Exogenous Feeding of Fructose and Phenylalanine Further Improves Betulin Production in Suspended Betula platyphylla Cells under Nitric Oxide Treatment
Abstract
:1. Introduction
2. Results
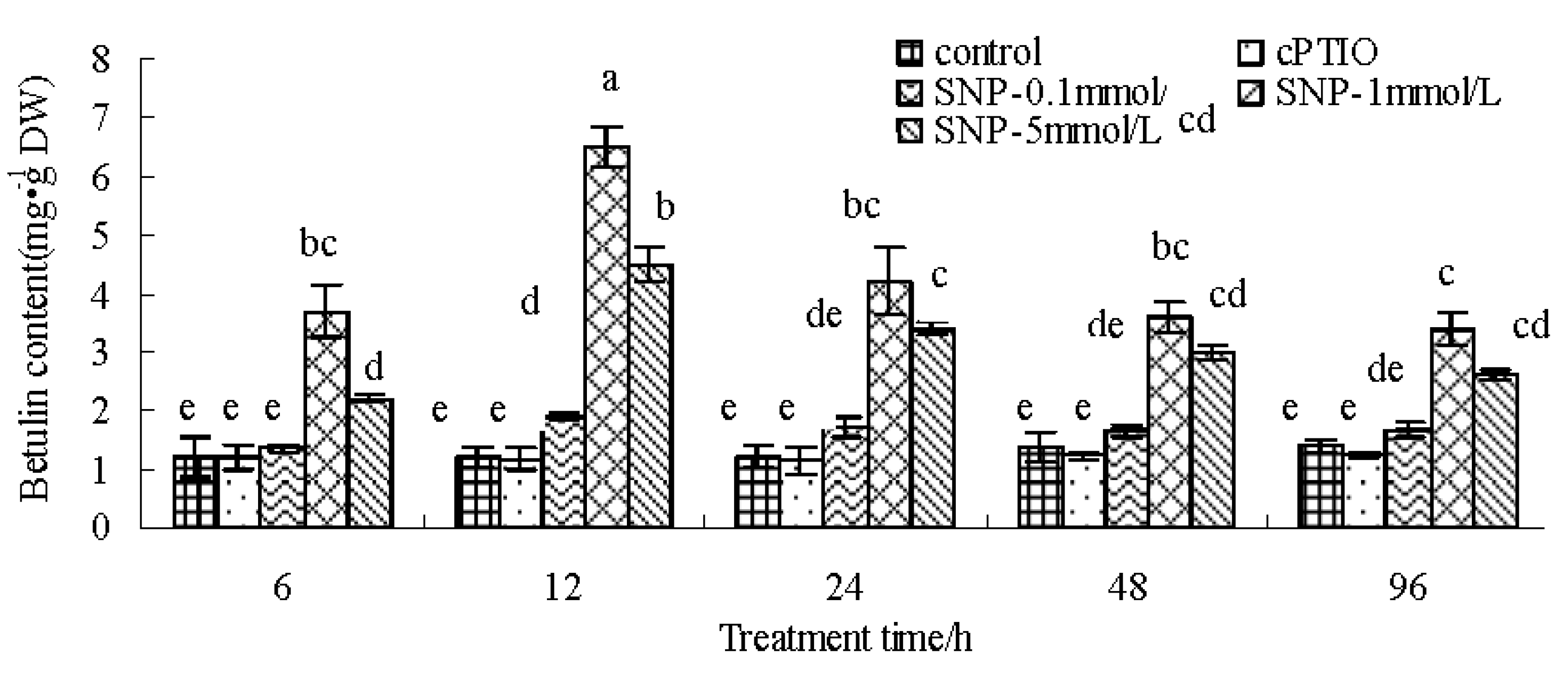
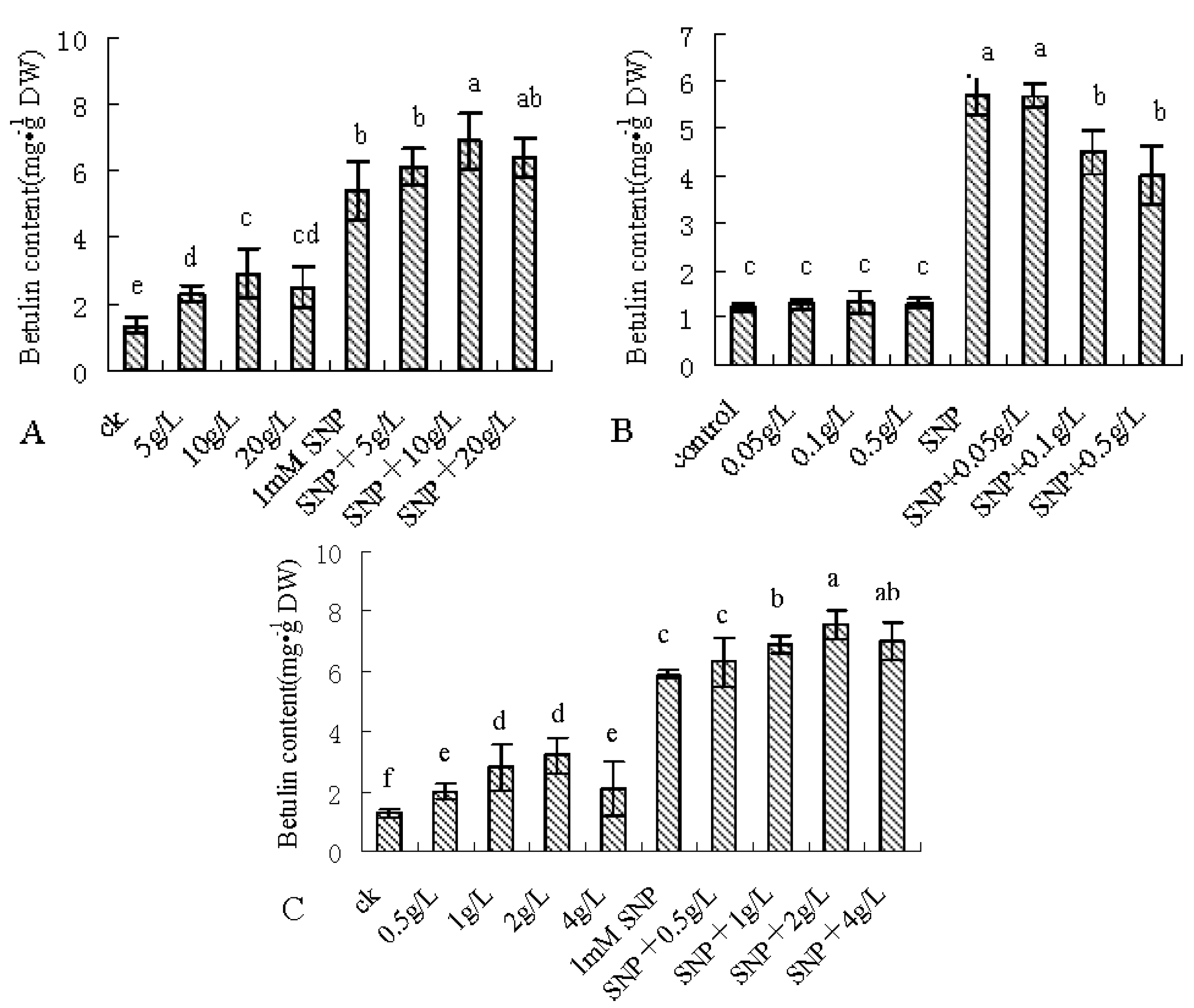
2.1. SNP Induces Betulin Accumulation
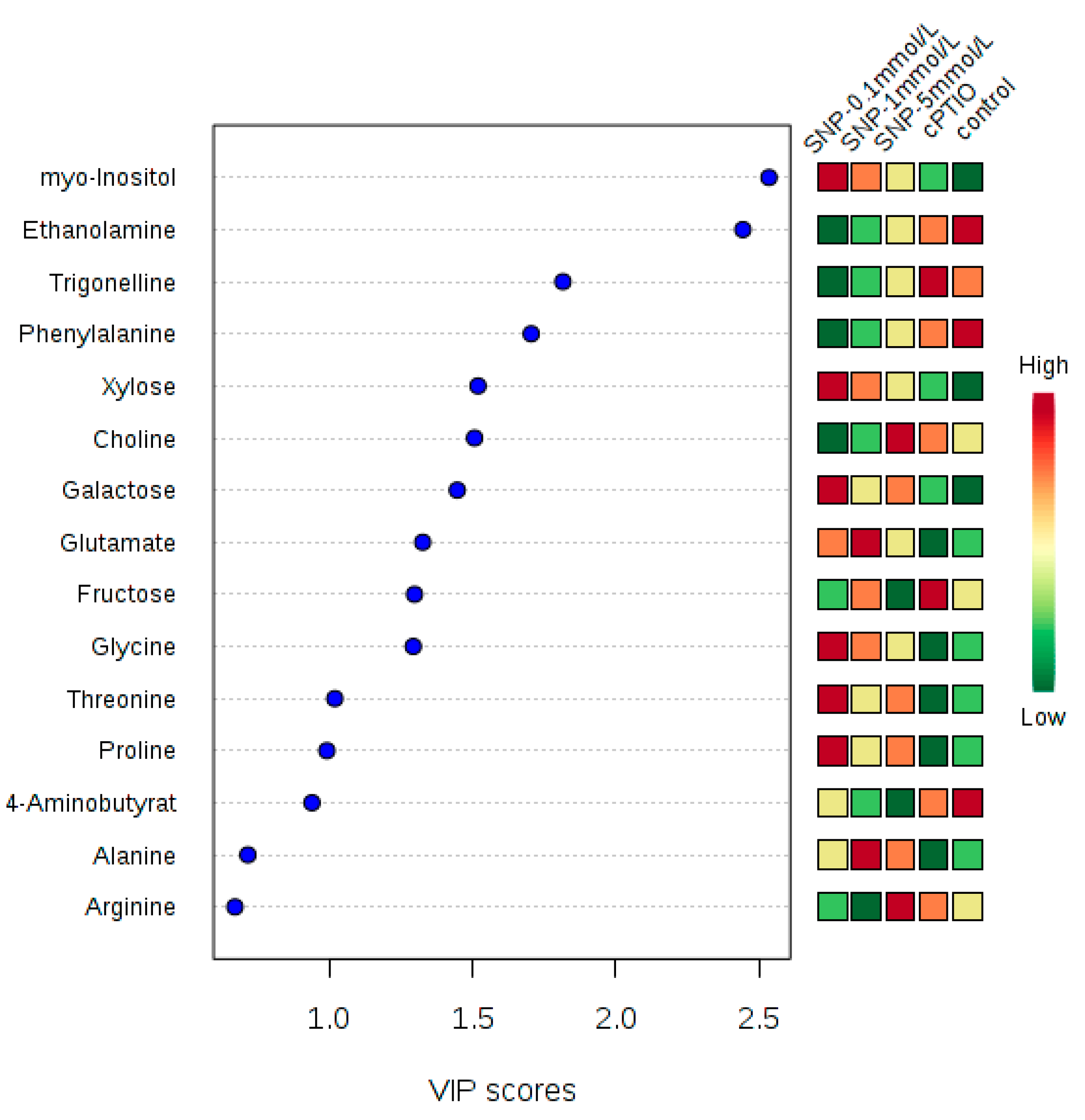
2.2. Identification and Analysis of Key Metabolites and Pathways under SNP Treatment
2.3. Enhancing the Betulin Production by Combinatorial Feeding Strategy
3. Discussion
4. Materials and Methods
4.1. Plant Cell Culture
4.2. Chemical Reagents and Treatment
4.3. Betulin Estimation
4.4. 1H-NMR Measurements
4.5. Data Processing and Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
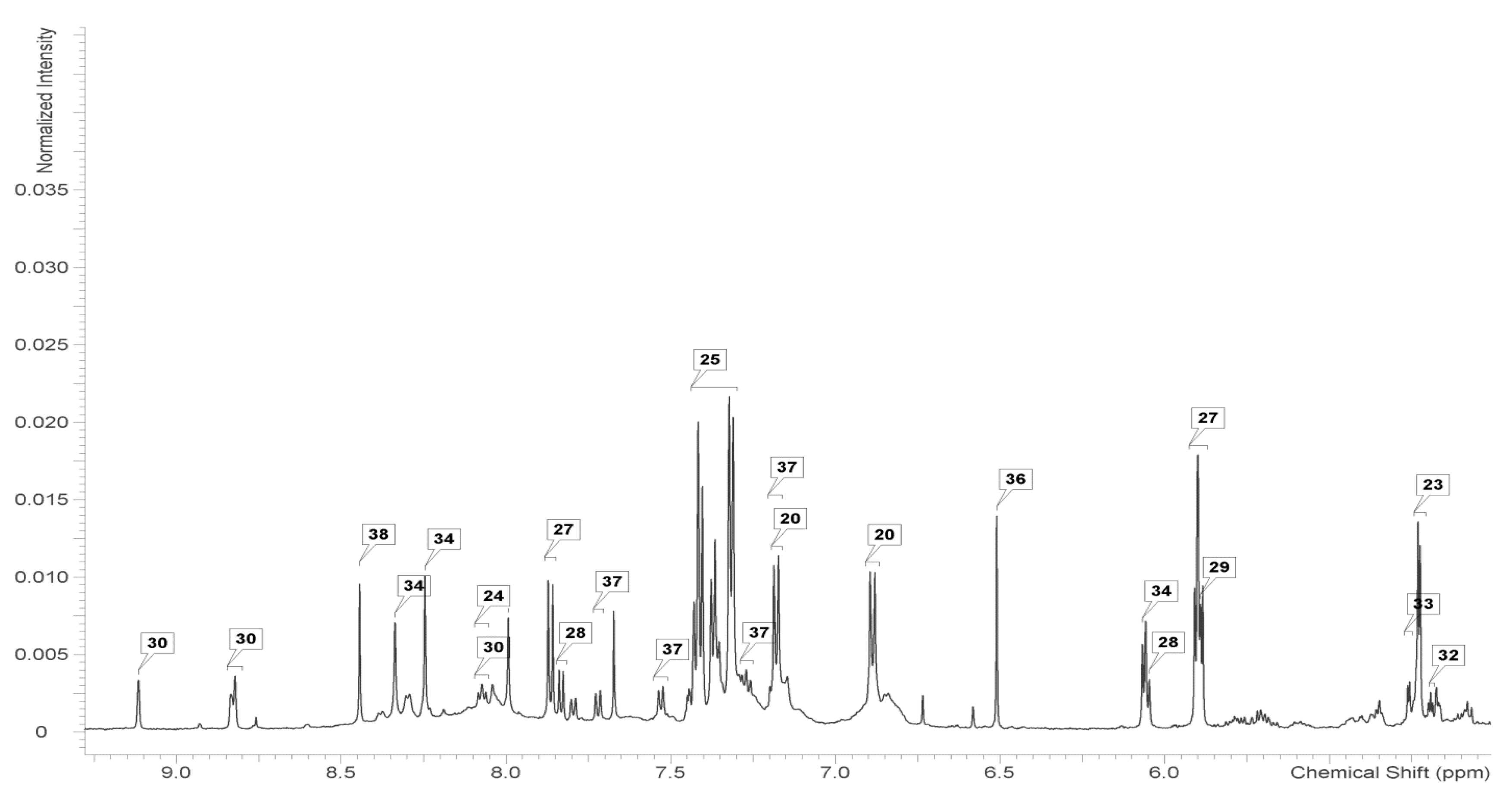
| Metabolite | Control | cPTIO | SNP 0.1 mmol/L | SNP 1 mmol/L | SNP 5 mmol/L |
|---|---|---|---|---|---|
| Sugars | |||||
| Fructose | 3.23 ± 0.56a | 4.14 ± 0.54a | 3.28 ± 0.77a | 3.90 ± 0.61a | 2.76 ± 0.45a |
| Fucose | 0.10 ± 0.01a | 0.11 ± 0.04a | 0.09 ± 0.01a | 0.08 ± 0.01a | 0.09 ± 0.02a |
| Galactose | 0.24 ± 0.07a | 0.24 ± 0.06a | 0.41 ± 0.10ab | 0.35 ± 0.11b | 0.32 ± 0.06ab |
| Glucose | 1.50 ± 0.11b | 1.89 ± 0.38b | 1.80 ± 0.24b | 1.87 ± 0.15b | 1.04 ± 0.12a |
| Xylose | 0.16 ± 0.01a | 0.19 ± 0.05a | 0.36 ± 0.07ab | 0.25 ± 0.04b | 0.18 ± 0.06a |
| Organic Acids | |||||
| Acetate | 0.05 ± 0.00a | 0.05 ± 0.01a | 0.03 ± 0.02a | 0.05 ± 0.01a | 0.06 ± 0.01a |
| Citrate | 0.07 ± 0.01a | 0.07 ± 0.01a | 0.06 ± 0.02a | 0.08 ± 0.01a | 0.06 ± 0.02a |
| Formate | 0.21 ± 0.03abc | 0.21 ± 0.0ab | 0.15 ± 0.04c | 0.29 ± 0.06a | 0.28 ± 0.05bc |
| Fumarate | 0.13 ± 0.04a | 0.13 ± 0.03a | 0.12 ± 0.03a | 0.16 ± 0.05a | 0.10 ± 0.01a |
| Malate | 5.62 ± 0.33a | 5.62 ± 0.54a | 6.42 ± 0.13a | 6.45 ± 0.71a | 4.95 ± 0.90a |
| Succinate | 0.34 ± 0.11a | 0.34 ± 0.03a | 0.37 ± 0.10a | 0.49 ± 0.09a | 0.32 ± 0.07a |
| Amino Acids and the Derivatives | |||||
| 4-Aminobutyrate | 0.17 ± 0.05c | 0.13 ± 0.01bc | 0.11 ± 0.02abc | 0.11 ± 0.02ab | 0.06 ± 0.01a |
| Alanine | 0.88 ± 0.07a | 0.78 ± 0.08a | 0.94 ± 0.10a | 1.13 ± 0.20a | 0.88 ± 0.09a |
| Arginine | 0.21 ± 0.04ab | 0.22 ± 0.03b | 0.21 ± 0.05a | 0.13 ± 0.01ab | 0.20 ± 0.05ab |
| Asparagine | 0.16 ± 0.04a | 0.15 ± 0.01a | 0.18 ± 0.05a | 0.21 ± 0.04a | 0.21 ± 0.03a |
| Aspartate | 0.18 ± 0.02a | 0.17 ± 0.02a | 0.24 ± 0.08a | 0.19 ± 0.06a | 0.24 ± 0.05a |
| Glutamate | 0.48 ± 0.07ab | 0.37 ± 0.08a | 0.61 ± 0.11b | 0.68 ± 0.10ab | 0.50 ± 0.09ab |
| Glutamine | 0.19 ± 0.05a | 0.17 ± 0.04a | 0.18 ± 0.06a | 0.17 ± 0.02a | 0.18 ± 0.03a |
| Glycine | 0.27 ± 0.06a | 0.20 ± 0.03a | 0.40 ± 0.12a | 0.38 ± 0.04a | 0.28 ± 0.09a |
| Isoleucine | 0.42 ± 0.07a | 0.35 ± 0.06a | 0.41 ± 0.09a | 0.43 ± 0.08a | 0.35 ± 0.08a |
| Leucine | 0.67 ± 0.09a | 0.53 ± 0.10a | 0.71 ± 0.13a | 0.75 ± 0.12a | 0.66 ± 0.12a |
| Lysine | 0.26 ± 0.09a | 0.20 ± 0.04a | 0.30 ± 0.05a | 0.25 ± 0.07a | 0.21 ± 0.05a |
| Methionine | 0.21 ± 0.05a | 0.20 ± 0.03a | 0.19 ± 0.03a | 0.20 ± 0.02a | 0.15 ± 0.01a |
| Phenylalanine | 0.69 ± 0.03a | 0.60 ± 0.12a | 0.52 ± 0.10a | 0.65 ± 0.07a | 0.52 ± 0.06a |
| Proline | 0.18 ± 0.02a | 0.14 ± 0.05a | 0.29 ± 0.03a | 0.23 ± 0.07a | 0.22 ± 0.06a |
| Serine | 0.35 ± 0.04a | 0.26 ± 0.02a | 0.36 ± 0.03a | 0.34 ± 0.06a | 0.29 ± 0.04a |
| Threonine | 0.33 ± 0.07a | 0.30 ± 0.04a | 0.44 ± 0.08a | 0.44 ± 0.01a | 0.36 ± 0.08a |
| Tryptophan | 0.11 ± 0.01a | 0.10 ± 0.01a | 0.09 ± 0.01a | 0.11 ± 0.03a | 0.10 ± 0.01a |
| Tyrosine | 0.19 ± 0.02a | 0.18 ± 0.03a | 0.23 ± 0.08a | 0.25 ± 0.06a | 0.21 ± 0.05a |
| Valine | 0.49 ± 0.04a | 0.43 ± 0.08a | 0.58 ± 0.02a | 0.58 ± 0.07a | 0.44 ± 0.06a |
| Alkaloids | |||||
| Choline | 0.54 ± 0.01ab | 0.61 ± 0.05b | 0.45 ± 0.08ab | 0.51 ± 0.02a | 0.54 ± 0.04ab |
| Trigonelline | 0.22 ± 0.02b | 0.26 ± 0.08b | 0.09 ± 0.02a | 0.13 ± 0.03a | 0.17 ± 0.02ab |
| Other Metabolites | |||||
| Ethanolamine | 0.85 ± 0.09bc | 0.88 ± 0.03c | 0.60 ± 0.08abc | 0.68 ± 0.04a | 0.64 ± 0.11ab |
| Uridine | 0.25 ± 0.04a | 0.30 ± 0.03a | 0.24 ± 0.01a | 0.30 ± 0.03a | 0.22 ± 0.02a |
| Adenosine | 0.13 ± 0.02a | 0.12 ± 0.02a | 0.10 ± 0.01a | 0.14 ± 0.03a | 0.13 ± 0.03a |
| Ethanol | 0.02 ± 0.00a | 0.03 ± 0.00a | 0.03 ± 0.00a | 0.02 ± 0.00a | 0.03 ± 0.00a |
| myo-Inositol | 0.66 ± 0.08a | 0.88 ± 0.14a | 1.12 ± 0.15b | 1.19 ± 0.06b | 0.84 ± 0.09a |
| Glycerol | 0.40 ± 0.05a | 0.34 ± 0.03a | 0.40 ± 0.01a | 0.37 ± 0.04a | 0.38 ± 0.08a |
| Cytidine | 0.12 ± 0.04a | 0.13 ± 0.02a | 0.10 ± 0.00a | 0.13 ± 0.01a | 0.13 ± 0.02a |
| Guanosine | 0.14 ± 0.02a | 0.12 ± 0.03a | 0.12 ± 0.01a | 0.16 ± 0.03a | 0.15 ± 0.02a |
| Methanol | 0.38 ± 0.01a | 0.26 ± 0.04a | 0.20 ± 0.03a | 0.31 ± 0.01a | 0.24 ± 0.04a |
| Metabolites | Control | cPTIO | SNP(1 mmol/L−1) |
|---|---|---|---|
| Myo−inositol | 0.015 | 0.992 | 0.443 |
| Ethanolamine | 0.249 | −0.347 | −0.697 |
| Trigonelline | −0.545 | −0.696 | −0.078 |
| Phenylalanine | 0.994 | 0.542 | −0.418 |
| Xylose | 0.998 | −0.907 | −0.681 |
| Choline | 0.719 | −0.243 | 0.217 |
| Galactose | 0.600 | −0.620 | −0.917 |
| Glutamate | 0.277 | 0.371 | −0.869 |
| Fructose | −0.246 | −0.866 | 0.530 |
| Glycine | −0.319 | 0.955 | −0.748 |
| Component | NT |
|---|---|
| MgSO4·7H2O | 1233 |
| CaC12·2H2O | 220 |
| KNO3 | 950 |
| NH4NO3 | 825 |
| KH2PO4 | 680 |
| FeSO4·7H2O | 27.8 |
| Na2-EDTA | 37.3 |
| MnSO4·4H2O | 22.3 |
| KI | 0.83 |
| CoC12·6H2O | 0.03 |
| ZnSO4·7H2O | 8.6 |
| CuSO4·5H2O | 0.25 |
| H3BO3 | 6.2 |
| Na2MoO4·2H2O | 0.25 |
| Inositol | 100 |
| VB1 | 1 |
| Glycine | 3 |
References
- Hussain, M.S.; Fareed, S.; Ansari, S.; Rahman, M.D.A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Sankarp, P.D. Plant cell culture technology and its entrée in to world of Ocimun. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. 2), 6–13. [Google Scholar]
- Goel, M.K.; Mehrotra, S.; Kukreja, A.K. Elicitor-Induced cellular and molecular events are responsible for productivity enhancement in hairy root cultures: An insight study. Appl. Biochem. Biotechnol. 2011, 165, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.H.; Mullauer, F.B.; de Roo, G.M.; Medema, J.P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007, 251, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Suresh, C.; Zhao, H.; Gumbs, A.; Chetty, C.S.; Bose, H.S. New ionic derivatives of betulinic acid as highly potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.Z.; Liu, Y.T.; Wang, X.D.; Zhan, Y.G. Cross-talk of polyamines and nitric oxide in endophytic fungus-induced betulin production in Betula platyphylla plantlets. Trees 2014, 28, 635–641. [Google Scholar] [CrossRef]
- Zeng, F.S.; Sun, F.K.; Li, L.L.; Liu, K.; Zhan, Y.G. Genome-scale transcriptome analysis in response to nitric oxide in birch cells: Implications of the triterpene biosynthetic pathway. PLoS ONE 2014, 9, 116–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vandelle, E.; Bellin, D.; Delledonne, M. Detection and function of nitric oxide during the hypersensitive response in Arabidopsis thaliana: Where there’s a will there’s a way. Nitric Oxide 2014, 43, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric Oxide: A multitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Dong, J.; Zhu, M.Y. Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol. 2005, 139, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Hollywood, K.; Brison, D.R.; Goodacre, R. Metabolomics: Current technologies and future trends. Proteomics 2006, 6, 4716–4723. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.Z.; Zhai, Q.L.; Zhan, Y.G. Gene Expression of Lupeol Synthase and Biosynthesis of Nitric Oxide in Cell Suspension Cultures of Betula platyphylla in Response to a Phomopsis Elicitor. Plant Mol. Biol. Rep. 2013, 31, 296–302. [Google Scholar] [CrossRef]
- Hajati, R.J.; Payamnoor, V.; Bezdi, K.G.; Chashmi, N.A. Optimization of Callus Induction and Cell Suspension Culture of Betula pendula Roth for Improved Production of Betulin, Betulinic Acid, and Antioxidant Activity. Cell. Dev. Biol. Plant 2016, 52, 400–407. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Berglund, T.; Kalbin, G.; Rydström, J.; Ohlsson, A.B. UV-B- and oxidative stress-induced increase in nicotinamide and trigonelline and inhibition of defensive metabolism induction by poly(ADP-ribose)polymerase inhibitor in plant tissue. FEBS Lett. 1996, 380, 188–193. [Google Scholar] [CrossRef]
- Tramontano, W.A.; Jouve, D. Trigonelline accumulation in salt-stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 1997, 44, 1037–1040. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Selvaraj, N.; Ganapathi, A.; Manickavasagam, M. Enhanced Biosynthesis of Withanolides by Elicitation and Precursor Feeding in Cell Suspension Culture of Withania somnifera (L.) Dunal in Shake-Flask Culture and Bioreactor. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; He, G.Y. Optimization of elicitors and precursors for paclitaxel production in cell suspension culture of Taxus chinensis in the presence of nutrient feeding. Process Biochem. 2004, 39, 1073–1079. [Google Scholar] [CrossRef]
- Qu, J.G.; Zhang, W.; Yu, X.J. A combination of elicitation and precursor feeding leads to increased anthocyanin synthesis in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult. 2011, 107, 261–269. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P.N. Myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Aharoni, A.; Galili, G. Metabolic engineering of the plant primary–secondary metabolism interface. Curr. Opin. Biotechnol. 2011, 22, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.O.; Shin, Y.S.; Hyun, S.H.; Cho, S.; Bang, K.H.; Lee, D.; Choi, S.P.; Choi, H.K. NMR-based metabolic profiling and differentiation of ginseng roots according to cultivation ages. J. Pharm. Biomed. Anal. 2012, 58, 19–26. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. Or not available |
 cPTIO treatment,
cPTIO treatment,  SNP treatment. Dash arrows skip one or several steps.
SNP treatment. Dash arrows skip one or several steps.
 cPTIO treatment,
cPTIO treatment,  SNP treatment. Dash arrows skip one or several steps.
SNP treatment. Dash arrows skip one or several steps.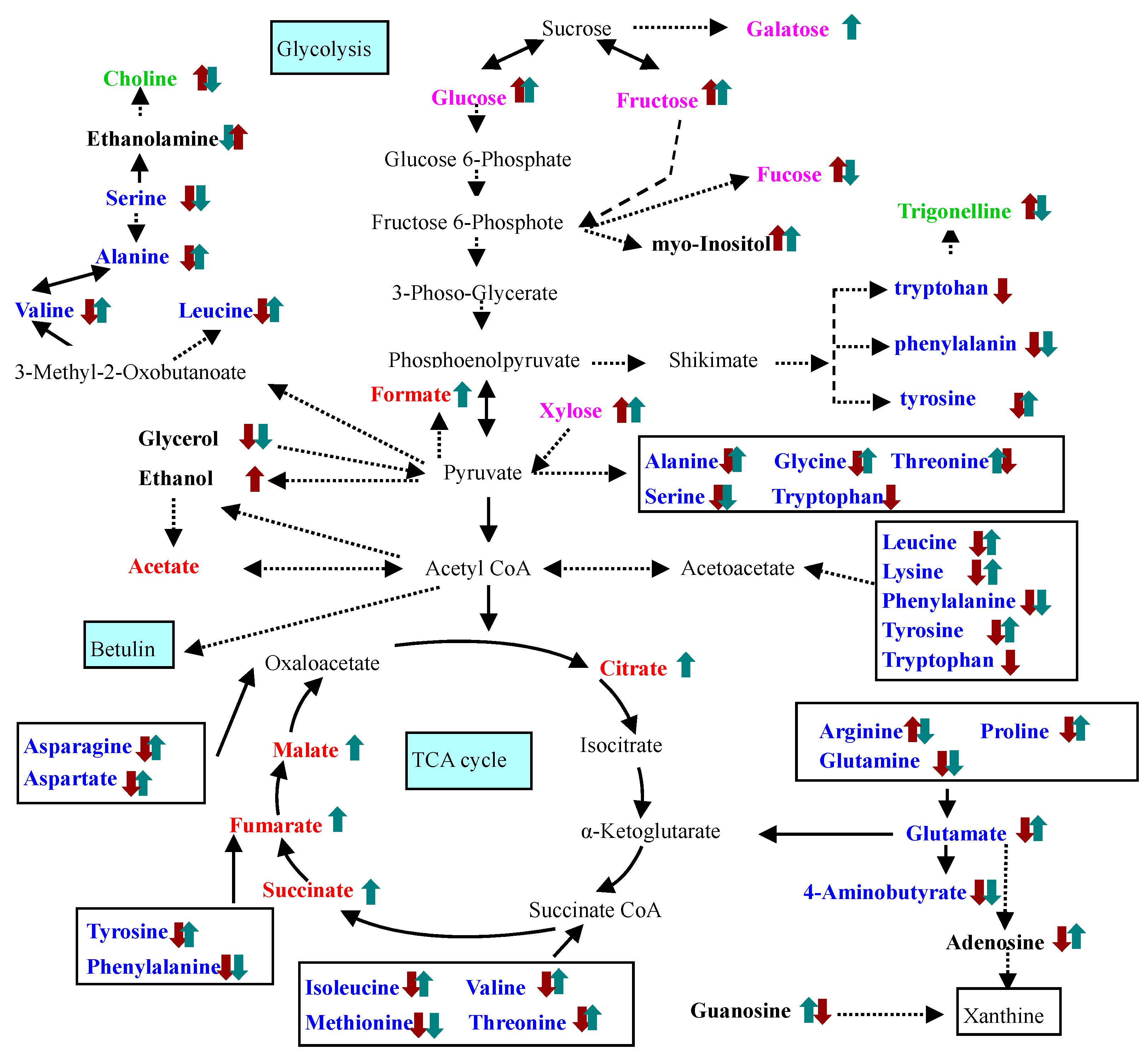

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, G.; Nie, T.; Fan, J.S.; Zhan, Y. Exogenous Feeding of Fructose and Phenylalanine Further Improves Betulin Production in Suspended Betula platyphylla Cells under Nitric Oxide Treatment. Molecules 2017, 22, 1035. https://doi.org/10.3390/molecules22071035
Fan G, Nie T, Fan JS, Zhan Y. Exogenous Feeding of Fructose and Phenylalanine Further Improves Betulin Production in Suspended Betula platyphylla Cells under Nitric Oxide Treatment. Molecules. 2017; 22(7):1035. https://doi.org/10.3390/molecules22071035
Chicago/Turabian StyleFan, Guizhi, Tingting Nie, Jin Sheng Fan, and Yaguang Zhan. 2017. "Exogenous Feeding of Fructose and Phenylalanine Further Improves Betulin Production in Suspended Betula platyphylla Cells under Nitric Oxide Treatment" Molecules 22, no. 7: 1035. https://doi.org/10.3390/molecules22071035
APA StyleFan, G., Nie, T., Fan, J. S., & Zhan, Y. (2017). Exogenous Feeding of Fructose and Phenylalanine Further Improves Betulin Production in Suspended Betula platyphylla Cells under Nitric Oxide Treatment. Molecules, 22(7), 1035. https://doi.org/10.3390/molecules22071035




