In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
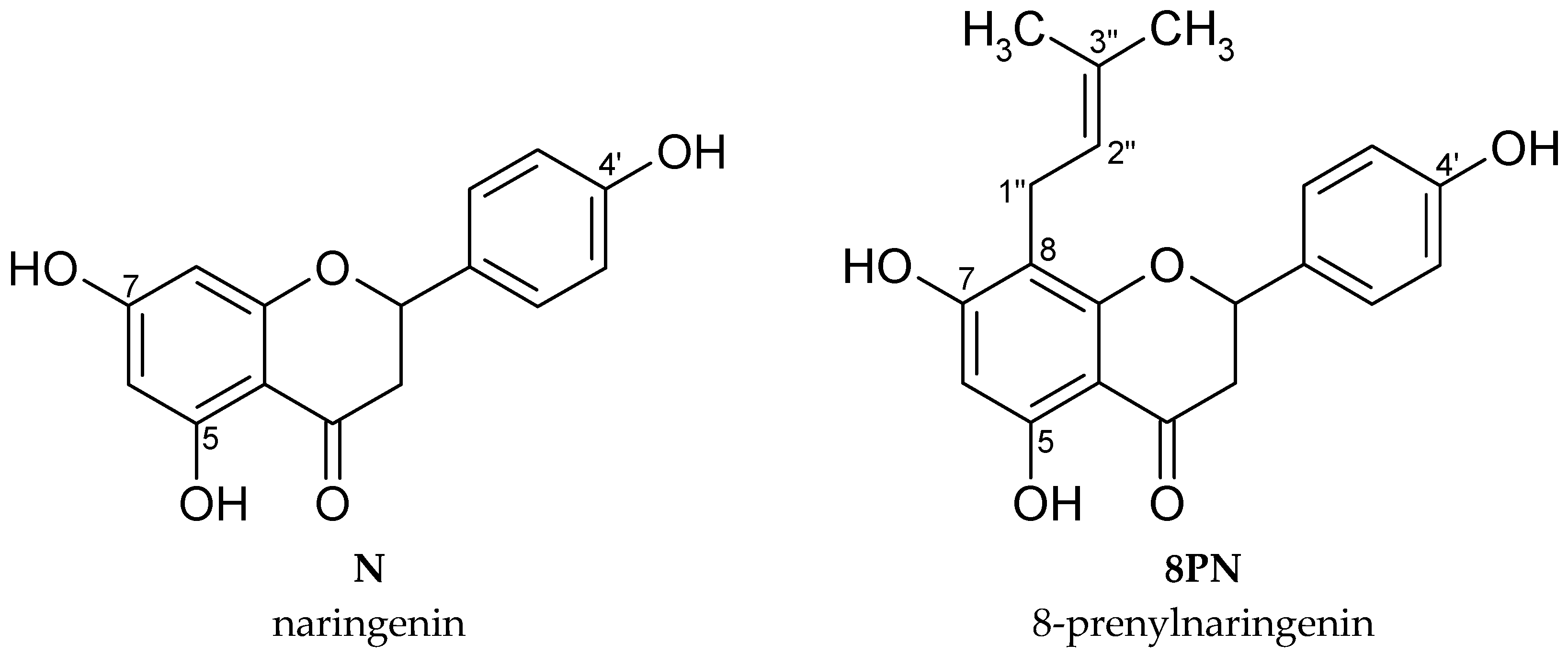
2.1. Synthesis of 8-Prenylnaringenin
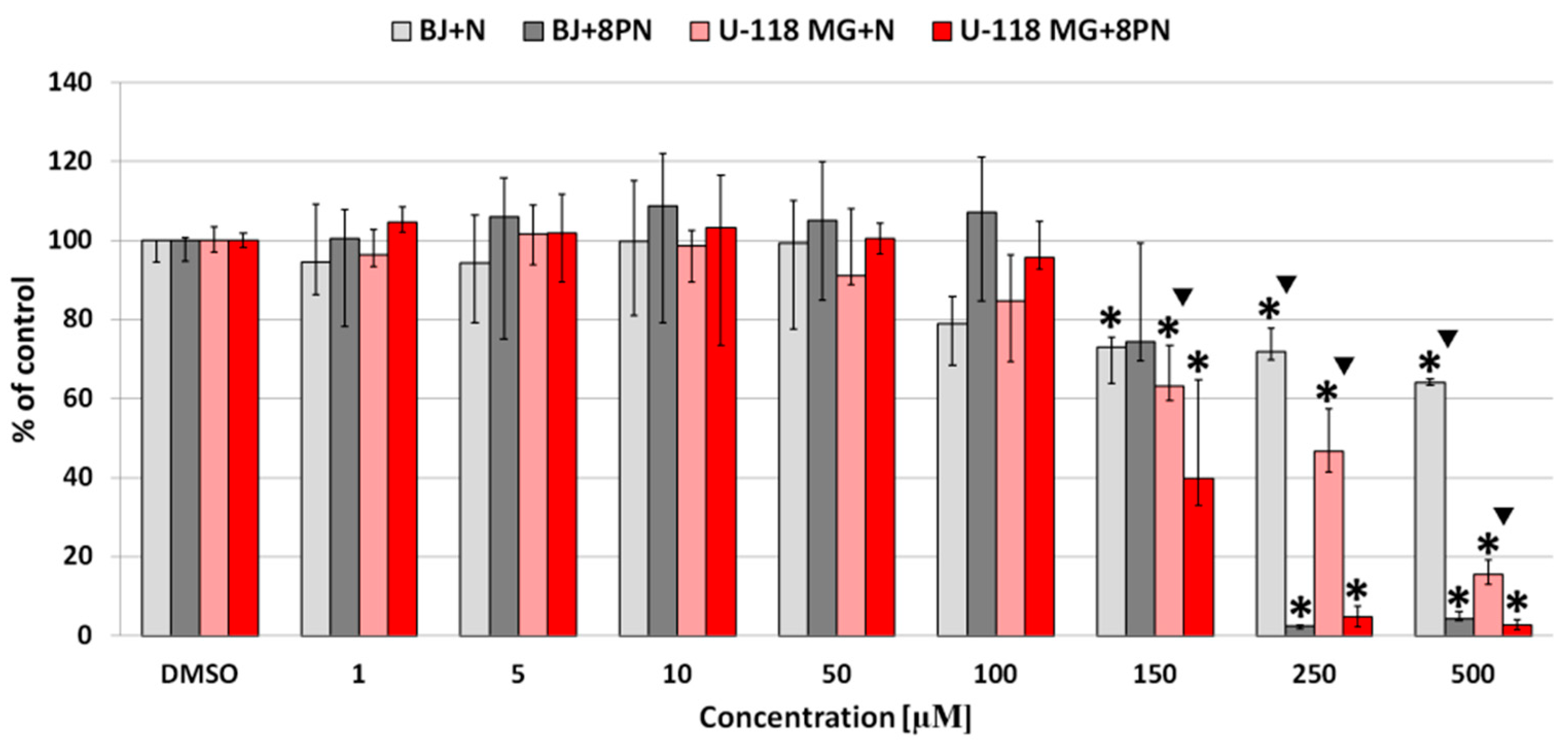
2.2. Cytotoxicity of Naringenin and 8-Prenylnaringenin
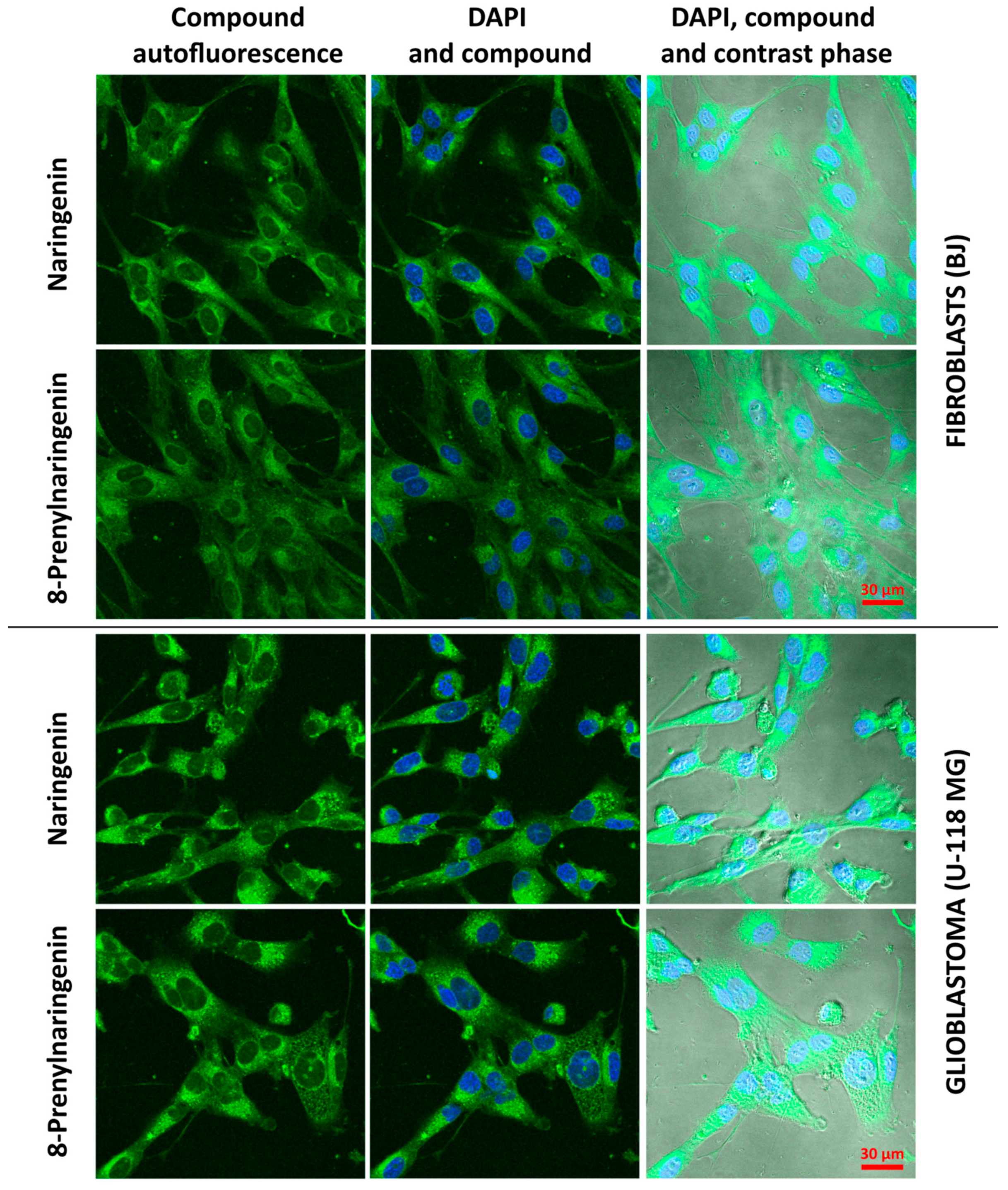
2.3. Cellular Accumulation of Naringenin and 8-Prenylnaringenin
3. Materials and Methods
3.1. Reagents
8-Prenylnaringenin
3.2. Cell Culture
3.3. Analytical Methods
3.4. Cytotoxicity Neutral Red (NR) Assay
3.5. Confocal Microscopy
3.6. Cellular Accumulation of Naringenin and 8-Prenylnaringenin
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflict of interest
References
- Li, J.Y.; Tang, C.; Li, L.W.; Li, R.J.; Fan, Y.W. Quercetin sensitizes glioblastoma to t-AUCB by dual inhibition of Hsp27 and COX-2 in vitro and in vivo. J. Exp. Clin. Cancer Res. 2016, 35, 61. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Ma, C.-M.; Han, Y.-Z.; Li, Y.; Liu, C.; Duan, Z.-H.; Wang, H.-L.; Liu, D.-Q.; Liu, R.-H. Protective effect of naringenin on glutamate-induced neurotoxicity in cultured hippocampal cells. Arch. Biol. Sci. 2015, 67, 639–646. [Google Scholar] [CrossRef]
- Kuete, V.; Ango, P.Y.; Yeboah, S.O.; Mbaveng, A.T.; Mapitse, R.; Kapche, G.D.; Ngadjui, B.T.; Efferth, T. Cytotoxicity of four Aframomum. species (A. arundinaceum, A. alboviolaceum, A. kayserianum and A. polyanthum) towards multi-factorial drug resistant cancer cell lines. BMC Complement. Altern. Med. 2014, 14, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Z.; Liu, J.; Zheng, Y. Xanthohumol, a novel anti-HIV-1 agent purified from hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Stompor, M.; Żarowska, B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-C.; You, S.-K.; Kim, H.J.; Cho, C.-W.; Lee, I.-S.; Kang, B.Y. Xanthohumol inhibits IL-12 production and reduces chronic allergic contact dermatitis. Int. Immunopharmcol. 2010, 10, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Stevens, J.F.; Helmrich, A.; Henderson, M.C.; Rodriguez, R.J.; Yang, Y.-H.; Deinzer, M.L.; Barnes, D.W.; Buhler, D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef]
- Gerhauser, C.; Alt, A.; Heiss, E.; Gamal-Eldeen, A.; Klimo, K.; Knauft, J.; Neumann, I.; Scherf, H.-R.; Frank, N.; Bartsch, H.; Becker, H. Cancer chemopreventive activity of xanthohumol, a natural product derived from hop. Mol. Cancer Ther. 2002, 1, 959–969. [Google Scholar] [PubMed]
- Ho, Y.-C.; Liu, C.-H.; Chen, C.-N.; Duan, K.-J.; Lin, M.-T. Inhibitory effects of xanthohumol from hops (Humulus. lupulus L.) on human hepatocellular carcinoma cell lines. Phytother. Res. 2008, 22, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Vanhoecke, B.; Derycke, L.; Marck, V.V.; Depypere, H.; Keukeleire, D.D.; Bracke, M. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus. lupulus L.) and beer. Int. J. Cancer 2005, 117, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Faria, A.; Azevedo, I.; Calhau, C. Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus. lupulus L.) flavonoids. J. Steroid Biochem. Mol. Biol. 2007, 105, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Choi, Y.H.; Lee, K.Y.; Jeong, H.G. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem. Biophys. Res. Commun. 2011, 409, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qui, C.; Nicoli, D.; Chen, S.N.; Huang, K.; Li, G.; Pauli, G.F.; van Breemen, R.B. Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 2014, 53, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; Minassi, A.; Appendino, G.; Moro, L. 8-Prenylnaringenin, inhibits estrogen receptor-α mediated cell growth and induces apoptosis in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2007, 107, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.-N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. 2016. [Google Scholar] [CrossRef]
- Festa, M.; Caputo, M.; Cipolla, C.; D’Acunto, C.; Rossi, A.; Tecce, M.; Capasso, A. The involvement of xanthohumol in the expression of annexin in human malignant glioblastoma cells. Open Biochem. J. 2013, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.; Damian, M.S.; Schindler, C.; Schachenmayr, W. Multidrug resistance in glioblastoma. Chemosensitivity testing and immunohistochemical demonstration of P-glycoprotein. Pathol. Res. Pract. 1998, 194, 149–155. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tani, E.; Kaba, K.; Shindo, H.; Miyaji, K. Expression of P-glycoprotein in human glioma cell lines and surgical glioma specimens. J. Neurosurg. 1991, 74, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.I.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Conseil, G.; Pérez-Victoria, J.M.; Dayan, G.; Baubichon-Cortay, H.; Trompier, D.; Steinfels, E.; Jault, J.M.; de Wet, H.; Maitrejean, M.; et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell. Mol. Life Sci. 2002, 59, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Sabarinathan, D.; Mahalakshmi, P.; Vanisree, A.J. Naringenin promote apoptosis in cerebrally implanted C6 glioma cells. Mol. Cell. Biochem. 2010, 345, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gester, S.; Metz, P.; Zierau, O.; Vollmer, G. An efficient synthesis of the potent phytoestrogens 8-prenylnaringenin and 6-(1,1-dimethylallyl) naringenin by europium(III)–catalysed Claisen rearrangement. Tetrahedron 2001, 57, 1015–1018. [Google Scholar] [CrossRef]
- Wilhelm, H.; Wessjohann, L.A. An efficient synthesis of the phytoestrogen 8-prenylnaringenin from xanthohumol by a novel demethylation process. Tetrahedron 2006, 62, 6961–6966. [Google Scholar] [CrossRef]
- Terao, J.; Mukai, R. Prenylation modulates the bioavailability and bioaccumulation of dietary flavonoids. Archiv. Biochem. Biophys. 2014, 559, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Colica, C.; Menichini, F. In vitro cytotoxic activity of extracts and isolated constituents of Salvia leriifolia Benth. against a panel of human cancer cell lines. Chem. Biodivers. 2011, 8, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Ayob, Z.; Mohd Bohari, S.P.; Abd Samad, A.; Jamil, S. Cytotoxic activities against breast cancer cells of local Justicia gendarussa crude extracts. Evid. Based Complement. Alternat. Med. 2014, 732980. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Gill, C.; Rowland, I. A comparison of the anticancer properties of isoxanthohumol and 8-prenylnaringenin using in vitro models of colon cancer. BioFactors 2013, 39, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Tokalov, S.V.; Henker, Y.; Schwab, P.; Metz, P.; Gutzeit, H.O. Toxicity and cell cycle effects of synthetic 8-prenylnaringenin and derivatives in human cells. Pharmacology 2014, 71, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mukthavavam, R.; Chao, Y.; Gharati, I.S.; Fogal, V.; Pastorino, S.; Cong, X.; Nomura, N.; Gallagher, M.; Abbasi, T.; et al. Novel anti-glioblastoma agents and therapeutic combinations identified a collection of FDA approved drugs. J. Transl. Med. 2014, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Terao, J.; Shirai, Y.; Saito, N.; Ashida, H. Determination of subcellular localization of flavonol in cultured cells by laser scanning. In Laser Scanning, Theory and Applications; Wang, C.-C., Ed.; INTECH Open Access Publisher, 2011; pp. 215–232. ISBN 978-953-307-205-0. Available online: http://www.intechopen.com/books/laser-scanning-theory-and-applications/determination-of-subcellularlocalization-of-flavonol-in-cultured-cells-by-laser-scanning (accessed on 20 June 2017). [CrossRef]
- Sudo, E.; Teranishi, M.; Hidema, J.; Taniuchi, T. Visualization of flavonol distribution in the abaxial epidermis of onion scales via detection of its autofluorescence in the absence of chemical processes. Biosci. Biotechnol. Biochem. 2009, 73, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Guidarelli, A.; Blasa, M.; Azzolini, C.; Candiracci, M.; Piatti, E.; Cantoni, O. Mitochondria accumulate large amounts of quercetin: Prevention of mitochondrial damage and release upon oxidation of the extra mitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Fujikura, Y.; Murota, K.; Uehara, M.; Minekawa, S.; Matsui, N.; Kawamura, T.; Nemoto, H.; Terao, J. Prenylation enhances quercetin uptake and reduces efflux in Caco-2 cells and enhances tissue accumulation in mice fed long-term. J. Nutr. 2013, 143, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Horikawa, H.; Fujikura, Y.; Kawamura, T.; Nemoto, H.; Nikawa, T.; Terao, J. Prevention of disuse muscle atrophy by dietary ingestion of 8-prenylnaringenin in denervated mice. PLoS ONE 2012, 7, e45048. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds N (naringenin) and 8PN (8-prenylnaringenin) are available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stompor, M.; Uram, Ł.; Podgórski, R. In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity. Molecules 2017, 22, 1092. https://doi.org/10.3390/molecules22071092
Stompor M, Uram Ł, Podgórski R. In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity. Molecules. 2017; 22(7):1092. https://doi.org/10.3390/molecules22071092
Chicago/Turabian StyleStompor, Monika, Łukasz Uram, and Rafał Podgórski. 2017. "In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity" Molecules 22, no. 7: 1092. https://doi.org/10.3390/molecules22071092
APA StyleStompor, M., Uram, Ł., & Podgórski, R. (2017). In Vitro Effect of 8-Prenylnaringenin and Naringenin on Fibroblasts and Glioblastoma Cells-Cellular Accumulation and Cytotoxicity. Molecules, 22(7), 1092. https://doi.org/10.3390/molecules22071092





