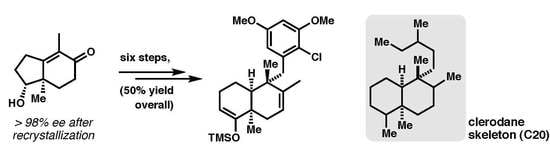
General Methodologies Toward cis-Fused Quinone Sesquiterpenoids. Enantiospecific Synthesis of the epi-Ilimaquinone Core Featuring Sc-Catalyzed Ring Expansion
Abstract
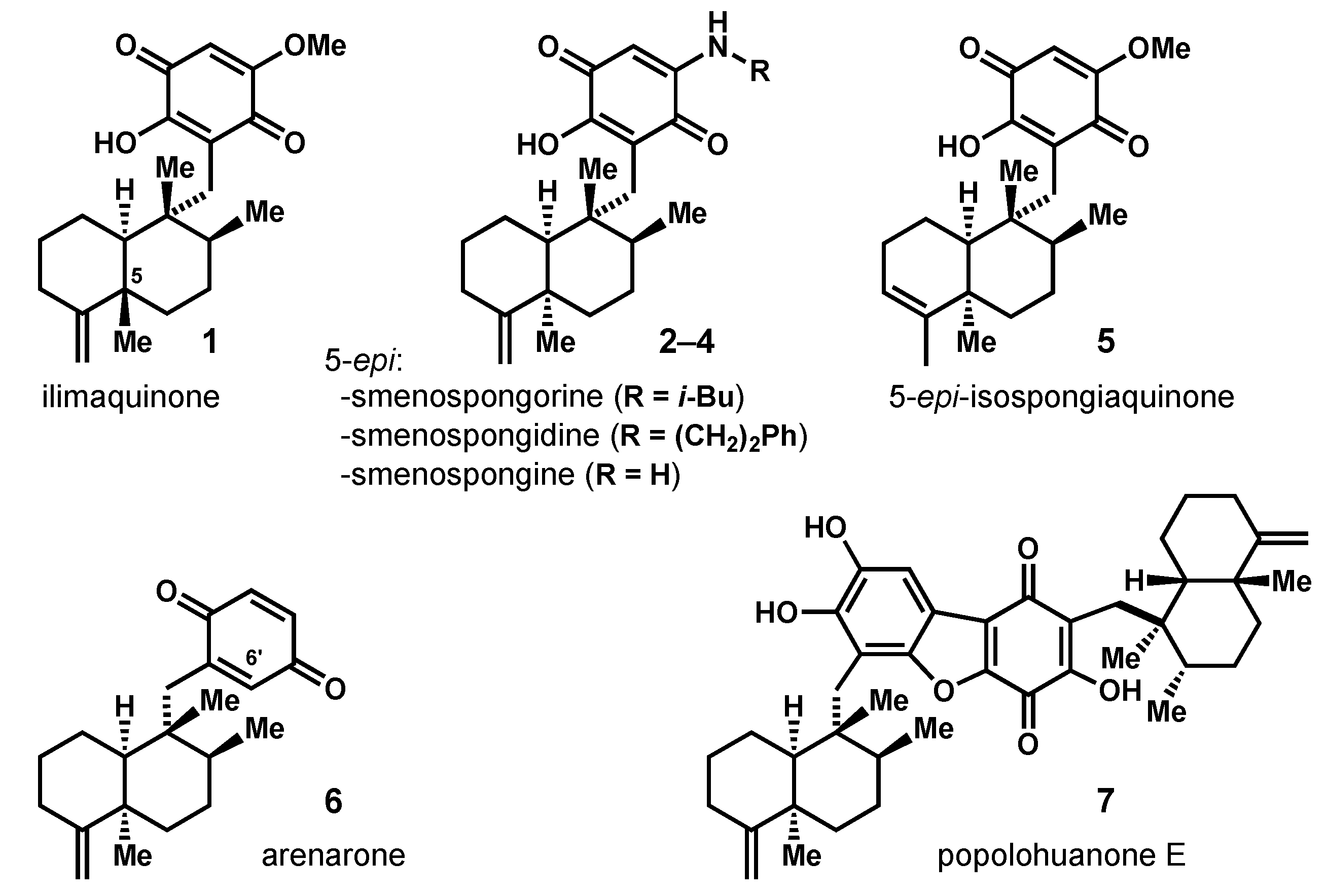
:1. Introduction
2. Results and Discussion
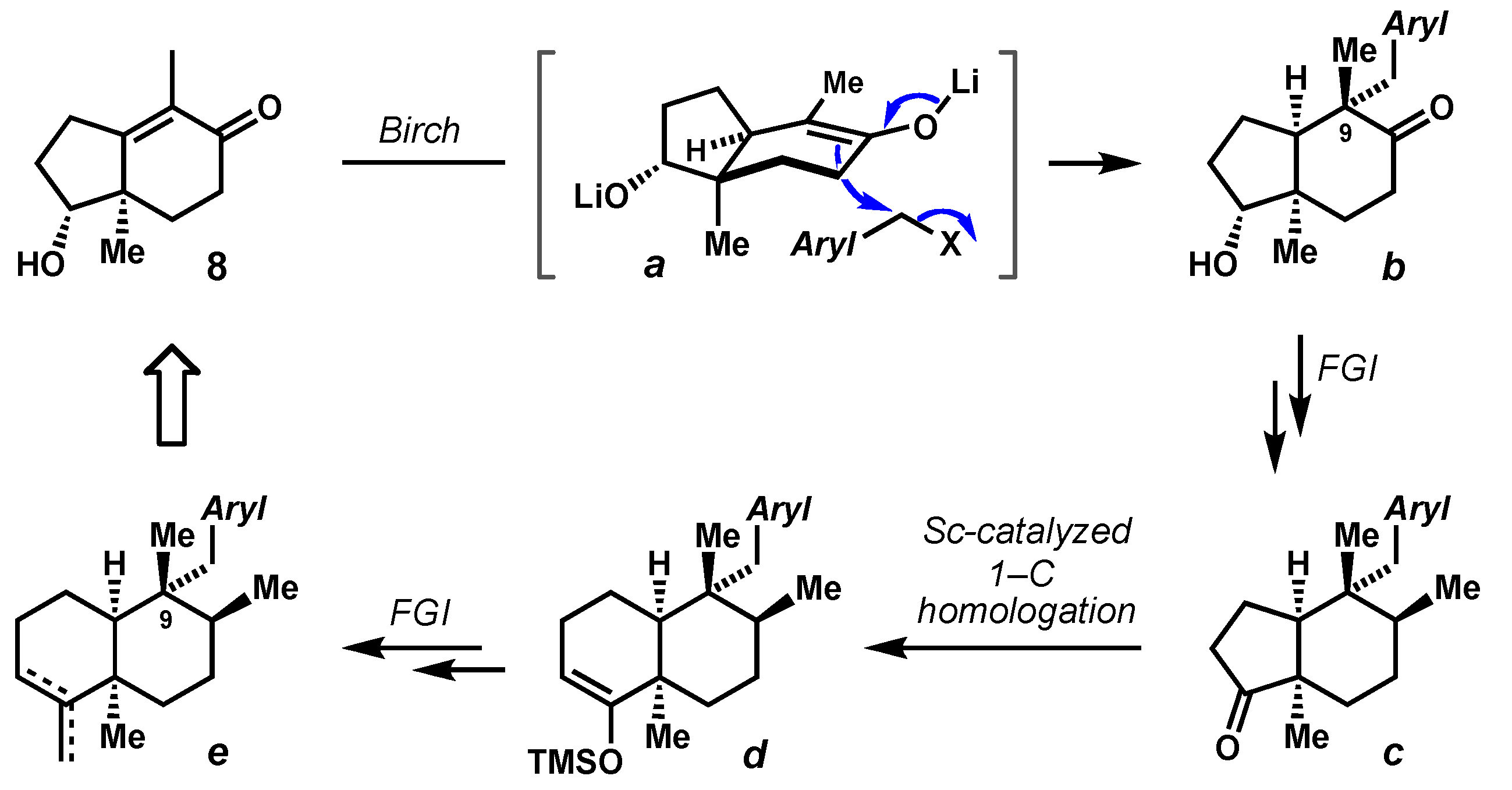
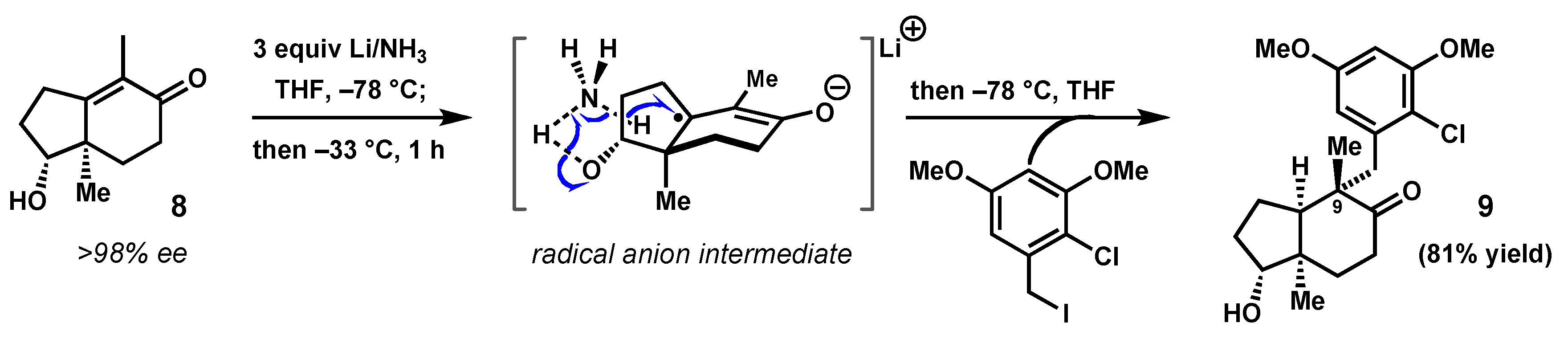
2.1. Diastereochemical Control at the C9 Quaternary Carbon by Birch Alkylation
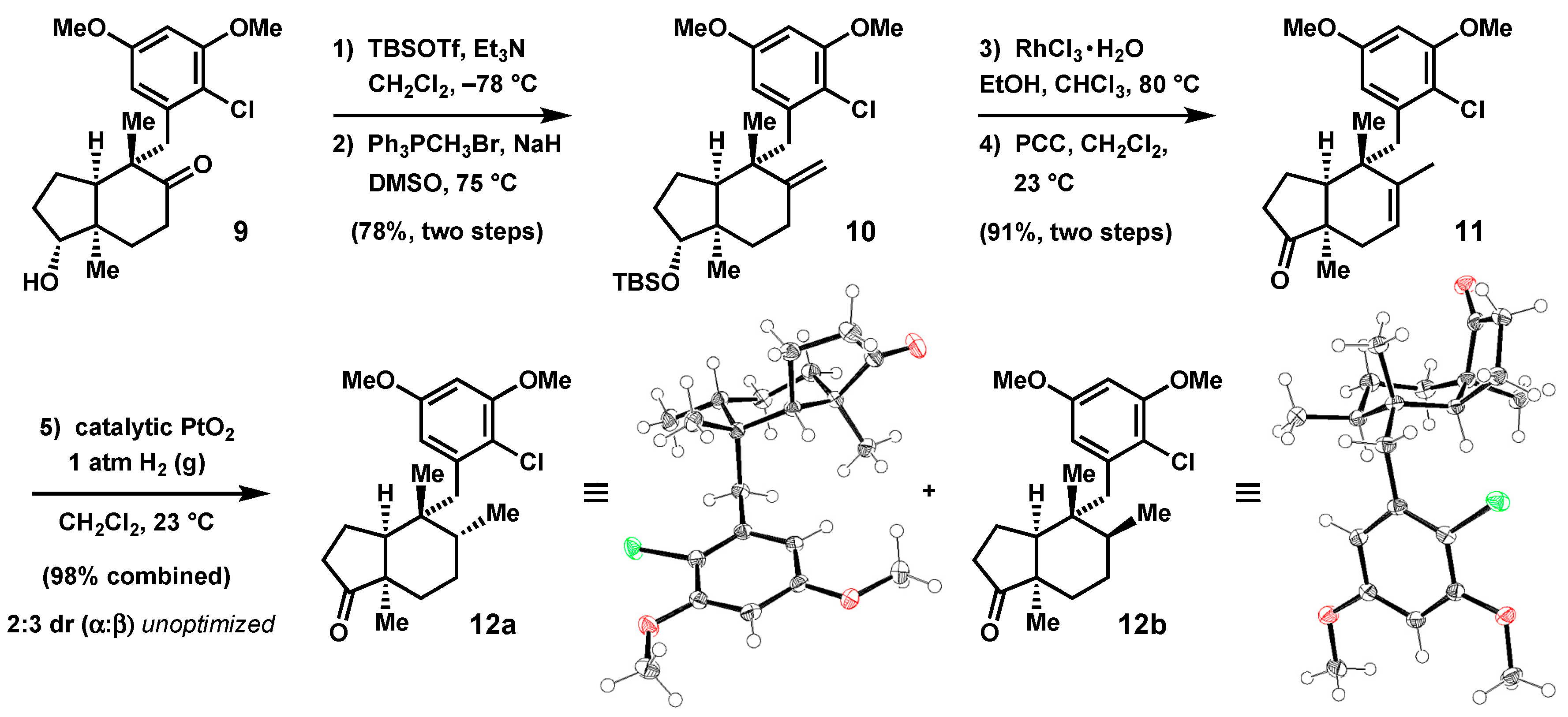
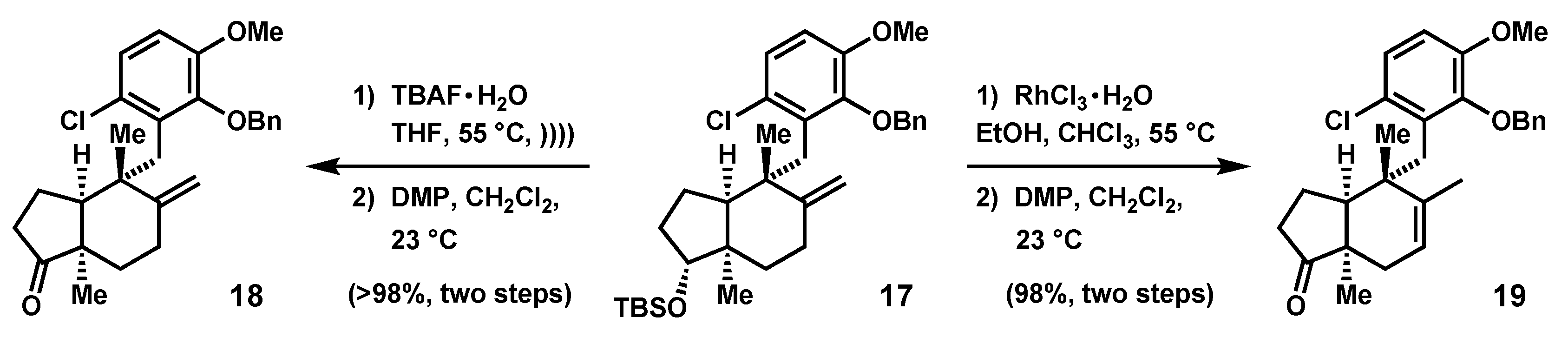
2.2. Further Elaboration of the Stereotriad Gives a Lower Homologue of the epi-Ilimaquinone Core
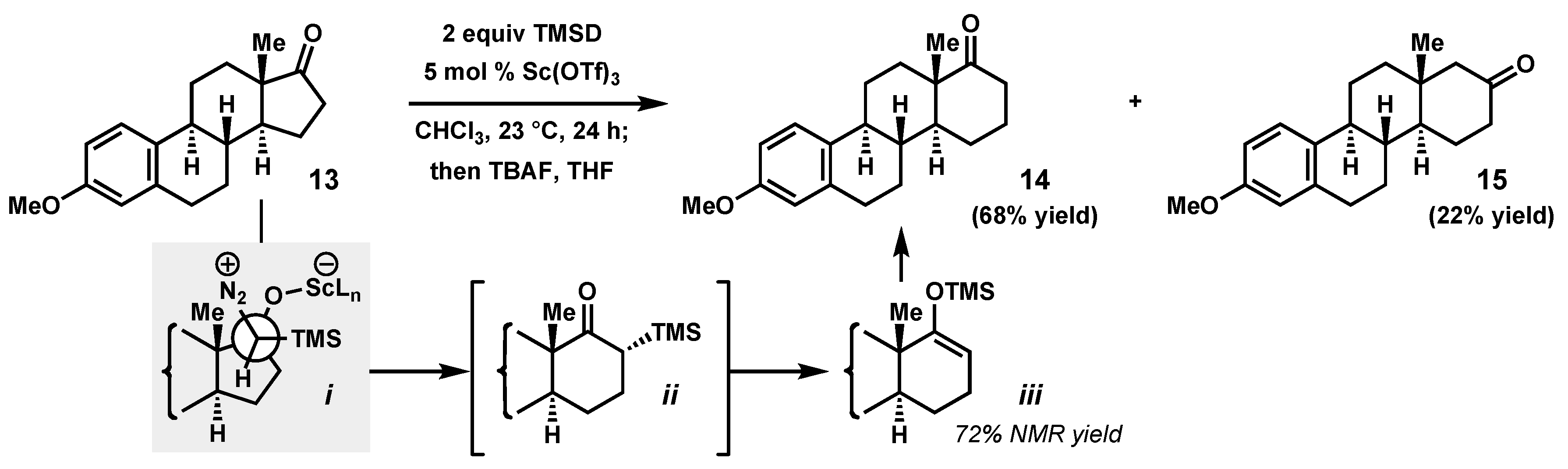
2.3. A Steroidal Model System for Catalysis of α-Quaternary Cyclopentanone Ring Enlargements
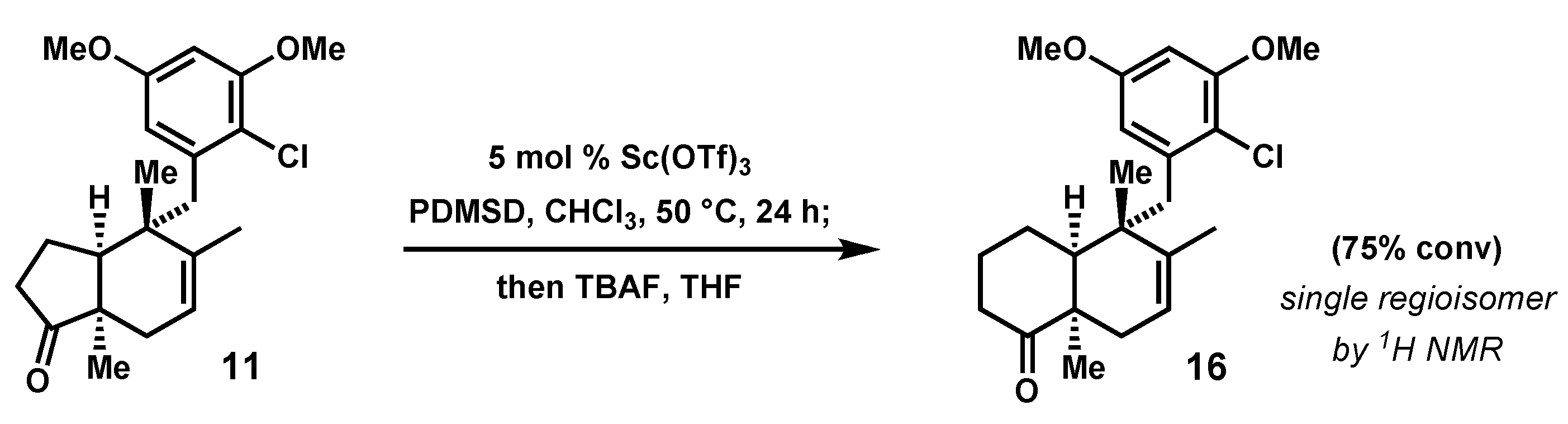
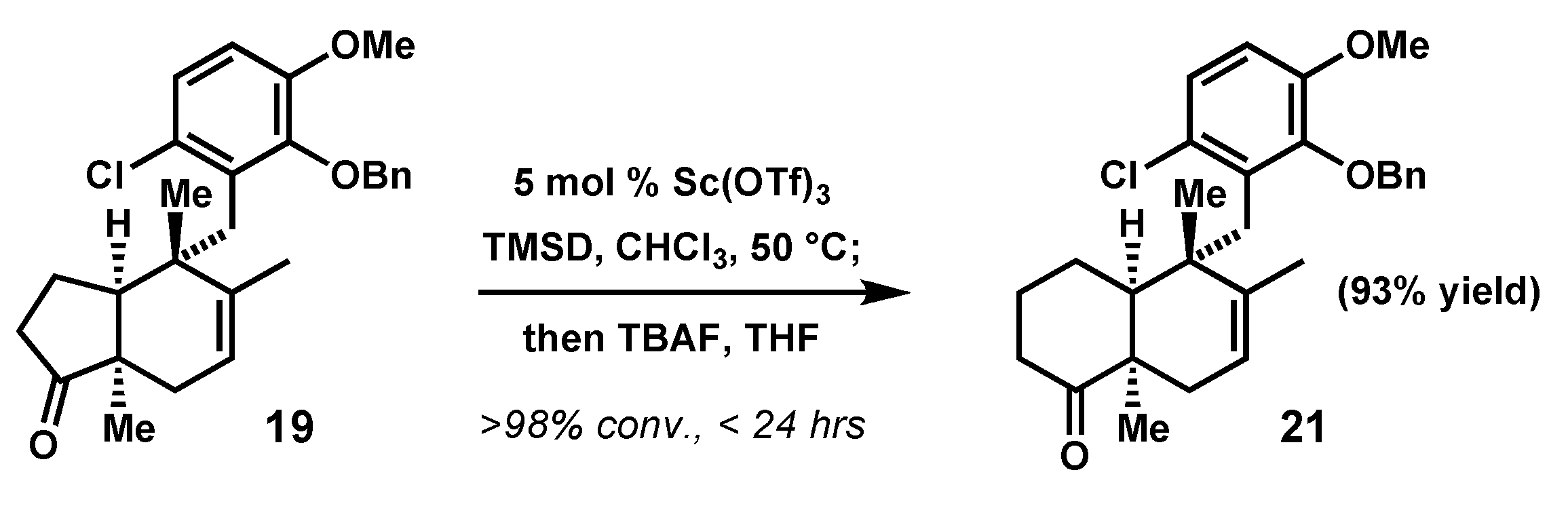
2.4. Advancement of Synthetic Bicyclopentanones to the epi-Ilimaquinone Core by Ring Expansion
3. Materials and Methods
3.1. General Remarks
3.2. Representative Procedure for Regioselective Sc-Catalyzed Ring Expansion with Estrone Model
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Capon, R.J. Marine bioprospecting−Trawling for treasure and pleasure. Eur. J. Org. Chem. 2001, 633–645. [Google Scholar] [CrossRef]
- Luibrand, R.T.; Erdman, T.R.; Vollmer, J.J.; Scheuer, P.J.; Finer, J.; Clardy, J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron 1979, 35, 609–612. [Google Scholar] [CrossRef]
- Kushlan, D.M.; Faulkner, D.J.; Parkanyi, L.; Clardy, J. Metabolites of the Palauan sponge Dactylospongia sp. Tetrahedron 1989, 45, 3307–3312. [Google Scholar] [CrossRef]
- Loya, S.; Tal, R.; Kashman, Y.; Hizi, A. Ilimaquinone, a selective inhibitor of the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1990, 34, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, M.P.; Wu, H.C. Ilimaquinone inhibits the cytotoxicities of ricin, diphtheria toxin, and other protein toxins in Vero cells. Exp. Cell Res. 1995, 219, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Talizawa, P.A.; Yucel, J.K.; Veit, B.; Faulkner, D.J.; Deerinck, T.; Soto, G.; Ellisman, M.; Malhotra, V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 1993, 73, 1079–1090. [Google Scholar]
- Bruner, S.D.; Radeke, H.S.; Tallarico, J.A.; Snapper, M.L. Total synthesis of (−)-ilimaquinone. J. Org. Chem. 1995, 60, 1114–1115. [Google Scholar] [CrossRef]
- Radeke, H.S.; Digits, C.A.; Bruner, S.D.; Snapper, M.L. New tools for studying vesicular-mediated protein trafficking: Synthesis and evaluation of ilimaquinone analogs in a non-radioisotope-based anti-secretory assay. J. Org. Chem. 1997, 62, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Poigny, S.; Guyot, M.; Samadi, M. Efficient total synthesis of (−)-ilimaquinone. J. Org. Chem. 1998, 63, 5890–5894. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Poupon, E.; Rueden, E.J.; Theodorakis, E.A. Synthesis of (−)-ilimaquinone via a radical decarboxylation and quinone addition reaction. Org. Lett. 2002, 4, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Radeke, H.S.; Digits, C.A.; Casaubon, R.L.; Snapper, M.L. Interactions of (−)-ilimaquinone with methylation enzymes: Implications for vesicular-mediated secretion. Chem. Biol. 1999, 6, 639–647. [Google Scholar] [CrossRef]
- Marcos, I.S.; Conde, A.; Moro, R.F.; Basabe, P.; Diez, D.; Urones, J.G. Quinone/hydroquinone sesquiterpenes. Mini-Rev. Org. Chem. 2010, 7, 230–254. [Google Scholar] [CrossRef]
- Theodorakis, E.A.; Ling, T.; Rueden, E.J.; Poupon, E.; Kim, S.H. Quinone sesquiterpenes: A challenge for the development of a new synthetic methodology. Strateg. Tact. Org. Synth. 2004, 5, 111–131. [Google Scholar] [CrossRef]
- Oda, T.; Wang, W.; Ukai, K.; Nakazawa, T.; Mochizuki, M. A sesquiterpene quinone, 5-epi-smenospongine, promotes TNF-α production in LPS-stimulated RAW 264.7 cells. Mar. Drugs 2007, 5, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.R.; Scheuer, P.J. Popolohuanone E, a topoisomerase II inhibitor with selective lung cytotoxicity from Pohnpei sponge Dysidea sp. Tetrahedron Lett. 1993, 34, 3727–3730. [Google Scholar] [CrossRef]
- Watson, A.T.; Park, K.; Wiemer, D.F. Application of the nickel-mediated neopentyl coupling in the total synthesis of the marine natural product arenarol. J. Org. Chem. 1995, 60, 5102–5106. [Google Scholar] [CrossRef]
- Kawano, H.; Itoh, M.; Katoh, T.; Terashima, S. Studies toward the synthesis of popolohuanone E: Synthesis of natural (+)-arenarol related to the proposed biogenetic precursor of popolohuanone E. Tetrahedron Lett. 1997, 38, 7769–7772. [Google Scholar] [CrossRef]
- Munday, R.H.; Denton, R.M.; Anderson, J.C. Asymmetric synthesis of 6′-hydroxyarenarol: The proposed biosynthetic precursor to popolohuanone E. J. Org. Chem. 2008, 73, 8033–8038. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Denton, R.M.; Wilson, C. A biomimetic strategy for the synthesis of the tricyclic dibenzofuran-1,4-dione core of popolohuanone E. Org. Lett. 2005, 7, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Rodgen, S.A.; Schaus, S.E. Efficient construction of the clerodane decalin core by an asymmetric Morita–Baylis–Hilman reaction/Lewis acid promoted annulation strategy. Angew. Chem. Int. Ed. 2006, 45, 4929–4932. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.S.; Chattopadhyay, P. Synthetic studies of trans-clerodane diterpenoids and congeners: Stereocontrolled total synthesis of (±)-avarol. J. Org. Chem. 1982, 47, 1727–1731. [Google Scholar] [CrossRef]
- An, J.; Wiemer, D.F. Stereoselective synthesis of (+)-avarol, (+)-avarone, and some nonracemic analogues. J. Org. Chem. 1996, 61, 8775–8779. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.; Kissau, L.; Mazitschek, R.; Huwe, A.; Furet, P.; Giannis, A.; Waldmann, H. Total synthesis and biological evaluation of the nakijiquinones. J. Am. Chem. Soc. 2001, 123, 11586–11593. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Poupon, E.; Rueden, E.J.; Kim, S.H.; Theodorakis, E.A. Unified synthesis of quinone sesquiterpenes based on a radical decarboxylation and quinone addition reaction. J. Am. Chem. Soc. 2002, 124, 12261–12267. [Google Scholar] [CrossRef] [PubMed]
- Slutskyy, Y.; Jamison, C.R.; Lackner, G.L.; Müller, D.S.; Dieskau, A.P.; Untiedt, N.L.; Overman, L.E. Short enantioselective total syntheses of trans-clerodane diterpenoids: Convergent fragment coupling using a trans-decalin tertiary radical generated from a tertiary alcohol precursor. J. Org. Chem. 2016, 81, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Aguilar, A.; Zou, B.; Bao, W.; Koldas, S.; Shi, A.; Desper, J.; Wangemann, P.; Xie, X.S.; Hua, D.H. Chemical synthesis of tetracyclic terpenes and evaluation of antagonistic activity on endothelin-A receptors and voltage-gated calcium channels. Bioorg. Med. Chem. 2015, 23, 5985–5998. [Google Scholar] [CrossRef] [PubMed]
- An exception to this is an exo-Diels–Alder approach to the rearranged drimane core of mamanuthaquinone; see: Yoon, T.; Danishefsky, S.J.; de Gala, S. A concise total synthesis of (±)-mamanuthaquinone by using an exo-Diels–Alder reaction. Angew. Chem. Int. Ed. 1994, 33, 853–855. [Google Scholar] [CrossRef]
- Paquette, L.A.; Wang, T.-Z.; Sivik, M.R. Total synthesis of (−)-austalide B. A generic solution to elaboration of the pyran/p-cresol/butenolide triad. J. Am. Chem. Soc. 1994, 116, 11323–11334. [Google Scholar] [CrossRef]
- Renoud-Grappin, M.; Vanucci, C.; Lhommet, G. Diastereoselective synthesis of a limonoid model related to the insect antifeedant genudin. J. Org. Chem. 1994, 59, 3902–3905. [Google Scholar] [CrossRef]
- Stork, G.; Darling, S.D. The stereochemistry of the lithium–ammonia reduction of α,β-unsaturated ketones. J. Am. Chem. Soc. 1964, 86, 1761–1768. [Google Scholar] [CrossRef]
- Dabrowski, J.A.; Moebius, D.C.; Wommack, A.J.; Kornahrens, A.F.; Kingsbury, J.S. Catalytic and regio-selective ring expansion of arylcyclobutanones with trimethylsilyldiazomethane. Ligand-dependent entry to α-ketosilane or enolsilane adducts. Org. Lett. 2010, 12, 3598–3601. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Virgil, S.C. Enantioselective synthesis of a protosterol, 3β,20-dihydroxyprotost-24-ene. J. Am. Chem. Soc. 1990, 112, 6429–6431. [Google Scholar] [CrossRef]
- Shigehisa, H.; Mizutani, T.; Tosaki, S.-Y.; Ohshima, T.; Shibasaki, M. Formal total synthesis of (+)-wortmannin using catalytic asymmetric intramolecular aldol condensation reaction. Tetrahedron 2005, 61, 5057–5065. [Google Scholar] [CrossRef]
- Kaplan, H.Z.; Rendina, V.L.; Kingsbury, J.S. Diastereoselective synthesis of complex cis-hexahydroindanes by reductive alkylation. J. Org. Chem. 2013, 78, 4620–4626. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.J. Reduction by dissolving metals. Part 1. J. Chem. Soc. 1944, 430–436. [Google Scholar] [CrossRef]
- Paquette, L.; Hofferberth, J.E. The α-hydroxy ketone (α-ketol) and related rearrangments. Org. React. 2003, 62, 477–567. [Google Scholar]
- Gutsche, C.D. The reaction of diazomethane and its derivatives with aldehydes and ketones. Org. React. 1954, 8, 364–403. [Google Scholar]
- Brook, A.G. Some molecular rearrangements of organosilicon compounds. Acc. Chem. Res. 1974, 7, 77–84. [Google Scholar] [CrossRef]
- Seto, H.; Fujioka, S.; Koshino, H.; Shimizu, T.; Yoshida, S.; Watanabe, T. Synthesis and biological activity of 6a-carbabrassinolide: B-ring homologation of 6-oxo-steroid to 6-oxo-7a-homosteroid with trimethylsilyl- diazomethane–boron trifluoride etherate. Tetrahedron Lett. 1999, 40, 2359–2362. [Google Scholar] [CrossRef]
- Rendina, V.L.; Moebius, D.C.; Kingsbury, J.S. An enantioselective synthesis of 2-aryl cycloalkanones by Sc-catalyzed carbon insertion. Org. Lett. 2011, 13, 2004–2007. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Nagayama, S.; Busujima, T. Lewis acid catalysts stable in water. Correlation between catalytic activity and in water and hydrolysis constants and exchange rate constants for substitution of inner-sphere water ligands. J. Am. Chem. Soc. 1998, 120, 8287–8288. [Google Scholar] [CrossRef]
- Shiori, T.; Aoyama, T.; Mori, S. Trimethylsilyldiazomethane. Org. Synth. 1990, 68, 1–4. [Google Scholar]
- Fleming, I.; Sanderson, P.E.J. A one-pot conversion of the phenyldimethylsilyl group into a hydroxyl group. Tetrahedron Lett. 1987, 28, 4229–4232. [Google Scholar] [CrossRef]
- Rendina, V.L. Development of Lewis-Acid Catalyzed Asymmetric Ring Expansion Reactions and Catalysis of Etherification Reactions with sp3 Electrophiles. Ph.D. Thesis, Boston College, Chestnut Hill, MA, USA, 2013. [Google Scholar]
- Moebius, D.C.; Rendina, V.L.; Kingsbury, J.S. Catalysis of diazoalkane–carbonyl homologation. How new developments in hydrazone oxidation enable a carbon insertion strategy for synthesis. Top. Curr. Chem. 2014, 346, 111–162. [Google Scholar] [PubMed]
- Reeder, L.M.; Hegedus, L.S. Effect of β-Substituents on the regioselectivity of the diazomethane ring expansion of α-methyl-α-methoxycyclobutanones to cyclopentanones. J. Org. Chem. 1999, 64, 3306–3311. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Wespe, D.A.; von Hof, J.M. A concise, stereocontrolled total synthesis of rippertenol. J. Am. Chem. Soc. 2011, 133, 8850–8853. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, K.; Concepcion, A.B.; Yamamoto, H. Selective homologation of ketones and aldehydes with diazoalkanes promoted by organoaluminum reagents. Synthesis 1994, 1283–1290. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds reported herein are available from the corresponding author. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, H.Z.; Rendina, V.L.; Kingsbury, J.S. General Methodologies Toward cis-Fused Quinone Sesquiterpenoids. Enantiospecific Synthesis of the epi-Ilimaquinone Core Featuring Sc-Catalyzed Ring Expansion. Molecules 2017, 22, 1041. https://doi.org/10.3390/molecules22071041
Kaplan HZ, Rendina VL, Kingsbury JS. General Methodologies Toward cis-Fused Quinone Sesquiterpenoids. Enantiospecific Synthesis of the epi-Ilimaquinone Core Featuring Sc-Catalyzed Ring Expansion. Molecules. 2017; 22(7):1041. https://doi.org/10.3390/molecules22071041
Chicago/Turabian StyleKaplan, Hilan Z., Victor L. Rendina, and Jason S. Kingsbury. 2017. "General Methodologies Toward cis-Fused Quinone Sesquiterpenoids. Enantiospecific Synthesis of the epi-Ilimaquinone Core Featuring Sc-Catalyzed Ring Expansion" Molecules 22, no. 7: 1041. https://doi.org/10.3390/molecules22071041