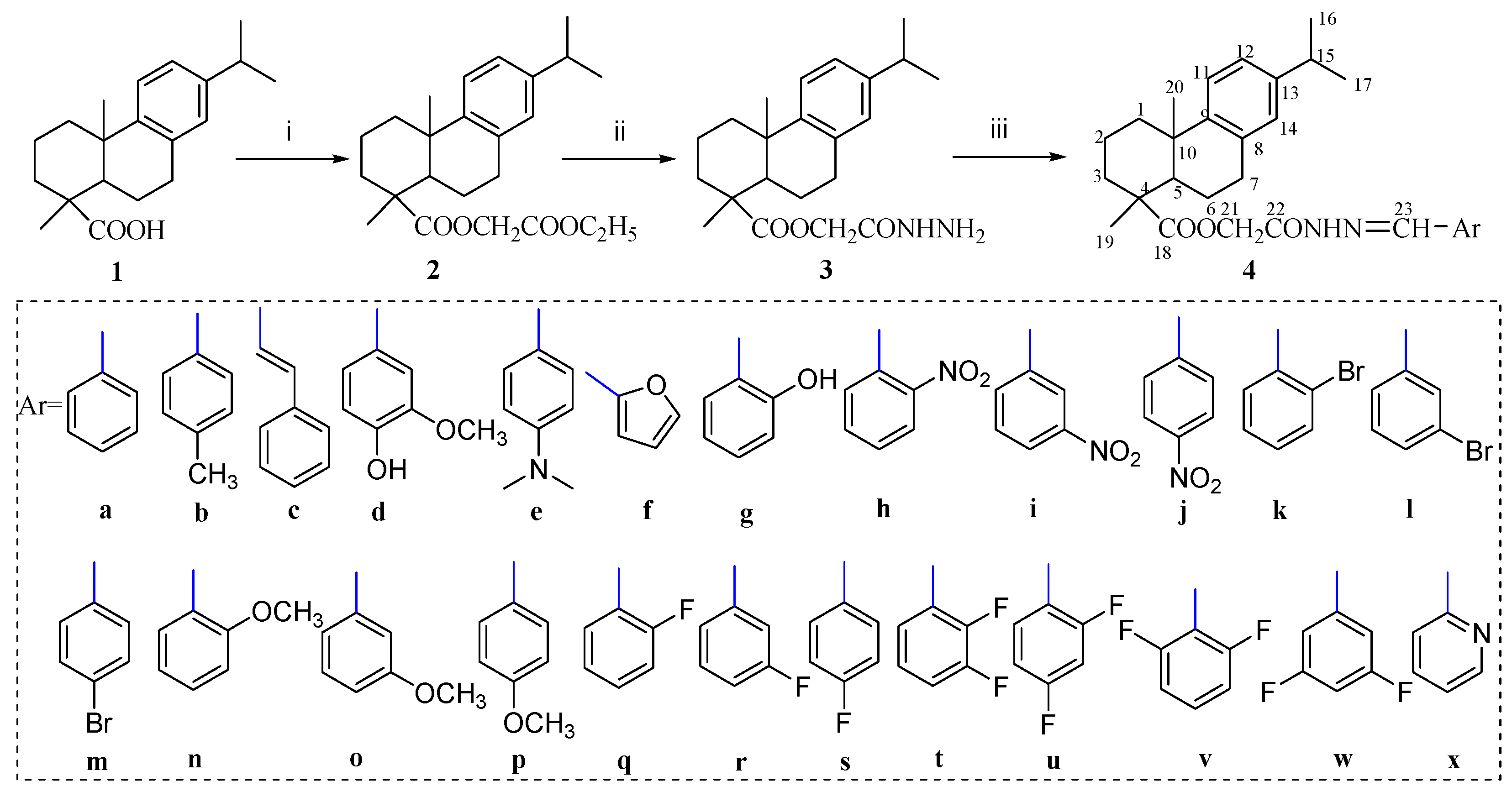
3.4. General Procedure for the Synthesis of Dehydroabietic Acid-Based Acylhydrazones 4a–x
A mixture of acylhydrazide 3 (2 mmol), the appropriate arylaldehyde (2.2 mmol) and glacial acetic acid (0.25 mL) in EtOH (20 mL) was refluxed for 6 h. After cooling, the formed precipitate was filtered off and purified by crystallization from anhydrous ethanol to afford the acylhydrazone derivatives.
N’-(Benzylidene)-2-(dehydroabietyloxy)acetohydrazide (4a): White solid; yield 87%, m.p. 155.1–156.7 °C. IR (KBr, cm−1): 3446, 3197 (N–H), 2951, 2864, 2366, 1732 (C=O), 1697 (C=O), 1614, 1492, 1411, 1296, 1232, 1174, 1118, 1022, 947, 819, 754, 690. 1H-NMR (600 MHz, CDCl3) δ 10.70 (s, 1H, CONH), 7.84 (s, 1H, N=CH), 7.64 (d, J = 9.2 Hz, 2H, H-2′ and H-6′), 7.40 (d, J = 6.2 Hz, 3H, H-3′, H-4′ and H-5′), 7.22 (d, J = 8.2 Hz, 1H, H-11), 7.03 (d, J = 8.2 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 5.19 (q, J = 16.0 Hz, 2H, H-21), 3.05 (m, 1H, H-15), 2.82–2.91 (m, 2H, H-7), 2.42 (dd, J = 12.3, 1.7 Hz, 1H, He-1), 2.36 (d, J = 12.9 Hz, 1H, H-5), 1.52–1.93 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.21 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.17 (C=O), 147.08, 145.85, 145.52, 135.02, 133.66, 130.53, 128.95, 127.37, 127.13, 124.36, 124.04, 61.54, 47.86, 44.88, 38.14, 37.12, 36.98, 33.62, 30.32, 25.46, 24.18, 21.82, 18.81, 16.81. MS (ESI) m/z 458.86 ([M − H]−).
N’-(4-Methylbenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4b): White solid; yield 89%, m.p. 170.0–172.1 °C. IR (KBr, cm−1): 2951, 2929, 1737 (C=O), 1691 (C=O), 1610, 1498, 1417, 1311, 1236, 1172, 1124, 954, 891, 813. 1H-NMR (600 MHz, CDCl3) δ 10.62 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.53 (d, J = 8.1 Hz, 2H, H-2′ and H-6′), 7.21 (d, J = 12.4 Hz, 3H, H-3′, H-5′ and H-11), 7.03 (d, J = 8.1 Hz, 1H, H-12), 6.88 (s, 1H, H-14), 5.17 (q, J = 16.0 Hz, 2H, H-21), 3.01 (d, J = 7.1 Hz, 1H, H-15), 2.79–2.92 (m, 2H, H-7), 2.42 (dd, J = 12.5, 2.0 Hz, 1H, He-1), 2.40 (s, 3H, Ph-CH3), 2.35 (d, J = 13.2 Hz, 1H, H-5), 1.53–2.01 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.21 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.50 (C=O), 170.00 (C=O), 147.09, 145.82, 145.57, 140.83, 135.05, 130.97, 129.67, 127.33, 127.13, 124.34, 124.01, 61.56, 47.85, 44.88, 38.13, 37.12, 36.97, 33.61, 30.32, 25.46, 24.16, 21.81, 21.70, 18.81, 16.81. MS (ESI) m/z 472.88 ([M − H]−).
N’-(3-Phenylallylidene)-2-(dehydroabietyloxy)acetohydrazide (4c): light yellow solid; yield 89%, m.p. 185.7–187.3 °C. IR (KBr, cm−1): 2924, 1734 (C=O), 1687 (C=O), 1496, 1417, 1296, 1238, 1170, 1124, 972, 750, 690, 599. 1H-NMR (600 MHz, CDCl3) δ 10.45 (s, 1H, CONH), 7.63 (d, J = 7.1 Hz, 1H, 1H, N=CH), 7.46 (d, J = 7.4 Hz, 2H, H-2′ and H-6′), 7.38 (t, 2H, H-3′ and H-5’), 7.34 (d, J = 7.1 Hz, 1H, H-4′), 7.21 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.9 Hz, 1H, H-12), 6.90 (s, 1H, H-14), 6.85 (s, 2H, HC=CH), 5.09 (q, J = 15.9 Hz, 2H, H-21), 2.99–3.05 (m, 1H, H-15), 2.81–2.93 (m, 2H, H-7), 2.41 (dd, J = 12.5, 2.0 Hz, 1H, He-1), 2.36 (d, J = 12.9 Hz, 1H, H-5), 1.54–1.99 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.22 (d, J = 6.9, 2.0 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.47 (C=O), 169.84 (C=O), 147.47, 147.06, 145.86, 139.99, 135.86, 135.00, 129.28, 129.03, 127.25, 127.14, 124.68, 124.33, 124.03, 61.47, 47.85, 44.88, 38.13, 37.11, 36.95, 33.61, 30.30, 25.44, 24.15, 21.82, 18.81, 16.81. MS (ESI) m/z 484.91 ([M − H]−).
N’-(3-Methoxy-4-hydroxylbenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4d): yellow solid; yield 85%, m.p. 117.6–119.0 °C. IR (KBr, cm−1): 3446, 3230 (N–H), 2954, 1730 (C=O), 1687 (C=O), 1598, 1516, 1462, 1425, 1282, 1238, 1170, 1122, 1029, 950, 862, 819, 756, 623. 1H-NMR (600 MHz, CDCl3) δ 10.35 (s, 1H, CONH), 7.72 (s, 1H, N=CH), 7.23 (s, 1H, H-6’), 7.19 (d, J = 8.2 Hz, 1H, H-2’), 7.01 (d, J = 8.0 Hz, 2H, H-5′ and H-11), 6.90 (d, J = 8.1 Hz, 1H, H-12), 6.87 (s, 1H, H-14), 5.15 (q, J = 15.9 Hz, 2H, H-21), 3.93 (s, 3H, OCH3), 2.98–3.02 (m, 1H, H-15), 2.76–2.93 (m, 2H, H-7), 2.39 (dd, J = 12.3, 1.5 Hz, 1H, He-1), 2.33 (d, J = 12.7 Hz, 1H, H-5), 1.48–2.03 (m, 7H, Ha-1, H-2, H-3, H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.58 (C=O), 169.65 (C=O), 148.24, 147.18, 147.04, 145.85, 145.66, 134.99, 127.10, 126.11, 124.31, 124.01, 122.89, 114.63, 107.78, 61.53, 56.11, 53.70, 47.86, 38.10, 37.09, 36.96, 33.60, 30.25, 25.42, 24.15, 21.81, 18.78, 16.79. MS (ESI) m/z 504.87 ([M − H]−).
N’-(4-Dimethylaminobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4e): brown red solid, yield 66%, m.p. 163.4–164.7 °C. IR (KBr, cm−1): 2953, 1735 (C=O), 1687 (C=O), 1606, 1531, 1417, 1359, 1298, 1234, 1174, 1116, 1047, 948, 815. 1H-NMR (600 MHz, CDCl3) δ 10.00 (s, 1H, CONH), 7.69 (s, 1H, N=CH), 7.50 (d, J = 8.5 Hz, 2H, H-2’ and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.2 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 6.70 (d, J = 8.5 Hz, 2H, H-3’ and H-5’), 5.14 (q, J =16.2 Hz, 2H, H-21), 3.03 (s, 6H, N(CH3)2), 2.94–3.00 (m, 1H, H-15), 2.80–2.90 (m, 2H, H-7), 2.40 (dd, J = 12.3, 1.4 Hz, 1H, He-1), 2.31 (d, J = 12.7 Hz, 1H, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3, H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.22 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.48 (C=O), 169.22 (C=O), 147.12, 146.94, 145.91, 145.76, 134.86, 128.77, 127.14, 124.35, 124.31, 123.94, 112.10, 61.64, 47.83, 44.78, 40.49, 38.04, 37.11, 36.92, 33.62, 30.30, 25.42, 24.16, 21.78, 18.74, 16.68. MS (ESI) m/z 501.89 ([M − H]−).
N’-((Furan-2-yl)methylene)-2-(dehydroabietyloxy)acetohydrazide (4f): yellow solid; yield 58%, m.p. 125.7–126.4 °C. IR (KBr, cm−1): 2968, 2868, 1730 (C=O), 1683 (C=O), 1625, 1541, 1469, 1419, 1386, 1334, 1284, 1228, 1170, 1124, 1012, 974, 937, 883, 837, 750. 1H-NMR (600 MHz, CDCl3) δ 10.68 (s, 1H, CONH), 7.71 (s, 1H, N=CH), 7.50 (s, 1H, H-4’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.01 (d, J = 8.1 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 6.67 (d, J = 3.4 Hz, 1H, H-4’), 6.48 (s, 1H, H-4’), 5.12 (q, J = 16.1 Hz, 2H, H-21), 2.96–3.02 (m, 1H, H-15), 2.81–2.91 (m, 2H, H-7), 2.39 (dd, J = 12.3, 1.6 Hz, 1H, He-1), 2.34 (d, J = 12.9 Hz, 1H, H-5), 1.52–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.44 (C=O), 170.16 (C=O), 149.15, 147.07, 145.83, 144.78, 135.24, 135.03, 127.11, 124.34, 124.01, 113.35, 112.09, 61.44, 47.82, 44.85, 38.11, 37.09, 36.89, 33.61, 30.27, 25.43, 24.16, 21.76, 18.78, 16.76. MS (ESI) m/z 448.86 ([M − H]−).
N’-(2-Hydroxylbenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4g): yellow solid; yield 73%, m.p. 214.9–215.6 °C. IR (KBr, cm−1): 3251 (N–H), 2953, 2870, 1726 (C=O), 1678 (C=O), 1616, 1552, 1292, 1390, 1274, 1220, 1166, 1124, 974, 896, 759. 1H-NMR (600 MHz, CDCl3) δ 10.81 (s, 1H, CONH), 9.87 (s, 1H, OH), 8.12 (s, 1H, N=CH), 7.34 (m, 1H, H-6’), 7.20 (d, J = 8.2 Hz, 1H and H-4’), 7.15 (d, J = 6.8 Hz, 1H, H-5’), 6.96–7.04 (m, 2H, H-11 and H-14), 6.84–6.91 (m, 7.4 Hz, 2H, H-12 and H-3’), 5.05 (q, J = 15.5 Hz, 2H, H-21), 2.95–3.02 (m, 1H, H-15), 2.78–2.90 (m, 2H, H-7), 2.38 (dd, J = 12.4, 1.2 Hz, 1H, He-1), 2.34 (d, J = 12.8 Hz, 1H, H-5),1.47–2.01 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.43 (C=O), 168.98 (C=O), 163.35, 157.91, 146.96, 145.91, 134.88, 132.39, 131.42, 127.12, 124.32, 124.07, 120.15, 119.53, 117.14, 60.92, 47.88, 44.83, 38.07, 37.08, 36.97, 33.61, 30.22, 25.40, 24.14, 21.85, 18.74, 16.77. MS (ESI) m/z 474.85 ([M − H]−).
N’-(2-Nitrobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4h): yellow solid; yield 62%; m.p. 94.9–96.7 °C. IR (KBr, cm−1): 3442, 3207 (N–H), 3101, 2954, 2927, 2868, 1728 (C=O), 1691 (C=O), 1597, 1527, 1463, 1417, 1346, 1301, 1238, 1170, 1126, 970, 935, 821, 783, 742. 1H-NMR (600 MHz, CDCl3) δ 10.48 (s, 1H, CONH), 8.38 (s, 1H, N=CH), 8.03 (d, J = 8.2 Hz, 2H, H-3’ and H-6’), 7.65 (t, J = 7.8 Hz, 1H, H-5’), 7.55 (t, J = 7.8 Hz, 1H, H-4’), 7.18 (d, J = 8.2 Hz, 1H, H-11), 7.00 (d, J = 8.1 Hz, 1H, H-12), 6.87 (s, 1H, H-14), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.95 (m, 1H, H-15), 2.85 (m, 2H, H-7), 2.35 (dd, J = 12.5, 1.7 Hz, 1H, He-1), 2.32 (d, J = 13.1 Hz, 1H, H-5), 1.49–1.93 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.59 (C=O), 169.97 (C=O), 148.30, 147.11, 145.77, 140.59, 135.06, 133.55, 130.64, 128.71, 128.49, 127.08, 125.02, 124.31, 123.96, 61.38, 47.85, 44.80, 38.07, 37.08, 36.92, 33.60, 30.19, 25.42, 24.16, 21.78, 18.71, 16.71. MS (ESI) m/z 503.88 ([M − H]−).
N’-(3-Nitrobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4i): light yellow solid; yield 82%, m.p. 99.6–101.9 °C. IR (KBr, cm−1): 3190, 2926, 1734 (C=O), 1693 (C=O), 1579, 1533, 1463, 1348, 1238, 1172, 1126, 981, 825, 734, 678. 1H-NMR (600 MHz, CDCl3) δ 10.85 (s, 1H, CONH), 8.46 (s, 1H, N=CH), 8.24 (s, 1H, H-2′), 7.92 (s, 2H, H-5′ and H-6′), 7.55 (s, 1H, H-3′), 7.19 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 9.3 Hz, 1H, H-14), 6.86 (s, 1H, H-12), 5.18 (q, J = 16.0 Hz, 2H, H-21), 2.96–3.02 (m, 1H, H-15), 2.77–2.91 (m, 2H, H-7), 2.31–2.42 (m, 2H, He-1 and H-5), 1.49–2.01 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.21 (d, J = 6.9, Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.60 (C=O), 170.44 (C=O), 148.76, 147.00, 145.90, 142.97, 135.39, 134.84, 132.84, 130.05, 127.07, 124.82, 124.34, 124.08, 121.84, 61.45, 47.86, 44.88, 38.10, 37.09, 36.93, 33.59, 30.23, 25.39, 24.15, 21.82, 18.74, 16.80. MS (ESI) m/z 503.87 ([M − H]−).
N’-(4-Nitrobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4j): yellow solid; yield 54%, m.p. 177.2–179.8 °C. IR (KBr, cm−1): 3099, 2947, 1739 (C=O), 1691 (C=O), 1591, 1523, 1463, 1413, 1342, 1234, 1170, 1111, 1014, 943, 833, 745. 1H-NMR (600 MHz, CDCl3) δ 10.63 (s, 1H, CONH), 8.24 (s, 2H, H-3’ and H-5’), 7.87 (s, 1H, N=CH), 7.75 (d, J = 8.6 Hz, 2H, H-2’ and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 6.86 (s, 1H, H-12), 7.02 (d, J = 9.3 Hz, 1H, H-14), 5.17 (q, J = 16.0 Hz, 2H, H-21), 2.95–3.01 (m, 1H, H-15), 2.77–2.91 (m, 2H, H-7), 2.34–2.39 (m, 2H, He-1 and H-5), 1.48–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.20 (d, J = 6.9, Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.53 (C=O), 170.36 (C=O), 148.71, 146.96, 145.96, 134.92, 132.51, 132.28, 128.70, 127.09, 124.79, 124.34, 124.06, 61.39, 47.86, 44.91, 38.09, 37.13, 36.93, 33.58, 30.26, 25.40, 24.13, 21.80, 18.77, 16.79. MS (ESI) m/z 503.87 ([M − H]−).
N’-(2-Bromobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4k): White solid; yield 70%; m.p. 184.9–185.7 °C. IR (KBr, cm−1): 3246 (N–H), 2935, 1730 (C=O), 1699 (C=O), 1668, 1602, 1496, 1462, 1402, 1234, 1172, 1128, 945, 877, 754, 711, 638. 1H-NMR (600 MHz, CDCl3) δ 10.54 (s, 1H, CONH), 8.21 (s, 1H, N=CH), 7.92 (d, J = 7.8 Hz, 1H, H-6’), 7.58 (d, J = 7.9 Hz, 1H, H-3’), 7.34 (t, J = 7.6 Hz, 1H, H-5’), 7.23–7.27 (m, 1H, H-4’), 7.19 (d, J = 8.2 Hz, 1H, H-11), 7.01 (d, J = 9.3 Hz, 1H, H-14), 6.87 (s, 1H, H-12), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.00 (m, 1H, H-15), 2.80–2.89 (m, 2H, H-7), 2.32–2.39 (m, 2H, He-1, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.00 (C=O), 147.07, 145.80, 144.04, 135.06, 133.38, 132.63, 131.60, 127.78, 127.71, 127.11, 124.38, 124.32, 123.98, 61.48, 47.84, 44.81, 38.10, 37.10, 37.00, 33.61, 30.25, 25.42, 24.16, 21.90, 18.80, 16.78. MS (ESI) m/z 538.72 ([M − H]−).
N’-(3-Bromobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4l): yellow solid; yield 61%; m.p. 178.3–179.8 °C. IR (KBr, cm−1): 3554, 3414, 3194 (N–H), 3111, 2947, 1730 (C=O), 1691 (C=O), 1560, 1409, 1236, 1170, 1120, 960, 877, 821, 734, 678. 1H-NMR (600 MHz, CDCl3) δ 9.85 (s, 1H, CONH), 7.64 (s, 1H, N=CH), 7.50 (dd, J = 21.1, 7.6 Hz, 1H, H-6’), 7.41 (d, J = 14.0 Hz, 1H, H-2’), 7.24 (s, 1H, H-4’), 7.20 (d, J = 8.1 Hz, 1H, H-5’), 7.02 (d, J = 7.9 Hz, 1H, H-11), 6.90 (s, 1H, H-14), 6.82 (s, 1H, H-12), 5.11–5.15 (m, 2H, H-21), 2.95–2.98 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.38 (d, J = 12.3 Hz, 1H, He-1), 2.34 (d, J = 12.7 Hz, 1H, H-5), 1.47–1.96 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.22 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.38 (C=O), 170.05 (C=O), 149.58, 147.07, 145.85, 138.47, 135.04, 132.22, 130.45, 129.89, 127.11, 125.69, 124.33, 124.02, 123.15, 61.80, 47.79, 44.80, 38.13, 37.10, 36.93, 33.61, 30.26, 25.42, 24.18, 21.82, 18.80, 16.78. MS (ESI) m/z 536.77 ([M − H]−).
N’-(4-Bromobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4m): White solid; yield 90%, m.p. 188.1–188.6 °C. IR (KBr, cm−1): 3201 (N–H), 3101, 2949, 1734 (C=O), 1691 (C=O), 1415, 1309, 1236, 1118, 1064, 1006, 952, 889, 817. 1H-NMR (600 MHz, CDCl3) δ 10.51 (s, 1H, CONH), 7.75 (s, 1H, N=CH), 7.51 (d, J = 8.5 Hz, 2H, H-3’ and H-5’), 7.46 (d, J = 8.5 Hz, 2H, H-2’ and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 9.3 Hz, 1H, H-12), 6.86 (s, 1H, H-14), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.02 (m, 1H, H-15), 2.79–2.90 (m, 2H, H-7), 2.38 (dd, J = 12.4, 1.7 Hz, 1H, He-1), 2.34 (d, J = 12.8 Hz, 1H, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.22 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.50 (C=O), 170.04 (C=O), 147.01, 145.88, 144.20, 134.92, 132.51, 132.28, 128.70, 127.09, 124.79, 124.34, 124.06, 61.47, 47.84, 44.88, 38.10, 37.10, 36.93, 33.60, 30.28, 25.43, 24.15, 21.80, 18.77, 16.79. MS (ESI) m/z 538.73 ([M − H]−).
N’-(2-Methoxybenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4n): White solid; yield 84%; m.p. 190.3–191.8 °C. IR (KBr, cm−1): 3442, 3217 (N-H), 3084, 2958, 1730 (C=O), 1691 (C=O), 1666, 1600, 1462, 1361, 1298, 1247, 1176, 1126, 1080, 1024, 968, 819, 761, 644. 1H-NMR (600 MHz, CDCl3) δ 10.38 (s, 1H, CONH), 8.24 (s, 1H, N=CH), 7.88 (d, J = 7.6 Hz, 1H, H-6’), 7.38 (t, J = 7.8 Hz, 1H, H-4’), 7.20 (d, J = 8.2 Hz, 1H, H-5’), 6.97–7.05 (d, 2H, H-11 and H-3’), 6.91 (d, J = 8.4 Hz, 1H, H-14), 6.87 (s, 1H, H-12), 5.18 (s, 2H, H-21), 3.85 (s, 3H, OCH3), 2.96–3.06 (m, 1H, H-15), 2.79–2.83 (m, 2H, H-7), 2.40 (d, J = 11.9 Hz, 1H, He-1), 2.34 (d, J = 12.5 Hz, 1H, H-5), 1.48–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.49 (C=O), 169.74 (C=O), 158.21, 147.09, 145.79 141.27, 135.19, 131.75, 127.15, 126.40, 124.31, 123.94, 122.21, 120.96, 111.18, 61.63, 55.62, 47.84, 44.91, 38.15, 37.12, 36.89, 33.61, 30.24, 25.47, 24.17, 21.76, 18.83 (C-6), 16.77. MS (ESI) m/z 488.86 ([M − H]−).
N’-(3-Methoxybenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4o): light yellow solid; yield 81%; m.p. 136.7–137.9 °C. IR (KBr, cm−1): 3412, 3080, 2958, 2927, 2870, 1726 (C=O), 1685 (C=O), 1573, 1458, 1419, 1311, 1286, 1249, 1130, 1043, 948, 873, 823, 775, 688. 1H-NMR (600 MHz, CDCl3) δ 10.71 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.31 (t, 1H, H-6’), 7.21 (d, J = 13.2 Hz, 2H, H-2’ and H-5’), 7.17 (d, J = 7.6 Hz, 1H, H-11), 7.03 (d, J = 8.0 Hz, 1H, H-12), 6.97 (d, J = 10.5 Hz, 1H, H-4’), 6.89 (s, 1H, H-14), 5.18 (q, J = 16.0 Hz, 2H, H-21), 3.85 (s, 3H, OCH3), 2.99–3.05 (s, 1H, H-15), 2.81–2.92 (m, 2H, H-7), 2.42 (dd, J = 12.3, 1.8 Hz, 1H, He-1), 2.36 (d, J = 12.8 Hz, 1H, H-5), 1.50–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.15 (C=O), 160.02, 147.06, 145.85, 145.41, 135.01, 129.98, 127.13, 124.34, 124.02, 120.55, 116.66, 111.53, 61.51, 55.47, 47.86, 44.86, 38.13, 37.11, 36.99, 33.62, 30.30, 25.45, 24.16, 21.83, 18.81, 16.81. MS (ESI) m/z 488.91 ([M − H]−).
N’-(4-Methoxybenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4p): White solid; yield 90%; m.p. 174.8–175.5 °C. IR (KBr, cm−1): 2931, 1737 (C=O), 1689 (C=O), 1604, 1504, 1419, 1303, 1247, 1168, 1124, 1035, 954, 893, 837. 1H-NMR (600 MHz, CDCl3) δ 10.49 (s, 1H, CONH), 7.76 (s, 1H, N=CH), 7.56 (d, 2H, H-2’, H-6’), 7.20 (d, 1H, H-11), 7.01 (d, 1H, H-12), 6.92 (d, 2H, H-3’, H-5’), 6.88 (s, 1H, H-14), 5.16 (q, J = 15.9 Hz, 2H, H-21), 3.85 (s, 3H, OCH3), 2.98–3.04 (m, 1H, H-15), 2.81–2.90 (m, 2H, H-7), 2.41 (d, J = 11.8 Hz, 1H, He-1), 2.35 (d, J = 12.8 Hz, 1H, H-5), 1.53–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9, Hz, 6H, H-16 and H-17).13C-NMR (151 MHz, CDCl3) δ 178.49 (C=O), 169.80 (C=O), 161.51, 147.08, 145.82, 145.18, 135.05, 128.90, 127.12, 126.41, 124.34, 124.00, 114.38, 61.56, 55.53, 47.84, 44.88, 38.13, 37.11, 36.95, 33.61, 30.31, 25.45, 24.16, 21.80, 18.80, 16.80. MS (ESI) m/z 488.88 ([M − H]−).
Dehydroabietic acid-based 2-fluorophenyl acylhydrazone (4q): light yellow solid; yield 72%; m.p. 130.2–130.5 °C. IR (KBr, cm−1): 2954, 2868, 1730 (C=O), 1691 (C=O), 1415, 1311, 1240, 1170, 1126, 1053, 877, 823, 756, 630. 1H-NMR (600 MHz, CDCl3) δ 10.86 (s, 1H, CONH), 8.10 (s, 1H, N=CH), 7.89 (d, J = 7.8 Hz, 1H, H-6’), 7.38 (d, J = 7.9 Hz, 1H, H-3’), 7.21 (d, J = 7.6 Hz, 1H, H-5’), 7.18 (d, J = 8.9 Hz, 1H, H-11), 7.12 (d, J = 8.2 Hz, 1H, H-4’), 7.02 (d, J = 9.3 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 5.17 (q, J = 16.0 Hz, 2H, H-21), 2.95–3.05 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.41 (dd, J = 12.4, 1.7 Hz, 1H, He-1), 2.35 (d, J = 12.9 Hz, 1H, H-5), 1.50–1.98 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.37 (s, 3H, H-19), 1.26 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.59 (C=O), 170.29 (C=O), 162.42, 147.14, 145.78, 138.62, 135.14, 132.02, 127.12, 126.72, 124.59, 124.34, 123.97, 121.60, 116.17, 61.44, 47.87, 44.78, 38.13, 37.10, 37.01, 33.62, 30.23, 25.43, 24.18, 21.79, 18.77, 16.72. MS (ESI) m/z 476.88 ([M − H]−).
N’-(3-Fluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4r): White solid; yield 82%, m.p. 166.0–167.3 °C. IR (KBr, cm−1): 3414, 3107, 2954, 1732 (C=O), 1697 (C=O), 1610, 1579, 1450, 1411, 1236, 1124, 943, 866, 785, 684. 1H-NMR (600 MHz, CDCl3) δ 10.75 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.39 (d, J = 9.2 Hz, 1H, H-5’), 7.35 (d, J = 5.3 Hz, 1H, H-2’), 7.34 (s, 1H, H-4’), 7.21 (d, J = 8.2 Hz, 1H, H-11), 7.10 (t, J = 7.7 Hz, 1H, H-5’), 7.02 (d, J = 7.9 Hz, 1H, H-12), 6.88 (s, 1H, H-14), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.07 (m, 1H, H-15), 2.78–2.93 (m, 2H, H-7), 2.40 (dd, J = 12.2, 1.2 Hz, 1H, He-1), 2.36 (d, J = 12.9 Hz, 1H, H-5), 1.51–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.22 (d, J = 6.8 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.31 (C=O), 163.96, 147.04, 145.88, 144.17, 135.92, 134.94, 130.58, 127.10, 124.35, 124.06, 123.67, 117.45, 113.20, 61.46, 47.85, 44.87, 38.11, 37.10, 36.96, 33.61, 30.29, 25.43, 24.13, 21.81, 18.78, 16.80. MS (ESI) m/z 476.89 ([M − H]−).
N’-(4-Fluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4s): White solid; yield 83%; m.p. 169.2–170.5 °C. IR (KBr, cm−1): 3224 (N–H), 3070, 2866, 1726 (C=O), 1687 (C=O), 1598, 1550, 1417, 1238, 1172, 1128, 1080, 945, 837, 723. 1H-NMR (600 MHz, CDCl3) δ 10.70 (s, 1H, CONH), 7.80 (s, 1H, N=CH), 7.61 (t, J = 8.3 Hz, 2H, H-2’ and H-6’), 7.21 (d, J = 8.1 Hz, 1H, H-11), 7.07 (t, 2H, J = 8.2 Hz, H-3’ and H-5’), 7.03 (d, J = 8.2 Hz, 1H, H-12), 6.87 (s, 1H, H-14), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.95–3.06 (m, 1H, H-15), 2.78–2.90 (m, 2H, H-7), 2.40 (d, J = 12.3 Hz, 1H, He-1), 2.35 (d, J = 12.9 Hz, 1H, H-5), 1.49–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.51 (C=O), 170.13 (C=O), 164.94, 147.04, 145.89, 144.37, 134.94, 129.89, 129.24, 127.10, 124.35, 124.07, 116.21, 61.50, 47.84, 44.90, 38.12, 37.11, 36.96, 33.61, 30.30, 25.44, 24.13, 21.81, 18.79, 16.80. MS (ESI) m/z 476.84 ([M − H]−).
N’-(2,3-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4t): White solid; yield 62%; m.p. 150.2–153.5 °C. IR (KBr, cm−1): 3097, 2966, 2868, 1732 (C=O), 1697 (C=O), 1612, 1487, 1415, 1305, 1238, 1170, 1124, 989, 786. 1H-NMR (600 MHz, CDCl3) δ 10.00 (s, 1H, CONH), 8.02 (s, 1H, H-23), 7.63 (dd, J = 7.5, 6.2 Hz, 1H, H-6’), 7.22 (dd, J = 20.1, 8.8 Hz, 2H, H-4’ and H-5’), 7.13 (dd, J = 12.4, 7.9 Hz, 1H, H-11), 7.02 (dd, J = 8.1, 1.5 Hz, 1H, H-12), 6.90 (s, 1H, H-14), 5.15 (q, J = 16.0 Hz, 2H, H-21), 2.96–3.04 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.39 (dd, J = 12.4, 1.9 Hz, 1H, He-1), 2.34 (d, J = 12.9 Hz, 1H, H-5), 1.53–1.95 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.24 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.36 (C=O), 169.52 (C=O), 151.49, 150.36, 148.76, 146.95, 145.67, 136.87, 134.91, 126.95, 124.35, 124.15, 123.83, 121.39, 118.73, 61.23, 47.73, 44.68, 37.97, 36.96, 36.80, 33.46, 30.05, 25.23, 23.97, 21.64, 18.59, 16.57. MS (ESI) m/z 495.1 ([M − H]−).
N’-(2,4-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4u): White solid; yield 77%; m.p. 181.9–184.5 °C. IR (KBr, cm−1): 3072, 2949, 2870, 1737 (C=O), 1691 (C=O), 1608, 1494, 1419, 1298, 1274, 1230, 1170, 1126, 962, 854, 794. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H, CONH), 7.97 (s, 1H, H-23), 7.89 (dd, J = 14.9, 8.4 Hz, 1H and H-6’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.0 Hz, 1H, H-12), 6.95 (t, J = 9.2 Hz, 1H, C-14), 6.92–6.83 (m, 2H, H-3’and H-5’), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.04 (m, 1H, H-15), 2.78–2.93 (m, 2H, C-7), 2.39 (dd, J = 12.3, 1.7 Hz, 1H, He-1), 2.34 (d, J = 13.0 Hz, 1H, H-5), 2.00–1.53 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.24 (d, J = 6.9 Hz, 6H, H-16 and H-17), 13C-NMR (151 MHz, CDCl3) δ 178.34 (C=O), 169.28 (C=O), 162.43, 160.74, 154.86, 146.95, 145.67, 137.02, 134.94, 126.95, 124.15, 123.83, 117.72, 112.44, 104.29, 61.26, 47.72, 44.68, 37.97, 36.96, 36.78, 33.46, 30.06, 25.24, 23.99, 21.63, 18.60, 16.57. MS (ESI) m/z 495.2 ([M − H]−).
N’-(2,6-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4v): Brown solid;yield 93%; m.p. 107.3–110.0 °C. IR (KBr, cm−1): 3101, 2954, 2868, 1728, 1693, 1620, 1467, 1415, 1240, 1170, 1126, 1012, 881, 785. 1H-NMR (600 MHz, CDCl3) δ 9.80 (s, 1H, CONH), 7.97 (s, 1H, H-23), 7.89 (dd, J = 14.9, 8.4 Hz, 1H, H-4’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.0 Hz, 1H, H-12), 6.95 (t, J = 9.2 Hz, 1H, H-14), 6.88 (q, J = 8.5 Hz, 2H, H-3’ and H-5’), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.94–3.04 (m, 1H, H-15), 2.78–2.94 (m, 2H, H-7), 2.39 (dd, J = 12.3, 1.7 Hz, 1H, He-1), 2.34 (d, J = 13.0 Hz, 1H, H-5), 1.54–2.00 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (d, J = 7.1 Hz, 3H, H-20), 1.24 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.29 (C=O), 170.00 (C=O), 162.04, 160.34, 153.60, 147.00, 145.62, 134.94, 131.20, 126.96, 124.14, 123.78, 117.72, 112.44, 104.29, 61.27, 47.68, 44.67, 37.98, 36.96, 36.78, 33.46, 30.06, 25.25, 23.97, 21.59, 18.61, 16.57. MS (ESI) m/z 495.2 ([M − H]−).
N’-(3,5-Difluorobenzylidene)-2-(dehydroabietyloxy)acetohydrazide (4w): White solid; yield 72%; m.p. 182.6–186.6 °C. IR (KBr,cm−1): 3091, 2949, 2885, 1724 (C=O), 1703 (C=O), 1612, 1583, 1436, 1415, 1363, 1298, 1240, 1174, 1122, 983, 850, 752.1H-NMR (600 MHz, CDCl3) δ 10.02 (s, 1H, CONH), 7.71 (s, 1H, H-23), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.16 (d, J = 5.8 Hz, 2H, H-2’ and H-6’), 7.02 (d, J = 8.0 Hz, 1H, H-12), 6.89 (s, 1H, H-14), 6.87 (t, J = 8.6 Hz, 1H, H-4’), 5.14 (q, J = 16.0 Hz, 2H, H-21), 2.96 –3.05 (m, 1H, H-15), 2.80–2.93 (m, 2H, H-7), 2.39 (d, J = 12.6, Hz, 1H, He-1), 2.36 (d, J = 13.0 Hz, 1H, H-5),1.53–1.97 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.38 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.31 (C=O), 169.65 (C=O), 164.00, 162.43, 162.35, 146.88, 145.74, 142.28, 136.58, 134.79, 126.94, 124.15, 123.89, 109.95, 105.67, 61.22, 47.72, 44.73, 37.97, 36.96, 36.77, 33.45, 30.08, 25.24, 23.97, 21.64, 18.60, 16.62. MS (ESI) m/z 495.1 ([M − H]−).
N’-((Pyridine-3-yl)methylene)-2-(dehydroabietyloxy)acetohydrazide (4x): White solid; yield 85%; m.p. 191.9–193.8 °C. IR (KBr, cm−1): 3084, 2956, 2866, 1723 (C=O), 1710 (C=O), 1614, 1462, 1402, 1288, 1240, 1170, 1126, 887, 704. 1H-NMR (600 MHz, CDCl3) δ 10.34 (s, 1H, CONH), 8.80 (s, 1H, H-2’), 8.65 (d, J = 4.1 Hz, 1H, H-4’), 8.00 (d, J = 7.9 Hz, 1H, H-6’), 7.83 (s, 1H, H-23), 7.35 (dd, J = 7.8, 4.9 Hz, 1H, H-5’), 7.20 (d, J = 8.2 Hz, 1H, H-11), 7.02 (d, J = 8.1 Hz, 1H, H-12), 6.88 (s, 1H, H-14), 5.16 (q, J = 16.0 Hz, 2H, H-21), 2.97–3.04 (m, 1H, H-15), 2.80–2.92 (m, 2H, H-7), 2.39 (d, J = 11.2 Hz, 1H, He-1), 2.35 (d, J = 12.8 Hz, 1H, H-5), 1.53–1.94 (m, 7H, Ha-1, H-2, H-3 and H-6), 1.39 (s, 3H, H-19), 1.27 (s, 3H, H-20), 1.23 (d, J = 6.9 Hz, 6H, H-16 and H-17). 13C-NMR (151 MHz, CDCl3) δ 178.34 (C=O), 169.75 (C=O), 151.13, 149.01, 146.89, 145.73, 141.81, 134.78, 133.50, 129.41, 126.94, 124.16, 123.89, 123.77, 61.26, 47.72, 44.73, 37.97, 36.96, 36.80, 33.46, 30.09, 25.24, 23.99, 21.66, 18.62, 16.63. MS (ESI) m/z 460.2 ([M − H]−).