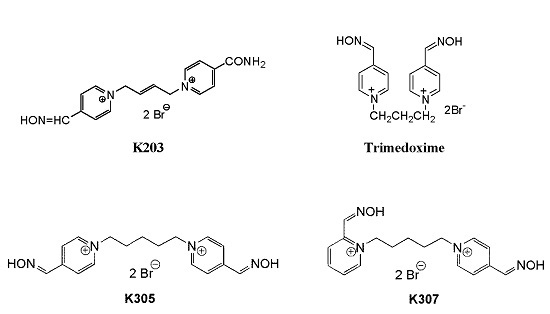
The Evaluation of the Reactivating and Neuroprotective Efficacy of Two Newly Prepared Bispyridinium Oximes (K305, K307) in Tabun-Poisoned Rats—A Comparison with Trimedoxime and the Oxime K203
Abstract
:1. Introduction
2. Results
2.1. The Evaluation of Neuprotective Efficacy of the Studied Oximes in Tabun-Poisoned Rats
2.2. The Evaluation of Reactivating Efficacy of the Studied Oximes in the Brain of Tabun-Poisoned Rats
2.3. Histopathological Evaluation of Tabun-Induced Brain Damage
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. In Vivo Experiments
5. Conclusions
Acknowledgements
Author Contributions
Conflict of Interest
References
- Bajgar, J. Organophosphate/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus compounds as chemical warfare agents: A review. J. Braz. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Jokanovic, M.; Prostran, M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr. Med. Chem. 2009, 16, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Kassa, J.; Musilek, K.; Zdarova Karasova, J.; Kuca, K.; Bajgar, J. Two possibilities how to increase the efficacy of antidotal treatment of nerve agent poisonings. Mini-Rev. Med. Chem. 2012, 12, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Cabal, J.; Bajgar, J. Tabun—Reappearance 50 years later. Chem. Listy 1999, 93, 27–31. (In Czech) [Google Scholar]
- Ekström, F.; Akfur, C.; Tunemalm, A.K.; Lundberg, S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochemistry 2006, 45, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Eisenkraft, A.; Finkelstein, A.; Schein, O.; Rotman, E.; Dushnitsky, T. A decade after the Tokyo sarin attack: A review of neurological follow-up of the victims. Mil. Med. 2007, 172, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Yamasue, H.; Abe, O.; Kasai, K.; Suga, M.; Iwanami, A.; Yamada, A.; Tochigi, M.; Ohtani, T.; Rogers, M.A.; Sasaki, T.; et al. Human brain structural changes related to acute single exposure to sarin. Ann. Neurol. 2007, 61, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cassel, G.; Karlsson, L.; Waara, L.; Wee Ang, K.; Goransson-Nyberg, A. Pharmacokinetics and effects of HI-6 in blood and brain of soman-intoxicated rats: A microdialysis study. Eur. J. Pharmacol. 1997, 332, 43–52. [Google Scholar] [CrossRef]
- Sakurada, K.; Matsubara, K.; Shimizu, K.; Shiono, H.; Seto, Y.; Tsuge, K.; Yoshino, M.; Sakai, I.; Mukoyama, H.; Takatori, E. Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochem. Res. 2003, 28, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Kalasz, H.; Petroianu, G.A.; Tekes, K. Entry of oximes into the brain: A review. Curr. Med. Chem. 2008, 15, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Jokanovic, M. Structure-activity relationship and efficacy of pyridinium oximes in the treatment of poisoning with organophosphorus compounds: A review of recent data. Curr. Top. Med. Chem. 2012, 12, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.M.; Snider, T.H.; Babin, M.C.; Jett, D.A.; Platoff, G.E., Jr.; Yeung, D.T. A comprehensive evaluation of the efficacy of leading oxime therapies in guinea pigs exposed to organophosphorus chemical warfare agents or pesticides. Toxicol. Appl. Pharmacol. 2014, 281, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, B.; Singh, N.; Acharya, J.R.; Musilek, K.; Kuca, K.; Ghosh, K.K. Development and structural modifications of cholinesterase reactivators against chemical warfare agents in last decade: A review. Mini-Rev. Med. Chem. 2015, 15, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Kassa, J.; Karasova, J.; Musilek, K.; Kuca, K. An evaluation of therapeutic and reactivating effects of newly developed oximes (K156, K203) with commonly used oximes (obidoxime, trimedoxime, HI-6) in tabun-poisoned rats and mice. Toxicology 2008, 243, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Wille, T.; Musilek, K.; Kuca, K.; Thiermann, H.; Worek, F. Investigation of the reactivation kinetics of a large series of bispyridinium oximes with organophosphate-inhibited human acetylcholinesterase. Toxicol. Lett. 2016, 244, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J.; Jun, D.; Kuca, K.; Bartosova, L.; Fusek, J. Cholinesterase reactivators: The fate and effects in the organism poisoned with organophosphates/nerve agents. Curr. Drug Metab. 2007, 8, 803–809. [Google Scholar] [CrossRef]
- Dolgin, E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013, 19, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Domingo, J. The use of chemical weapons in the Syrian conflict. Toxics 2014, 2, 391–402. [Google Scholar] [CrossRef]
- Shih, T.M.; Duniho, S.M.; McDonough, J.H. Control of NA-induced seizures is critical for neuroprotection and survival. Toxicol. Appl. Pharmacol. 2003, 188, 69–80. [Google Scholar] [CrossRef]
- Marrs, T.C. Toxicology of Organophosphate Nerve Agents. In Chemical Warfare Agents: Toxicology and Treatment; Marrs, T.C., Maynard, R.L., Sidell, F.R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 191–221. [Google Scholar]
- Chen, Y. Organophosphate-induced brain damage: Mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. NeuroToxicology 2012, 33, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Weissman, B.A.; Raveh, L. Therapy against organophosphate poisoning: The importance of anticholinergic drugs with antiglutamatergic properties. Toxicol. Appl. Pharmacol. 2008, 232, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kassa, J.; Kunesova, G. Comparison of the neuroprotective effects of the newly developed oximes (K027, K048) with trimedoxime in tabun-poisoned rats. J. Appl. Biomed. 2006, 4, 123–134. [Google Scholar]
- McDonough, J.H., Jr.; Zoeffel, L.D.; McMonagle, J.; Copeland, T.L.; Smith, C.D.; Shih, T.-M. Anticonvulsant treatment of nerve agent seizures: Anticholinergics versus diazepam in soman-intoxicated guinea-pigs. Epilepsy Res. 2000, 38, 1–14. [Google Scholar] [CrossRef]
- Antonijevic, B.; Stojiljkovic, P. Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin. Med. Res. 2007, 5, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Nurulain, S.M. Efficacious oxime for organophosphorus poisoning: A minireview. Trop. J. Pharm. Res. 2011, 10, 341–349. [Google Scholar] [CrossRef]
- Kassa, J.; Krejcova, G. Neuroprotective effects of currently used antidotes in tabun-poisoned rats. Pharmacol. Toxicol. 2003, 92, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kassa, J.; Karasova, J.; Vasina, L.; Bajgar, J.; Kuca, K.; Musilek, K. A comparison of neuroprotective efficacy of newly developed oximes (K203, K206) and commonly used oximes (obidoxime, HI-6) in tabun-poisoned rats. Drug Chem. Toxicol. 2009, 32, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Widmann, R.; Knopff, O.; Szinicz, L. Reactivating potency of obidoxime, pralidoxime, HI-6 and HLö-7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch. Toxicol. 1998, 72, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zdarova Karasova, J.; Pohanka, M.; Musilek, K.; Zemek, F.; Kuca, K. Passive diffusion of acetylcholinesterase oxime reactivators through the blood-brain barrier: Influence of molecular structure. Toxicol. In Vitro 2010, 24, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Cabal, J.; Kuca, K.; Kassa, J. Specification of the structure of oximes able to reactivate tabun-inhibited acetylcholinesterase. Basic Clin. Pharmacol. Toxicol. 2004, 95, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Jun, D.; Musilek, K. Structural requirements of acetylcholinesterase reactivators. Mini Rev. Med. Chem. 2006, 6, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Musilek, K.; Kuca, K.; Jun, D.; Dolezal, M. Progress in synthesis of new acetylcholinesterase reactivators during the period 1990–2004. Curr. Org. Chem. 2007, 11, 229–238. [Google Scholar] [CrossRef]
- Biljana, A.; Slavica, V.; Cupic, V. Protective effect of HI-6 and trimedoxime combination in mice acutely poisoned with tabun, dichlorvos and heptenophos. Acta Vet. Beogr. 2012, 62, 123–135. [Google Scholar] [CrossRef]
- Kassa, J.; Sepsova, V.; Tumova, M.; Horova, A.; Musilek, K. A comparison of the reactivating and therapeutic efficacy of two newly developed oximes (K727, K733) with oxime K203 and trimedoxime in tabun-poisoned rats and mice. Basic Clin. Pharmacol. Toxicol. 2015, 116, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Kadar, T.; Shapira, S.; Cohen, G.; Sahar, R.; Alkalay, D.; Raveh, L. Sarin-induced neuropathology in rats. Hum. Exp. Toxicol. 1995, 14, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jun, D.; Kuca, K.; Stodulka, P.; Koleckar, V.; Dolezal, B.; Simon, P.; Veverka, M. HPLC analysis of HI-6 dichloride and dimethanesulfonate - antidotes against nerve agents and organophosphorus pesticides. Anal. Lett. 2007, 40, 2783–2787. [Google Scholar] [CrossRef]
- Kassa, J.; Sepsova, V.; Horova, A.; Musilek, K. A comparison of the reactivating and therapeutic efficacy of two novel bispyridinium oximes (K305, K307) with the oxime K203 and trimedoxime in tabun-poisoned rats and mice. J. Appl. Biomed. 2017, 15, 49–53. [Google Scholar] [CrossRef]
- Moser, V.C.; Tilson, H.; McPhail, R.C.; Becking, G.C.; Cuomo, V.; Frantik, E.; Kulig, B.M.; Winneke, G. The IPCS collaborative study on neurobehavioral screening methods: II. Protocol design and testing procedures. NeuroToxicology 1997, 18, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, D.K.; Andres, V., Jr.; Feartherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–93. [Google Scholar] [CrossRef]
- Clement, J.G.; Hansen, A.S.; Boulet, C.A. Efficacy of HLö-7 and pyrimidoxime as antidotes of nerve agent poisoning in mice. Arch. Toxicol. 1992, 66, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotactic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2006; 456p. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| 2 h | Controls | Tabun + A + K203 | Tabun + A + Trimedoxime | Tabun + A + K307 | Tabun + A + K305 | Tabun | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Marker | x/M | ±s | x/M | ±s | x/M | ±s | x/M | ±s | x/M | ±s | x/M | ±s |
| 1 | Posture | 1.00 | 4.00 * | 3.00 | 3.00 | 4.00 * | 7.00 * | ||||||
| 2 | Catch Difficulty | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 3 | Ease of Handling | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | ||||||
| 4 | Muscular Tonus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | -2.00 * | ||||||
| 5 | Lacrimation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 6 | Palpebral Closure | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 7 | Endo/Exophtalmus | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 * | ||||||
| 8 | Fur Abnormalities | 0.00 | 2.00 * | 0.00 | 0.00 | 0.00 | 7.00 * | ||||||
| 9 | Skin Abnormalities | 0.00 | 1.00 * | 0.00 | 0.00 | 0.00 | 1.00 * | ||||||
| 10 | Salivation | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | 2.00 * | ||||||
| 11 | Nose Secretion | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 * | ||||||
| 12 | Rearing | 7.88 | 8.41 | 2.50 | 5.24 | 4.38 | 5.42 | 1.13 * | 2.47 | 0.13 * | 0.35 | 0.00 * | 0.00 |
| 13 | Urination | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 14 | Defecation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 15 | Hyperkinesis | 0.00 | 0.00 | 0.00 | 0.00 | 6.00 * | 7.00 * | ||||||
| 16 | Tremors | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | 5.00 * | ||||||
| 17 | Clonic Movements | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 * | 2.00 * | ||||||
| 18 | Tonic Movements | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | 5.00 * | ||||||
| 19 | Gait | 0.00 | 7.00 * | 0.00 | 1.00 * | 7.00 * | 7.00 * | ||||||
| 20 | Ataxia | 0.00 | 2.00 * | 0.00 | 1.00 * | 2.00 * | 2.00 * | ||||||
| 21 | Gait Score | 1.00 | 1.00 | 1.00 | 1.00 | 2.00 * | 2.00 * | ||||||
| 22 | Mobility Score | 1.00 | 1.00 | 1.00 | 1.00 | 4.00 * | 4.00 * | ||||||
| 23 | Activity | 4.00 | 4.00 | 4.00 | 4.00 | 1.00 * | 1.00 * | ||||||
| 24 | Tension | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | ||||||
| 25 | Vocalisation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 26 | Stereotypy | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 27 | Bizzare Behavior | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | ||||||
| 28 | Approach Response | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 29 | Touch Response | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 30 | Click Response | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | ||||||
| 31 | Tail-Pinch Response | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 32 | Pupil Size | 0.00 | −2.00 * | −2.00 * | −2.00 * | −2.00 * | −2.00 * | ||||||
| 33 | Pupil Response | 1.00 | 0.00 * | 0.00 * | 0.00 * | 0.00 * | 0.00 * | ||||||
| 34 | RRF | 1.00 | 4.00 * | 2.00 * | 2.00 * | 2.00 * | 4.00 * | ||||||
| 35 | RRV | 1.00 | 1.00 | 1.00 | 3.00 * | 4.00 * | 4.00 * | ||||||
| 36 | Landing Foot Splay (mm) | 9.41 | 2.51 | 7.86 | 2.10 | 8.05 | 2.21 | 7.15 | 2.40 | 4.76 * | 3.75 | 3.51 * | 3.15 |
| 37 | Forelimb Grip Strength (kg) | 8.54 | 2.32 | 5.54 | 4.26 | 6.00 | 3.49 | 5.17 | 3.49 | 1.39 * | 2.98 | 0.94 * | 1.10 |
| 38 | Hindlimb Grip Strength (Kg) | 2.08 | 0.50 | 0.87 * | 0.55 | 1.04 * | 0.47 | 1.03 * | 0.89 | 0.34 * | 0.32 | 0.47 * | 0.43 |
| 39 | Grip Strength of all Limbs (kg) | 13.89 | 2.25 | 7.40 * | 4.58 | 11.68 | 6.51 | 10.50 | 6.59 | 3.40 * | 4.38 | 2.41 * | 2.10 |
| 40 | Vertical Activity | 53.88 | 32.70 | 5.13 * | 4.82 | 3.38 * | 4.50 | 13.13 * | 3.52 | 0.75 * | 2.12 | 0.00 * | 0.00 |
| 41 | Horizontal Activity | 316.13 | 120.85 | 249.00 | 45.39 | 327.13 | 219.49 | 278.63 | 216.51 | 136.63 * | 154.11 | 1.63 * | 4.60 |
| 42 | Total Motor Activity | 370.00 | 147.64 | 254.13 | 45.80 | 330.50 | 218.87 | 291.75 | 238.41 | 137.38 * | 155.60 | 1.63 * | 4.60 |
| n = 8 | n = 7 | n = 8 | n = 8 | n = 6 | n = 3 | ||||||||
| 24 h | Controls | Tabun + A + K203 | Tabun + A + Trimedoxime | Tabun + A + K307 | Tabun + A + K305 | Tabun | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Marker | x/M | ±s | x/M | ±s | x/M | ±s | x/M | ±s | x/M | ±s | x/M | ±s |
| 1 | Posture | 1.00 | 1.00 | 1.00 | 1.00 | 7.00 * | 7.00 * | ||||||
| 2 | Catch Difficulty | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 3 | Ease of Handling | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | 1.00 * | ||||||
| 4 | Muscular Tonus | 0.00 | 0.00 | 0.00 | 0.00 | −2.00 * | −2.00 * | ||||||
| 5 | Lacrimation | 0.00 | 0.00 | 0.00 | 0.00 | 4.00 * | 4.00 * | ||||||
| 6 | Palpebral Closure | 1.00 | 1.00 | 1.00 | 1.00 | 5.00 * | 5.00 * | ||||||
| 7 | Endo/Exophtalmus | 0.00 | 0.00 | 0.00 | 0.00 | −1.00 * | −1.00 * | ||||||
| 8 | Fur Abnormalities | 0.00 | 0.00 | 0.00 | 0.00 | 7.00 * | 7.00 * | ||||||
| 9 | Skin Abnormalities | 0.00 | 0.00 | 0.00 | 0.00 | 4.00 * | 4.00 * | ||||||
| 10 | Salivation | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | 2.00 * | ||||||
| 11 | Nose Secretion | 0.00 | 0.00 | 0.00 | 1.00 * | 3.00 * | 3.00 * | ||||||
| 12 | Rearing | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 13 | Urination | 0.63 | 1.19 | 3.13 | 3.72 | 0.38 | 1.06 | 3.00 | 4.04 | 0.25 | 0.46 | 0.13 | 0.35 |
| 14 | Defecation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| 15 | Hyperkinesis | 0.00 | 0.00 | 0.00 | 0.00 | 7.00 * | 7.00 * | ||||||
| 16 | Tremors | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | 5.00 * | ||||||
| 17 | Clonic Movements | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | 2.00 * | ||||||
| 18 | Tonic Movements | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | 5.00 * | ||||||
| 19 | Gait | 0.00 | 0.00 | 0.00 | 0.00 | 7.00 * | 7.00 * | ||||||
| 20 | Ataxia | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | 2.00 * | ||||||
| 21 | Gait Score | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | 2.00 * | ||||||
| 22 | Mobility Score | 1.00 | 1.00 | 1.00 | 1.00 | 4.00 * | 4.00 * | ||||||
| 23 | Activity | 4.00 | 4.00 | 4.00 | 4.00 | 1.00 * | 1.00 * | ||||||
| 24 | Tension | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 * | 2.00 * | ||||||
| 25 | Vocalisation | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 * | 3.00 * | ||||||
| 26 | Stereotypy | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | 5.00 * | ||||||
| 27 | Bizzare Behavior | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 * | 5.00 * | ||||||
| 28 | Approach Response | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | 1.00 * | ||||||
| 29 | Touch Response | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 30 | Click Response | 2.00 | 2.00 | 2.00 | 2.00 | 1.00 * | 1.00 * | ||||||
| 31 | Tail-Pinch Response | 2.00 | 2.00 | 1.00 * | 1.00 * | 1.00 * | 1.00 * | ||||||
| 32 | Pupil Size | 0.00 | 0.00 | 0.00 | 0.00 | −2.00 * | −2.00 * | ||||||
| 33 | Pupil Response | 1.00 | 1.00 | 1.00 | 1.00 | 0.00 * | 0.00 * | ||||||
| 34 | RRF | 1.00 | 1.00 | 1.00 | 1.00 | 4.00 * | 4.00 * | ||||||
| 35 | RRV | 1.00 | 1.00 | 2.00 * | 1.00 | 4.00 * | 4.00 * | ||||||
| 36 | Landing Foot Splay (mm) | 10.20 | 1.39 | 5.89 * | 4.14 | 5.83 * | 3.66 | 6.22 * | 4.49 | 3.80 * | 4.09 | 1.08 * | 2.06 |
| 37 | Forelimb Grip Strength (kg) | 8.78 | 2.35 | 6.68 | 5.16 | 7.06 | 4.55 | 5.72 | 4.37 | 1.94 * | 2.90 | 0.33 * | 0.61 |
| 38 | Hindlimb Grip Strength (kg) | 2.54 | 0.41 | 1.40 * | 1.01 | 1.78 | 1.25 | 1.43 * | 1.08 | 0.54 * | 0.71 | 0.24 * | 0.49 |
| 39 | Grip Strength of all Limbs (kg) | 12.67 | 2.14 | 9.29 | 5.99 | 10.23 | 6.51 | 11.80 | 9.68 | 4.82 * | 5.97 | 1.41 * | 2.61 |
| 40 | Vertical Activity | 40.38 | 29.81 | 19.38 | 27.54 | 11.00 * | 12.36 | 5.75 * | 6.61 | 0.63 * | 0.77 | 0.00 * | 0.00 |
| 41 | Horizontal Activity | 224.63 | 91.36 | 94.25 * | 85.62 | 99.25 * | 101.98 | 94.63 * | 119.90 | 23.63 * | 47.51 | 2.38 * | 5.29 |
| 42 | Total Motor Activity | 265.00 | 109.52 | 113.63 * | 111.23 | 110.25 * | 113.60 | 100.38 * | 125.72 | 24.25 * | 49.20 | 2.38 * | 5.29 |
| n = 8 | n = 6 | n = 6 | n = 6 | n = 4 | n = 3 | ||||||||
| Treatment | AChE Activity (μ kat/kg) |
|---|---|
| Brain | |
| Controls | 123.1 ± 3.86 |
| Tabun | 18.05 ± 3.59 a,* |
| Tabun + atropine + K203 (% reactivation b) | 27.22 ± 3.55 * (8.7) |
| Tabun + atropine + trimedoxime (% reactivation) | 32.59 ± 6.02 * (13.8) |
| Tabun + atropine + K305 (% reactivation) | 23.09 ± 6.93 (4.8) |
| Tabun + atropine + K307 (% reactivation) | 28.99 ± 3.24 * (10.4) |
| AMG | CRBL | CTX | HIPP | HYPOTH | PIRI | TH | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score scale | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Controls | 4 | 4 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 |
| Tabun | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 2 |
| Tabun + A + K203 | 5 | 0 | 0 | 1 | 0 | 3 | 3 | 0 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 4 | 1 | 1 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 4 | 1 | 1 | 0 | 0 |
| Tabun + A + TRI | 5 | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| Tabun + A + K305 | 0 | 0 | 1 | 0 | 3 | 1 | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 1 |
| Tabun + A + K307 | 1 | 2 | 2 | 1 | 0 | 5 | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 1 | 0 | 1 | 4 | 0 | 1 | 0 | 1 | 2 | 1 | 2 | 0 | 1 | 2 | 2 | 0 | 1 | 2 | 1 | 2 | 1 | 0 |
| AMG | CRBL | CTX | HIPP | HYPOTH | PIRI | TH | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score scale | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Controls | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 |
| Tabun | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 |
| Tabun + A + K203 | 4 | 0 | 1 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 1 | 4 | 1 | 0 | 0 | 1 | 3 | 2 | 0 | 1 | 0 | 5 | 0 | 0 | 0 | 1 | 4 | 0 | 1 | 0 | 1 |
| Tabun + A + TRI | 6 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 3 | 1 | 2 | 0 | 0 |
| Tabun + A + K305 | 1 | 0 | 0 | 2 | 1 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 2 | 1 |
| Tabun + A + K307 | 3 | 2 | 0 | 0 | 1 | 5 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 1 | 4 | 0 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 1 | 3 | 1 | 1 | 0 | 1 |
| Marker | Scored Values Only | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Posture | sitting or standing | rearing | asleep | flattened | lying on side | crouched over | head bobbing | |||
| Catch Difficulty | passive | normal | defense | flight | escape | aggrression | ||||
| Ease of Handling | very easy | easy | moderately difficult | difficult | ||||||
| Muscular Tonus | atonia | hypotonia | normal | hypertonia | rigidity | fasciculations | ||||
| Lacrimation | none | slight | severe | crusta | coloured crusta | |||||
| Palpebral Closure | open | slightly | half-way | completely | ptosis | |||||
| Endo-Exophthalmus | endo | normal | exo | |||||||
| Piloerection | no | yes | ||||||||
| Skin Abnormalities | normal | pale | erythema | cyanosis | pigmented | cold | injury | |||
| Salivation | none | sllight | severe | |||||||
| Nose Secretion | none | slight | severe | coloured | ||||||
| Clonic Movements | normal | repetitive | nonrhythmic | mild tremors | severe tremors | myoclonic | clonic | |||
| Tonic Movements | normal | contraction of extensors | opisthotonus | emprostho- tonus | explosive jumps | tonic convulsions | ||||
| Gait | normal | ataxia | overcompen- sation of hindlimbs movements | feet point outwards from body | forelimbs are extended | walks on tiptoes | hunched body | body is flattened against surface | ||
| Gait Score | normal | slightly impaired | somewhat impaired | totally impaired | ||||||
| Mobility Score | normal | slightly impaired | somewhat impaired | totally impaired | ||||||
| Arousal (Level of Unprovoked Activity) | very low | sporadic | reduced | normal | enhanced | permanent | ||||
| Tension | none | partial (ears) | stupor | |||||||
| Stereotypy | none | head weaving | body weaving | grooming | circling | others | ||||
| Bizarre Behavior | none | head | body | self-mutilation | abnormal movements | others | ||||
| Approach Response | no reaction | normal | slow reaction | energetic reaction | exaggerated reaction | |||||
| Touch Response | no reaction | normal | slow reaction | energetic reaction | exaggerated reaction | |||||
| Click Response | no reaction | normal | slow reaction | energetic reaction | exaggerated reaction | |||||
| Tail-Pinch Response | no reaction | normal | slow reaction | energetic reaction | exaggerated reaction | |||||
| Pupil Size | miosis | normal | mydriasis | |||||||
| Pupil Response | no reaction | normal reaction | ||||||||
| Righting Reflex | normal | slightly uncoordin. | lands on side | lands on back | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassa, J.; Misik, J.; Hatlapatkova, J.; Zdarova Karasova, J.; Sepsova, V.; Caisberger, F.; Pejchal, J. The Evaluation of the Reactivating and Neuroprotective Efficacy of Two Newly Prepared Bispyridinium Oximes (K305, K307) in Tabun-Poisoned Rats—A Comparison with Trimedoxime and the Oxime K203. Molecules 2017, 22, 1152. https://doi.org/10.3390/molecules22071152
Kassa J, Misik J, Hatlapatkova J, Zdarova Karasova J, Sepsova V, Caisberger F, Pejchal J. The Evaluation of the Reactivating and Neuroprotective Efficacy of Two Newly Prepared Bispyridinium Oximes (K305, K307) in Tabun-Poisoned Rats—A Comparison with Trimedoxime and the Oxime K203. Molecules. 2017; 22(7):1152. https://doi.org/10.3390/molecules22071152
Chicago/Turabian StyleKassa, Jiri, Jan Misik, Jana Hatlapatkova, Jana Zdarova Karasova, Vendula Sepsova, Filip Caisberger, and Jaroslav Pejchal. 2017. "The Evaluation of the Reactivating and Neuroprotective Efficacy of Two Newly Prepared Bispyridinium Oximes (K305, K307) in Tabun-Poisoned Rats—A Comparison with Trimedoxime and the Oxime K203" Molecules 22, no. 7: 1152. https://doi.org/10.3390/molecules22071152