- Caillet, S.; Yu, H.; Lessard, S.; Lamoureu, G.; Ajdukovic, D.; Lacroix, M. Fenton reaction applied for screening natural antioxidants. Food Chem. 2007, 100, 542–552. [Google Scholar] [CrossRef]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part I.-The ferrous ion reaction. Trans. Faraday Soc. 1951, 47, 462–500. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. A 1934, 147, 332–351. [Google Scholar] [CrossRef]
- Mordechai, L.K. Promotion of the Fenton reaction by Cu2+ ions: Evidence for intermediates. Int. J. Chem. Kinet. 2006, 38, 725–736. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Treatment | L* | a* | b* | C* | h° | ΔE | Browning |
|---|---|---|---|---|---|---|---|
| control | 4.5 ± 0.9 c | 21.1 ± 3.5 a | 0.7 ± 0.4 a | 21.1 ± 3.4 a | 0.03 ± 0.2 b | – | 0.5 ± 0.2 b |
| 40% 10 min | 5.01 ± 0.62 c | 25.1 ± 2.3 a | 0.8 ± 0.2 a | 25.2 ± 2.3 a | 0.03 ± 0.0 b | 4.0 ± 1.4 abc | 0.7 ± 0.2 ab |
| 40% 15 min | 6.3 ± 0.5 cb | 23.8 ± 3.0 a | 0.7 ± 0.6 a | 23.8 ± 3.0 a | 0.02 ± 0.0 b | 5.8 ± 2.2 ab | 0.7 ± 0.2 ab |
| 40% 25 min | 11.3 ± 3.8 a | 21.8 ± 8.9 a | 0.4 ± 0.3 a | 21.8 ± 8.9 a | 0.02 ± 0.1 b | 4.1 ± 1.0 abc | 0.7 ± 0.3 ab |
| 60% 10 min | 5.2 ± 0.6 c | 25.1 ±1.8 a | 0.8 ± 0.5 a | 25.1 ± 1.8 a | 0.03 ± 0.2 b | 4.2 ± 1.8 abc | 0.7 ± 0.1 ab |
| 60% 15 min | 8.0 ± 4.1 cb | 25.6 ± 3.9 a | 0.4 ± 0.2 a | 25.6 ± 3.9 a | 0.01 ± 0.1 b | 6.3 ± 2.5 a | 0.5 ± 0.1 ab |
| 60% 25 min | 6.6 ± 0.8 cb | 25.8 ± 2.8 a | 0.7 ± 0.2 a | 25.8 ± 2.8 a | 0.03 ± 0.0 b | 6.4 ± 1.7 a | 0.7 ± 0.1 ab |
| 80% 3 min | 4.5 ± 0.7 c | 23.9 ± 2.9 a | 0.9 ± 0.2 a | 23.5 ± 3.3 a | 0.03 ± 0.0 b | 2.5 ± 1.0 c | 0.7 ± 0.1 ab |
| 80% 5 min | 4.9 ± 0.7 c | 24.0 ± 3.2 a | 0.7 ± 0.1 a | 24.0 ± 3.2 a | 0.02 ± 0.0 b | 2.9 ± 1.0 cb | 0.8 ± 0.2 a |
| 80% 8 min | 5.3 ± 0.7 c | 25.4 ± 2.3 a | 0.8 ± 0.4 a | 25.4 ± 2.3 a | 0.03 ± 0.0 b | 4.4 ± 1.1 abc | 0.6 ± 0.1 ab |
| 80% 10 min | 5.5 ± 0.7 c | 25.9 ± 2.3 a | 0.6 ± 0.4 a | 25.9 ± 2.3 a | 0.02 ± 0.2 b | 5.5 ± 1.5 abc | 0.7 ± 0.1 ab |
| 80% 15 min | 9.3 ± 4.2 ba | 21.9 ±4.3 a | 0.5 ± 0.3 a | 21.9 ± 4.3 a | 0.02 ± 0.1 b | 5.8 ± 2.4 ab | 0.7 ±0.7 ab |
| 80% 25 min | 6.6 ± 0.5 cb | 28.3 ± 2.3 a | 1.7 ± 0.5 b | 28.4 ± 2.3 a | 0.06 ± 0.0 a | 5.8 ± 2.9 ab | 0.6 ± 0.3 ab |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Hernández, I.; Cruz-Cansino, N.D.S.; Santander-Martínez, I.R.; Alanís-García, E.; Delgado-Olivares, L.; Ramírez-Moreno, E.; Ariza-Ortega, J.A.; Omaña-Covarrubias, A.; Torres-Valencia, J.M.; Manríquez-Torres, J.D.J. Fenton Discoloration of Ultrasonicated Purple Cactus Pear Juice. Molecules 2017, 22, 1344. https://doi.org/10.3390/molecules22081344
Reyes-Hernández I, Cruz-Cansino NDS, Santander-Martínez IR, Alanís-García E, Delgado-Olivares L, Ramírez-Moreno E, Ariza-Ortega JA, Omaña-Covarrubias A, Torres-Valencia JM, Manríquez-Torres JDJ. Fenton Discoloration of Ultrasonicated Purple Cactus Pear Juice. Molecules. 2017; 22(8):1344. https://doi.org/10.3390/molecules22081344
Chicago/Turabian StyleReyes-Hernández, Isidro, Nelly Del S. Cruz-Cansino, Ingrid Renata Santander-Martínez, Ernesto Alanís-García, Luis Delgado-Olivares, Esther Ramírez-Moreno, José A. Ariza-Ortega, Ariana Omaña-Covarrubias, Jesús Martín Torres-Valencia, and José De Jesús Manríquez-Torres. 2017. "Fenton Discoloration of Ultrasonicated Purple Cactus Pear Juice" Molecules 22, no. 8: 1344. https://doi.org/10.3390/molecules22081344






