Synthesis and Evaluation of New Quinoxaline Derivatives of Dehydroabietic Acid as Potential Antitumor Agents
Abstract
:1. Introduction
2. Results and Discussion
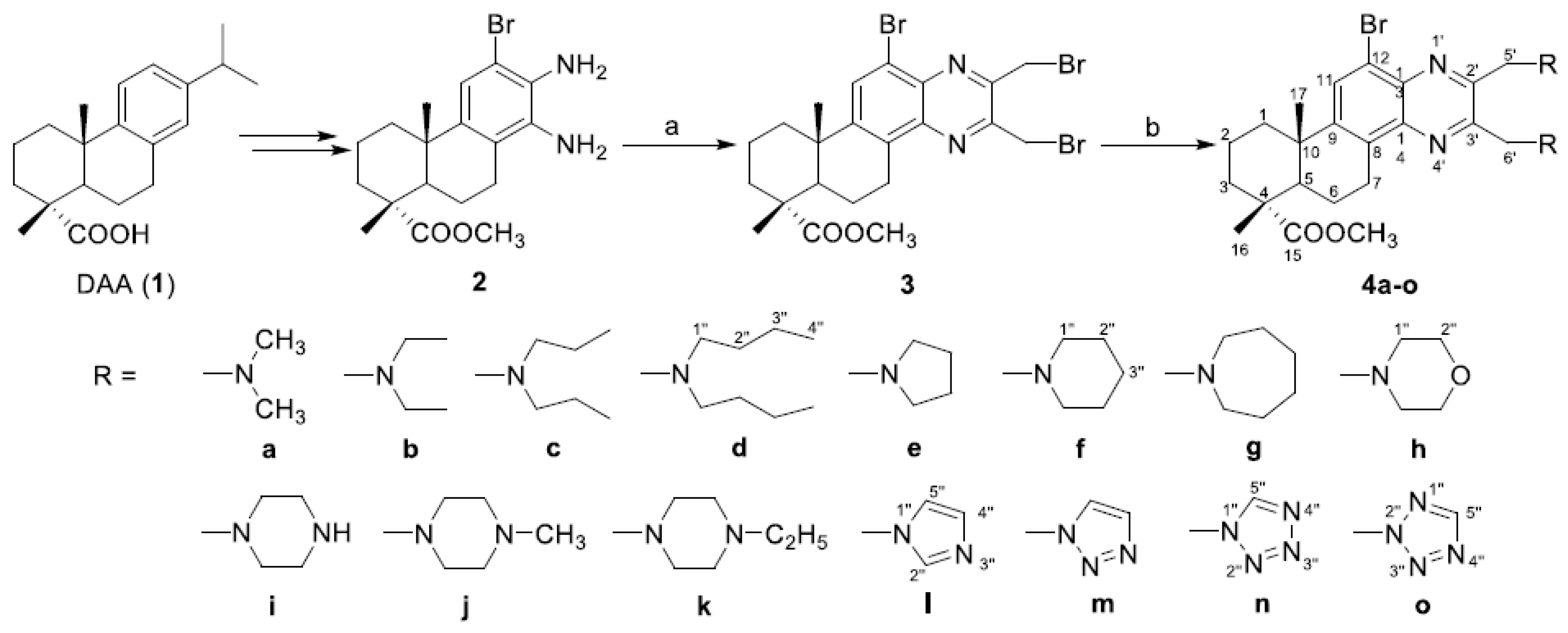
2.1. Chemistry
2.2. Antiproliferative Effects of Dehydroabietic Acid (DAA) and Its Derivatives
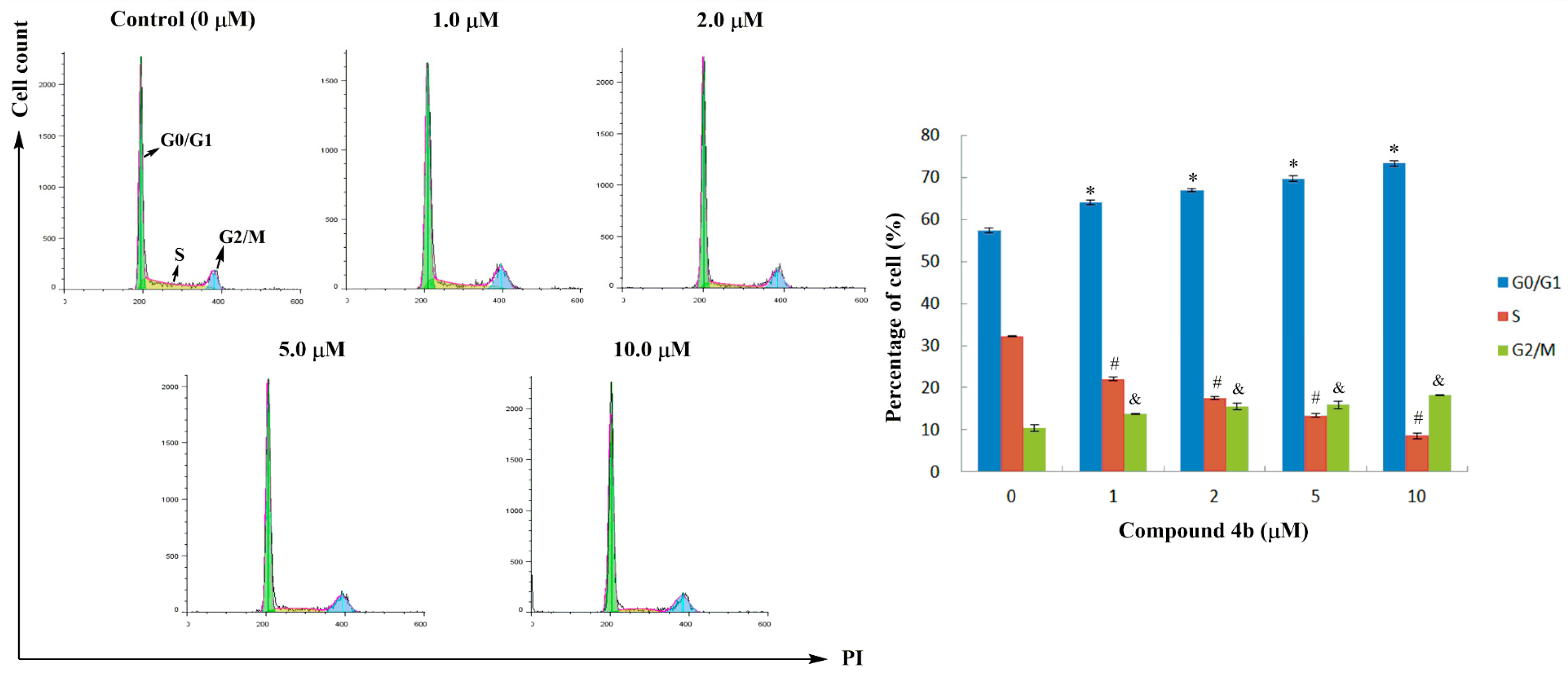
2.3. Cell Cycle Analysis
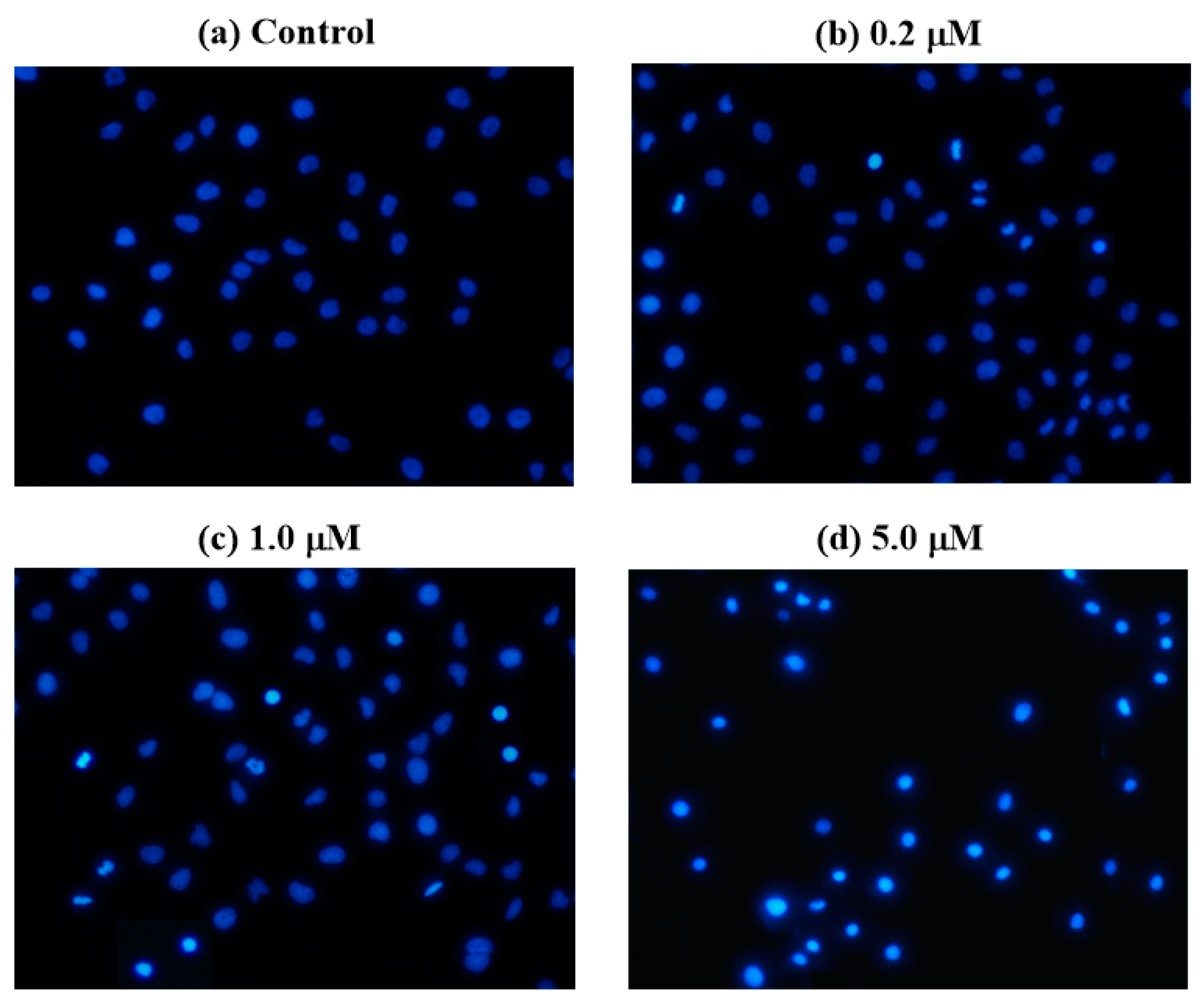
2.4. Hoechst 33258 Staining Assay
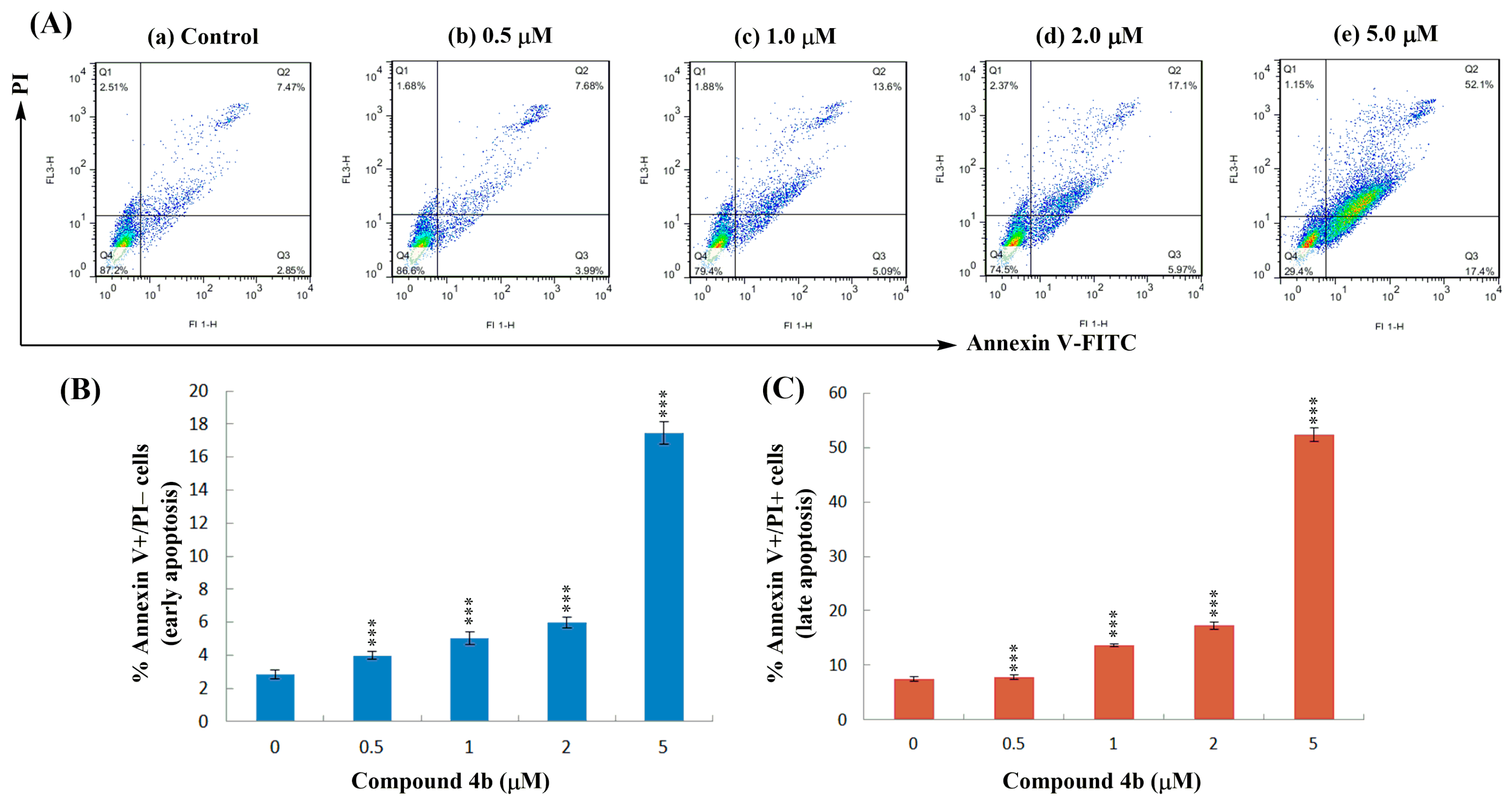
2.5. Annexin V-FITC/PI Dual Staining Assay
3. Experimental Section
3.1. Materials and Methods
3.2. Procedure for the Synthesis of Compound 3
3.3. General Procedures for the Synthesis of Compounds 4a–o
3.4. Cytotoxic Assay
3.5. Cell Cycle Analysis
3.6. Hoechst 33258 Staining Assay
3.7. Annexin V-FITC/PI Dual Staining Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhai, S.; Li, L.; Li, X.; Zhou, H.; Liu, A.; Su, G.; Mu, Q.; Du, Y.; Yan, B. Anti-tumor selectivity of a novel tubulin and HSP90 dual-targeting inhibitor in non-small cell lung cancer models. Biochem. Pharmacol. 2013, 86, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Oh, H.M.; Choi, S.K.; Han, D.C.; Kwon, B.M. Anti-tumor abietane diterpenes from the cones of Sequoia sempervirens. Bioorg. Med. Chem. Lett. 2005, 15, 2019–2021. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Huang, R.Z.; Liao, Z.X.; Pan, Y.M.; Gou, S.H.; Wang, H.S. Synthesis and pharmacological evaluation of dehydroabietic acid thiourea derivatives containing bisphosphonate moiety as an inducer of apoptosis. Eur. J. Med. Chem. 2016, 108, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.F.; Cardoso, M.J.; Silva, S.D.; Souza, M.G.; Veneziani, R.C.; Ambrosio, S.R.; Martins, C.H. Antibacterial activity of Pinus elliottii and its major compound, dehydroabietic acid, against multidrug-resistant strains. J. Med. Microbiol. 2014, 63, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.Y.; Duan, W.G.; Liu, L.Z.; Li, F.Y.; Lu, M.P.; Liu, B.M. Synthesis and antifungal activity of dehydroabietic acid-based thiadiazole-phosphonates. Holzforschung 2015, 69, 1069–1075. [Google Scholar] [CrossRef]
- Li, W.S.; McChesney, J.D. Preparation of potential anti-inflammatory agents from dehydroabietic dehydroabietic acid. J. Pharm. Sci. 1992, 81, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, B.; Astudillo, L.; Rodriguez, J.A.; Yanez, T.; Theoduloz, C.; Schmeda-Hirschmann, G. Gastroprotective and cytotoxic effect of dehydroabietic acid derivatives. Pharmacol. Res. 2005, 52, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Zapata, B.; Rojas, A.; Betancur-Galvis, L.; Mesa-Arango, A.C.; Pérez-Guaitac, D.; González, M.A. Cytotoxic, immunomodulatory, antimycotic, and antiviral activities of semisynthetic 14-hydroxyabietane derivatives and triptoquinone C-4 epimers. MedChemComm 2013, 4, 1239–1246. [Google Scholar] [CrossRef]
- Roa-Linares, V.C.; Brand, Y.M.; Agudelo-Gomez, L.S.; Tangarife-Castaño, V.; Betancur-Galvis, L.A.; Gallego-Gomez, J.C.; González, M.A. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem. 2016, 108, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, Y.G.; Lee, J.Y.; Choi, D.H.; Cho, Y.U.; Shin, J.M.; Park, J.S.; Lee, J.H.; Kim, W.G.; Seo, D.B.; et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015, 412, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.L.; Xu, W.S.; Gao, W.L.; Zhang, J.; Zhao, Y.; Long, J.Y.; Anson, C.E.; Powell, A.K. DNA binding and cytotoxicity activity of a chiral iron(III) triangle complex based on a natural rosin product. J. Photochem. Photobiol. B 2015, 142, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Qu, H.E.; Huang, X.C.; Pan, Y.M.; Liang, D.; Chen, Z.F.; Wang, H.S.; Zhang, Y. Synthesis and biological evaluation of novel dehydroabietic acid derivatives conjugated with acyl-thiourea peptide moiety as antitumor agents. Int. J. Mol. Sci. 2015, 16, 14571–14593. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.J.; Ni, Q.; Ji, A.L.; Gu, W.; Wu, J.H.; Jiang, C.P. Dehydroabietic acid derivative QC4 induces gastric cancer cell death via oncosis and apoptosis. BioMed Res. Int. 2016, 2016, 2581061. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Schumann, D.; Linne, U.; Koert, U.; Marahiel, M.A. Rational design of bacitracin A derivatives by incorporating natural product derived heterocycles. J. Am. Chem. Soc. 2006, 128, 10513–10520. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Zhang, Y.Q.; Wang, Y.; McAlpine, S.R. Design, synthesis and anticancer mechanistic studies of linked azoles. MedChemComm 2015, 6, 300–305. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Xu, F.F.; Cheng, G.Y.; Hao, H.H.; Wang, Y.L.; Wang, X.; Chen, D.M.; Peng, D.P.; Liu, Z.L.; Yuan, Z.H.; Dai, M.H. Mechanisms of antibacterial action of quinoxaline 1,4-di-N-oxides against Clostridium perfringens and Brachyspira hyodysenteriae. Front. Microbiol. 2016, 7, 1948. [Google Scholar] [CrossRef] [PubMed]
- Loughran, H.M.; Han, Z.Y.; Wrobel, J.E.; Decker, S.E.; Ruthel, G.; Freedman, B.D.; Harty, R.N.; Reitz, A.B. Quinoxaline-based inhibitors of Ebola and Marburg VP40 egress. Bioorg. Med. Chem. Lett. 2016, 26, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Dias, E.; Di Maio, R.; González, M.; Pacce, S.; Saenz, P.; Seoane, G.; Suescun, L.; Mombrú, A.; Fernández, G.; et al. Synthesis and herbicidal activity of N-oxide derivatives. J. Agric. Food Chem. 2000, 48, 2995–3002. [Google Scholar] [CrossRef] [PubMed]
- Guirado, A.; Sánchez, J.I.L.; Ruiz-Alcaraz, A.J.; Bautista, D.; Galvez, J. Synthesis and biological evaluation of 4-alkoxy-6,9-dichloro[1,2,4]triazolo[4,3-a]quinoxalines as inhibitors of TNF-α and IL-6. Eur. J. Med. Chem. 2012, 54, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zghaib, Z.; Guichou, J.F.; Vappiani, J.; Bec, N.; Hadj-Kaddour, K.; Vincent, L.A.; Paniagua-Gayraud, S.; Larroque, C.; Moarbess, G.; Cuq, P.; et al. New imidazoquinoxaline derivatives: Synthesis, biological evaluation on melanoma, effect on tubulin polymerization and structure–activity relationships. Bioorg. Med. Chem. 2016, 24, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Corona, P.; Carta, A.; Loriga, M.; Vitale, G.; Paglietti, G. Synthesis and in vitro antitumor activity of new quinoxaline derivatives. Eur. J. Med. Chem. 2009, 44, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Ghattass, K.; El-Sitt, S.; Zibara, K.; Rayes, S.; Haddadin, M.J.; El-Sabban, M.; Gali-Muhtasib, H. The quinoxaline di-N-oxide DCQ blocks breast cancer metastasis in vitro and in vivo by targeting the hypoxia inducible factor-1 pathway. Mol. Cancer 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Kumar, R.N.; Reddy, G.M.; Swaroop, D.K.; Poornachandra, Y.; Kumar, C.G.; Narsaiah, B. Synthesis of novel hydrazone and azole functionalized pyrazolo[3,4-b]pyridine derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4427–4432. [Google Scholar] [CrossRef] [PubMed]
- Mareddy, J.; Suresh, N.; Kumar, C.G.; Kapavarapu, R.; Jayasree, A.; Pal, S. 1,2,3-Triazole-nimesulide hybrid: Their design, synthesis and evaluation as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Cao, R.H.; Shi, B.X.; Zhou, X.T.; Ma, Q.; Sun, J.; Guo, L.; Yi, W.; Chen, Z.Y.; Song, H.C. Synthesis and cytotoxic evaluation of 1-carboxamide and 1-amino side chain substituted β-carbolines. Eur. J. Med. Chem. 2010, 45, 5513–5519. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.; Gigante, B.; Marques, M.M.; Gilchrist, T.L.; de Clercq, E. Synthesis and antiviral evaluation of benzimidazoles, quinoxalines and indoles from dehydroabietic acid. Bioorg. Med. Chem. 2004, 12, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Delcros, J.G.; Biggerstaff, J.; Phanstiel, O. Synthesis and biological evaluation of N-(anthracen-9-ylmethyl)triamines as molecular recognition elements for the polyamine transporter. J. Med. Chem. 2003, 46, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.G.; Reddy, T.S.; Nayak, V.L.; Prasad, B.; Reddy, A.P.; Ravikumar, A.; Taj, S.; Kamal, A. Design, synthesis and biological evaluation of N-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)-1,3-diphenyl-1H-pyrazole-4-carboxamides as CDK1/Cdc2 inhibitors. Eur. J. Med. Chem. 2016, 122, 164–177. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4a–o are available from the authors. |
| Compound | CLogP | IC50 (μM) | |||
|---|---|---|---|---|---|
| MCF-7 | SMMC-7721 | HeLa | LO2 | ||
| 1 | − | >50 | >50 | >50 | >50 |
| 2 | − | >50 | >50 | 43.32 ± 3.26 | >50 |
| 3 | − | >50 | 45.83 ± 5.28 | 37.72 ± 3.75 | >50 |
| 4a | 4.69 | 2.36 ± 0.29 | 1.65 ± 0.22 | 2.08 ± 0.43 | 15.72 ± 0.65 |
| 4b | 6.81 | 1.78 ± 0.36 | 0.72 ± 0.09 | 1.08 ± 0.12 | 11.09 ± 0.57 |
| 4c | 8.92 | 21.02 ± 1.27 | 10.96 ± 0.58 | 8.67 ± 0.65 | >50 |
| 4d | 11.04 | 28.13 ± 0.92 | 32.70 ± 2.69 | 25.32 ± 3.01 | >50 |
| 4e | 5.96 | 20.45 ± 1.51 | 18.64 ± 2.37 | 12.79 ± 1.26 | >50 |
| 4f | 7.08 | 8.95 ± 0.63 | 7.71 ± 0.82 | 5.98 ± 0.37 | 42.23 ± 2.78 |
| 4g | 8.20 | >50 | 27.89 ± 2.91 | 32.01 ± 4.21 | >50 |
| 4h | 4.52 | 12.32 ± 1.03 | 16.28 ± 1.85 | 9.67 ± 1.13 | 36.64 ± 3.12 |
| 4i | 4.49 | 5.89 ± 0.56 | 4.32 ± 0.64 | 5.05 ± 0.72 | 21.95 ± 2.70 |
| 4j | 5.40 | 12.21 ± 0.75 | 8.03 ± 0.70 | 6.62 ± 0.59 | 29.52 ± 3.61 |
| 4k | 6.58 | 38.66 ± 2.87 | 26.45 ± 3.73 | 13.51 ± 2.21 | >50 |
| 4l | 3.75 | 22.84 ± 3.21 | 15.30 ± 2.06 | 12.73 ± 1.35 | >50 |
| 4m | 3.21 | 3.73 ± 0.62 | 4.66 ± 0.42 | 2.18 ± 0.37 | 18.36 ± 1.73 |
| 4n | 3.86 | 15.76 ± 0.81 | 10.88 ± 1.68 | 13.26 ± 2.71 | >50 |
| 4o | 3.44 | 24.38 ± 1.69 | 16.92 ± 1.95 | 20.46 ± 3.12 | >50 |
| Etoposide | − | 0.73 ± 0.07 | 0.69 ± 0.18 | 0.87 ± 0.16 | 8.89 ± 0.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, W.; Wang, S.; Jin, X.; Zhang, Y.; Hua, D.; Miao, T.; Tao, X.; Wang, S. Synthesis and Evaluation of New Quinoxaline Derivatives of Dehydroabietic Acid as Potential Antitumor Agents. Molecules 2017, 22, 1154. https://doi.org/10.3390/molecules22071154
Gu W, Wang S, Jin X, Zhang Y, Hua D, Miao T, Tao X, Wang S. Synthesis and Evaluation of New Quinoxaline Derivatives of Dehydroabietic Acid as Potential Antitumor Agents. Molecules. 2017; 22(7):1154. https://doi.org/10.3390/molecules22071154
Chicago/Turabian StyleGu, Wen, Shuang Wang, Xiaoyan Jin, Yaliang Zhang, Dawei Hua, Tingting Miao, Xubing Tao, and Shifa Wang. 2017. "Synthesis and Evaluation of New Quinoxaline Derivatives of Dehydroabietic Acid as Potential Antitumor Agents" Molecules 22, no. 7: 1154. https://doi.org/10.3390/molecules22071154





