Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length
Abstract
:1. Introduction
2. Results and Discussion
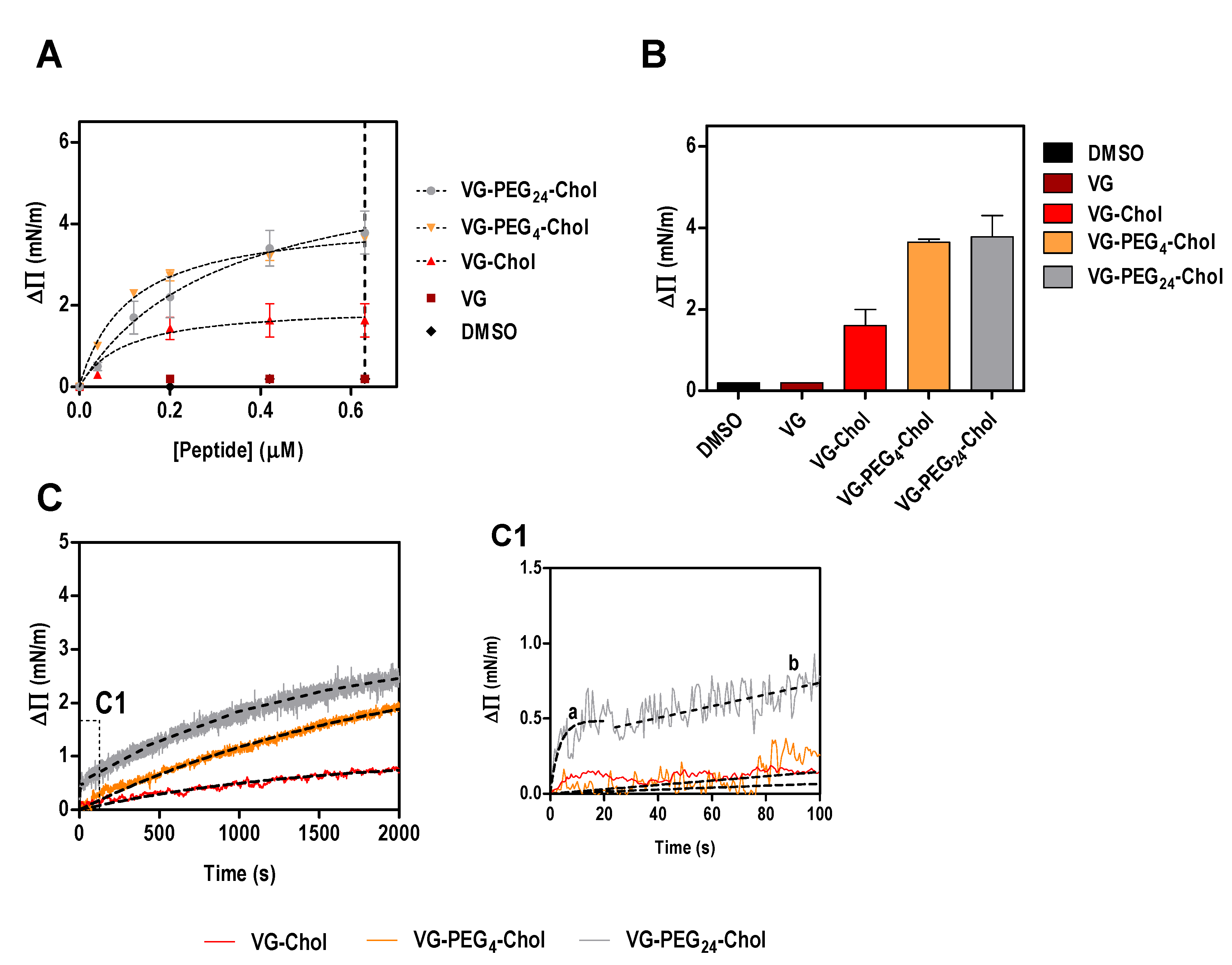
2.1. PEG Linker Influences Peptide-Lipid Interactions
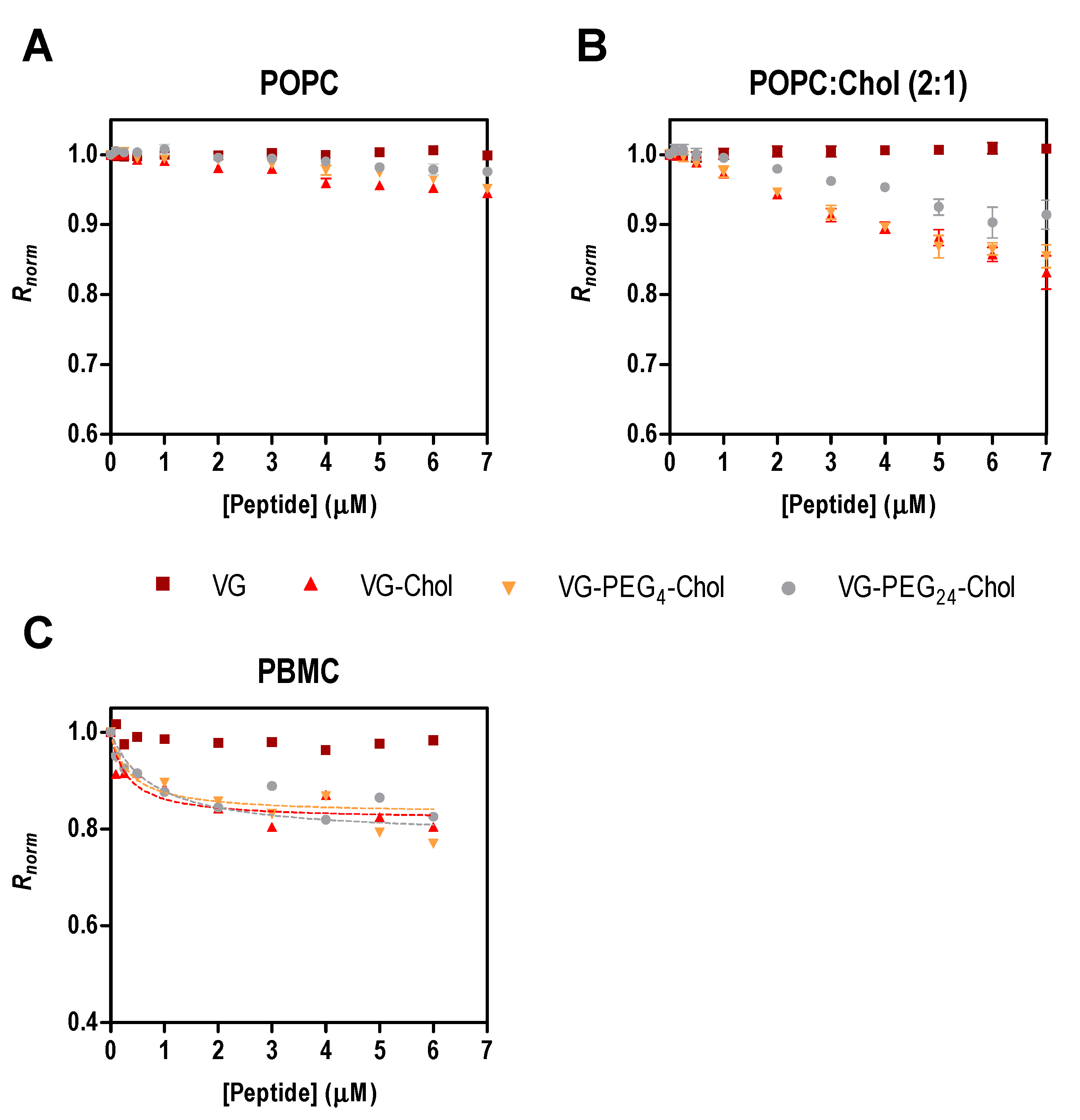
2.2. HPIV3 HRC Peptides Interact with PBMC
3. Materials and Methods
3.1. Peptide Synthesis and Lipids
3.2. Surface Pressure
3.3. Membrane Dipole Potential Assessed by Di-8-ANEPPS
3.4. Data Analysis and Fitting
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Englund, J.A.; Moscona, A. Paramyxoviruses: Parainfluenza Viruses. In Viral Infections of Humans; Kaslow, R.A., Stanberry, L.R., Le Duc, J.W., Eds.; Springer US: New York, NY, USA, 2014; pp. 579–600. [Google Scholar]
- Clayton, B.A. Nipah virus: Transmission of a zoonotic paramyxovirus. Curr. Opin. Virol. 2017, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Satterfield, B.A.; Dawes, B.E.; Milligan, G.N. Status of vaccine research and development of vaccines for Nipah virus. Vaccine 2016, 34, 2971–2975. [Google Scholar] [CrossRef] [PubMed]
- St Vincent, M.R.; Colpitts, C.C.; Ustinov, A.V; Muqadas, M.; Joyce, M.A.; Barsby, N.L.; Epand, R.F.; Epand, R.M.; Khramyshev, S.A.; Valueva, O.A.; et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. USA 2010, 107, 17339–17344. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Lee, J.; Hollmann, A.; Tanner, L.B.; Akyol Ataman, Z.; Yun, T.; Shui, G.; Aguilar, H.C.; Zhang, D.; Meriwether, D.; et al. A mechanistic paradigm for broad-spectrum antivirals that target virus-cell fusion. PLoS Pathog. 2013, 9, e1003297. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Hollmann, A.; Lee, J.; Santos, N.C.; Jung, M.E.; Lee, B. The rigid amphipathic fusion inhibitor dUY11 acts through photosensitization of viruses. J. Virol. 2014, 88, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, A.; Castanho, M.A.R.B.; Lee, B.; Santos, N.C. Singlet oxygen effects on lipid membranes: Implications for the mechanism of action of broad-spectrum viral fusion inhibitors. Biochem. J. 2014, 459, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, A.; Gonçalves, S.; Augusto, M.T.; Castanho, M.A.R.B.; Lee, B.; Santos, N.C. Effects of singlet oxygen generated by a broad-spectrum viral fusion inhibitor on membrane nanoarchitecture. Nanomedicine 2015, 11, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Schang, L.M. Biophysical approaches to entry inhibitor antivirals with a broad spectrum of action. Future Virol. 2014, 9, 283–299. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Vigant, F.; Santos, N.C.; Lee, B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015, 13, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Lee, J.; Cho, N.-J. Nanomedicine for infectious disease applications: Innovation towards broad-spectrum treatment of viral infections. Small 2016, 12, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Castanho, M.A.R.B.; Santos, N.; Wiley InterScience (Online service). Peptide Drug Discovery and Development; Castanho, M., Santos, N.C., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 9783527636730. [Google Scholar]
- Matos, P.M.; Franquelim, H.G.; Castanho, M.A.R.B.; Santos, N.C. Quantitative assessment of peptide-lipid interactions.: Ubiquitous fluorescence methodologies. Biochim. Biophys. Acta 2010, 1798, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Matos, P.M.; Castanho, M.A.R.B.; Santos, N.C. HIV-1 fusion inhibitor peptides enfuvirtide and T-1249 interact with erythrocyte and lymphocyte membranes. PLoS ONE 2010, 5, e9830. [Google Scholar] [CrossRef] [PubMed]
- Franquelim, H.G.; Loura, L.M.S.; Santos, N.C.; Castanho, M.A.R.B. Sifuvirtide screens rigid membrane surfaces. establishment of a correlation between efficacy and membrane domain selectivity among HIV fusion inhibitor peptides. J. Am. Chem. Soc. 2008, 130, 6215–6223. [Google Scholar] [CrossRef] [PubMed]
- Franquelim, H.G.; Veiga, A.S.; Weissmüller, G.; Santos, N.C.; Castanho, M.A.R.B. Unravelling the molecular basis of the selectivity of the HIV-1 fusion inhibitor sifuvirtide towards phosphatidylcholine-rich rigid membranes. Biochim. Biophys. Acta 2010, 1798, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Badani, H.; Garry, R.F.; Wimley, W.C. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta 2014, 1838, 2180–2197. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Doctor, L.; Carta, P.; Fornabaio, M.; Greengard, O.; Kellogg, G.E.; Moscona, A. Inhibition of hendra virus fusion. J. Virol. 2006, 80, 9837–9849. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Rockx, B.; Yokoyama, C.C.; Talekar, A.; Devito, I.; Palermo, L.M.; Liu, J.; Cortese, R.; Lu, M.; Feldmann, H.; et al. Inhibition of Nipah virus infection in vivo: Targeting an early stage of paramyxovirus fusion activation during viral entry. PLoS Pathog. 2010, 6, e1001168. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Augusto, M.T.; Niewiesk, S.; Horvat, B.; Palermo, L.M.; Sanna, G.; Madeddu, S.; Huey, D.; Castanho, M.A.R.B.; Porotto, M.; et al. Broad spectrum antiviral activity for paramyxoviruses is modulated by biophysical properties of fusion inhibitory peptides. Sci. Rep. 2017, 7, 43610. [Google Scholar] [PubMed]
- Ingallinella, P.; Bianchi, E.; Ladwa, N.A.; Wang, Y.-J.; Hrin, R.; Veneziano, M.; Bonelli, F.; Ketas, T.J.; Moore, J.P.; Miller, M.D.; et al. Addition of a cholesterol group to an HIV-1 peptide fusion inhibitor dramatically increases its antiviral potency. Proc. Natl. Acad. Sci. USA 2009, 106, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Pessi, A.; Gui, L.; Santoprete, A.; Talekar, A.; Moscona, A.; Porotto, M. Capturing a fusion intermediate of influenza hemagglutinin with a cholesterol-conjugated peptide, a new antiviral strategy for influenza virus. J. Biol. Chem. 2011, 286, 42141–42149. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, A.; Matos, P.M.; Augusto, M.T.; Castanho, M.A.R.B.; Santos, N.C. Conjugation of cholesterol to HIV-1 fusion inhibitor C34 increases peptide-membrane interactions potentiating its action. PLoS ONE 2013, 8, e60302. [Google Scholar] [CrossRef] [PubMed]
- Welsch, J.C.; Talekar, A.; Mathieu, C.; Pessi, A.; Moscona, A.; Horvat, B.; Porotto, M. Fatal measles virus infection prevented by brain-penetrant fusion inhibitors. J. Virol. 2013, 87, 13785–13794. [Google Scholar] [CrossRef] [PubMed]
- Augusto, M.T.; Hollmann, A.; Castanho, M.A.R.B.; Porotto, M.; Pessi, A.; Santos, N.C. Improvement of HIV fusion inhibitor C34 efficacy by membrane anchoring and enhanced exposure. J. Antimicrob. Chemother. 2014, 69, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Huey, D.; Jurgens, E.; Welsch, J.C.; DeVito, I.; Talekar, A.; Horvat, B.; Niewiesk, S.; Moscona, A.; Porotto, M. Prevention of measles virus infection by intranasal delivery of fusion inhibitor peptides. J. Virol. 2015, 89, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Figueira, T.N.; Palermo, L.M.; Veiga, A.S.; Huey, D.; Alabi, C.A.; Santos, N.C.; Welsch, J.C.; Mathieu, C.; Horvat, B.; Niewiesk, S.; et al. In vivo efficacy of measles virus fusion protein-derived peptides is modulated by the properties of self-assembly and membrane residence. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Silvius, J.R. Role of cholesterol in lipid raft formation: Lessons from lipid model systems. Biochim. Biophys. Acta 2003, 1610, 174–183. [Google Scholar] [CrossRef]
- Campbell, S.M.; Crowe, S.M.; Mak, J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J. Clin. Virol. 2001, 22, 217–227. [Google Scholar] [CrossRef]
- Takeda, M.; Leser, G.P.; Russell, C.J.; Lamb, R.A. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. 2003, 100, 14610–14617. [Google Scholar] [CrossRef] [PubMed]
- Leser, G.P.; Lamb, R.A. Influenza virus assembly and budding in raft-derived microdomains: A quantitative analysis of the surface distribution of HA, NA and M2 proteins. Virology 2005, 342, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Manié, S.N.; de Breyne, S.; Debreyne, S.; Vincent, S.; Gerlier, D. Measles virus structural components are enriched into lipid raft microdomains: A potential cellular location for virus assembly. J. Virol. 2000, 74, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, J.P.; McGinnes, L.W.; Morrison, T.G. Incorporation of functional HN-F glycoprotein-containing complexes into newcastle disease virus is dependent on cholesterol and membrane lipid raft integrity. J. Virol. 2007, 81, 10636–10648. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.H.; Kolokoltsov, A.A.; Davey, R.A.; Nichols, J.E.; Roberts, N.J. Respiratory syncytial virus F envelope protein associates with lipid rafts without a requirement for other virus proteins. J. Virol. 2006, 80, 12160–12170. [Google Scholar] [CrossRef] [PubMed]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Openshaw, P.J. Antiviral B cell and T cell immunity in the lungs. Nat. Immunol. 2014, 16, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Hiraldo, P.I.; Arasaratnam, R.J.; Tzannou, I.; Kuvalekar, M.; Lulla, P.; Naik, S.; Martinez, C.A.; Piedra, P.A.; Vera, J.F.; Leen, A.M. Characterizing the cellular immune response to Parainfluenza virus 3. J. Infect. Dis. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Pohl, C.; Szecsi, J.; Trajkovic-Bodennec, S.; Devergnas, S.; Raoul, H.; Cosset, F.-L.; Gerlier, D.; Wild, T.F.; Horvat, B. Nipah virus uses leukocytes for efficient dissemination within a host. J. Virol. 2011, 85, 7863–7871. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, D.; Underhaug Gjerde, A.; Petit-Paris, I.; Mauco, G.; Holmsen, H. Polyethylene glycols interact with membrane glycerophospholipids: Is this part of their mechanism for hypothermic graft protection? J. Chem. Biol. 2009, 2, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Gursahani, H.; Riggs-Sauthier, J.; Pfeiffer, J.; Lechuga-Ballesteros, D.; Fishburn, C.S. Absorption of polyethylene glycol (PEG) polymers: The effect of PEG size on permeability. J. Pharm. Sci. 2009, 98, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Porotto, M.; Yokoyama, C.C.; Palermo, L.M.; Mungall, B.; Aljofan, M.; Cortese, R.; Pessi, A.; Moscona, A. viral entry inhibitors targeted to the membrane site of action. J. Virol. 2010, 84, 6760–6768. [Google Scholar] [CrossRef] [PubMed]
- Matos, P.M.; Freitas, T.; Castanho, M.A.R.B.; Santos, N.C. The role of blood cell membrane lipids on the mode of action of HIV-1 fusion inhibitor sifuvirtide. Biochem. Biophys. Res. Commun. 2010, 403, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Bedlack, R.S.; Loew, L.M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 1994, 67, 208–216. [Google Scholar] [CrossRef]
- Clarke, R.J.; Kane, D.J. Optical detection of membrane dipole potential: Avoidance of fluidity and dye-induced effects. Biochim. Biophys. Acta 1997, 1323, 223–239. [Google Scholar] [CrossRef]
- Cladera, J.; O’Shea, P. Intramembrane molecular dipoles affect the membrane insertion and folding of a model amphiphilic peptide. Biophys. J. 1998, 74, 2434–2442. [Google Scholar] [CrossRef]
| Peptide Name | Sequence and Modifications (N-to-C) |
|---|---|
| HPIV3 F protein residues 449–484 | VALDPIDISIELNKAKSDLEESKEWIRRSNQKLDSI |
| VG | Ac-VALDPIDISIVLNKAKSDLEESKEWIRRSNGKLDSI-GSGSG-C-NH2 |
| VG-Chol | Ac-VALDPIDISIVLNKAKSDLEESKEWIRRSNGKLDSI-GSGS-C(Chol)-NH2 |
| VG-PEG4-Chol | Ac-VALDPIDISIVLNKAKSDLEESKEWIRRSNGKLDSI-GSGS-C(PEG4-Chol)-NH2 |
| VG-PEG24-Chol | Ac-VALDPIDISIVLNKAKSDLEESKEWIRRSNGKLDSI-GSGS-C(PEG24-Chol)-NH2 |
| VG-Chol | VG-PEG4-Chol | VG-PEG24-Chol | ||
|---|---|---|---|---|
| ΔΠmax (mN/m) | 1.26 ± 0.38 | 4.16 ± 0.16 | 5.01 ± 0.80 | |
| Kd (10−2 µM) | 9.83 ± 1.59 | 10.90 ± 1.59 | 30.89 ± 14.52 | |
| k (10−4 s−1) | 2.06 ± 0.01 | 4.96 ± 0.05 | 2417 ± 767 1 | 7.34 ± 0.09 2 |
| Peptides | Kd (µM) | Rmin, norm |
|---|---|---|
| VG | - | - |
| VG-Chol | 0.32 ± 0.11 | −0.18 ± 0.01 |
| VG-PEG4-Chol | 0.77± 0.38 | −0.21 ± 0.03 |
| VG-PEG24-Chol | 0.36 ± 0.14 | −0.17 ± 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto, M.T.; Hollmann, A.; Porotto, M.; Moscona, A.; Santos, N.C. Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length. Molecules 2017, 22, 1190. https://doi.org/10.3390/molecules22071190
Augusto MT, Hollmann A, Porotto M, Moscona A, Santos NC. Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length. Molecules. 2017; 22(7):1190. https://doi.org/10.3390/molecules22071190
Chicago/Turabian StyleAugusto, Marcelo T., Axel Hollmann, Matteo Porotto, Anne Moscona, and Nuno C. Santos. 2017. "Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length" Molecules 22, no. 7: 1190. https://doi.org/10.3390/molecules22071190





