New Strigolactone Mimics as Exogenous Signals for Rhizosphere Organisms
Abstract
:1. Introduction
2. Results and Discussion
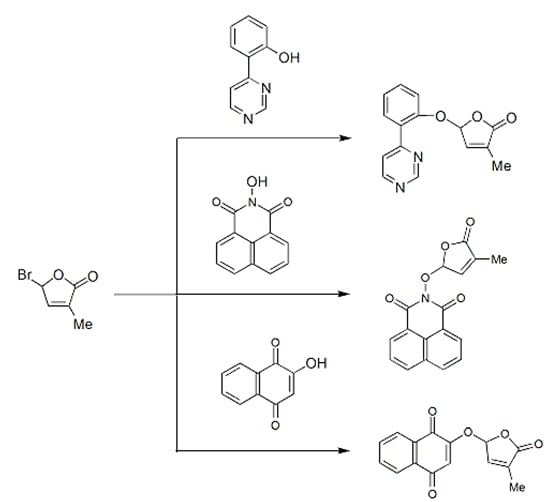
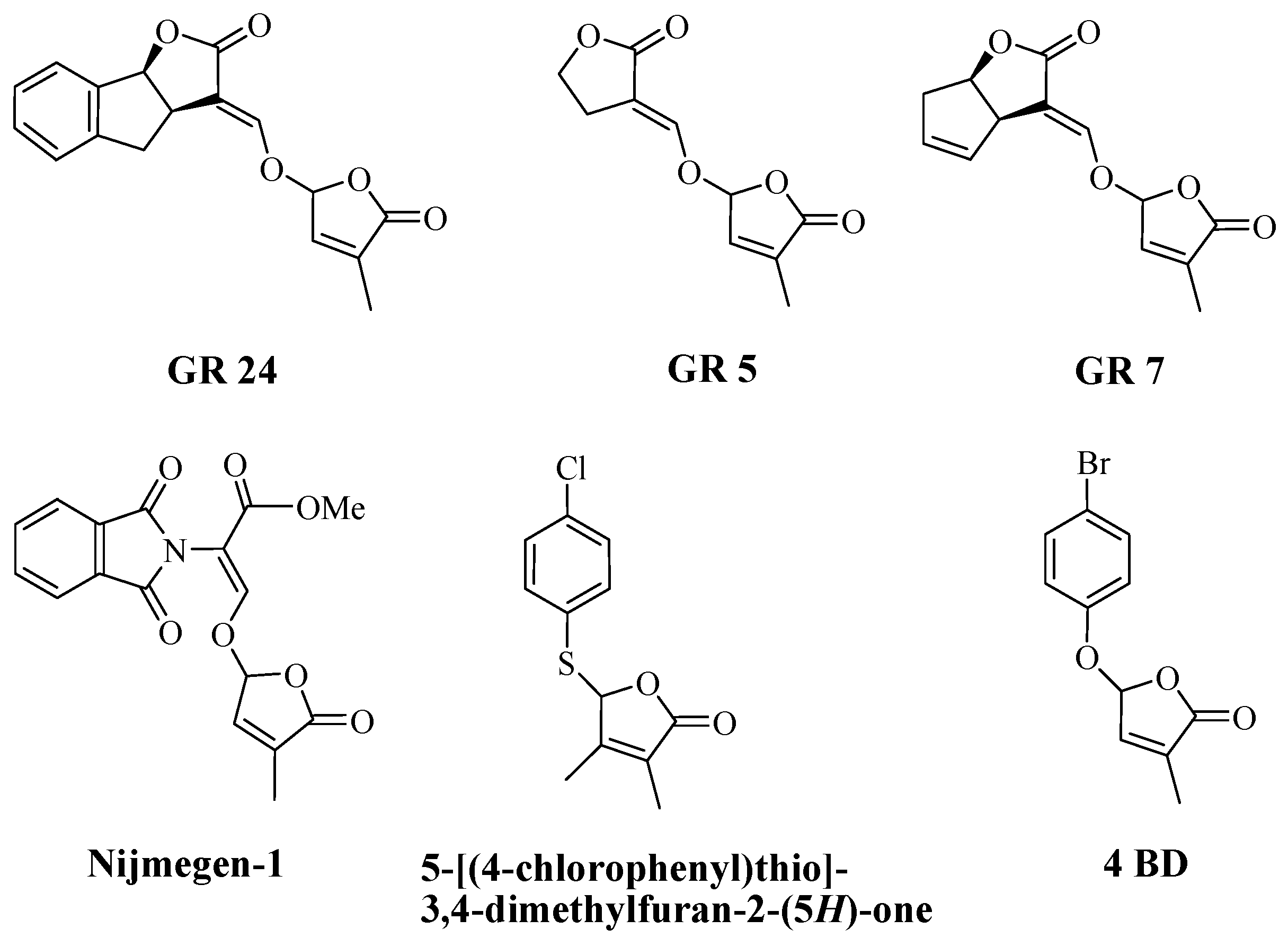
2.1. Synthesis of New Strigolactone Mimics
2.2. The Biological Activity of Synthesized Compounds
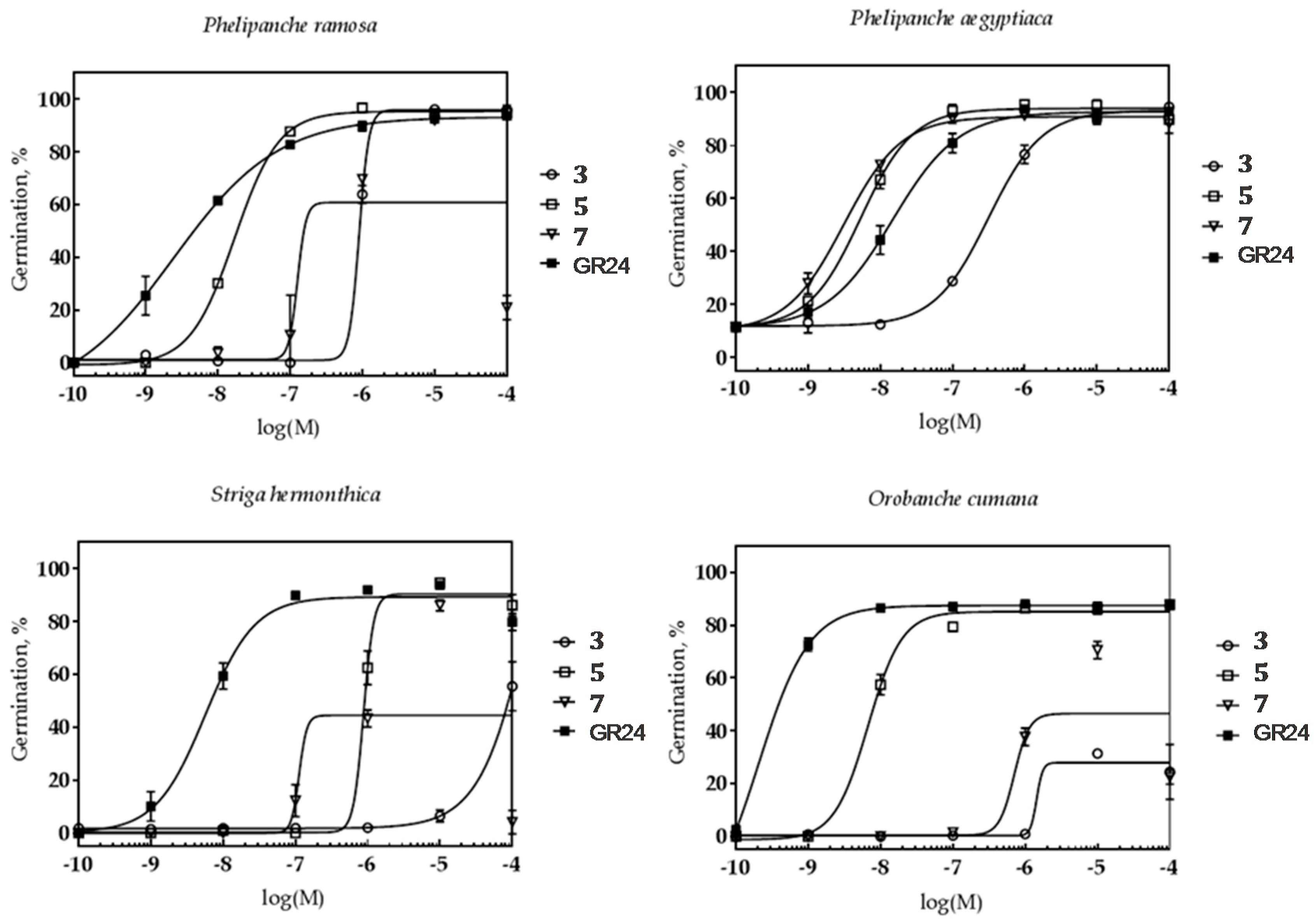
2.2.1. The Effect on Parasitic Weed Seeds
2.2.2. The Effect on Plant Pathogenic Fungi
2.3. Chemical Stability
3. Materials and Methods
3.1. General Information
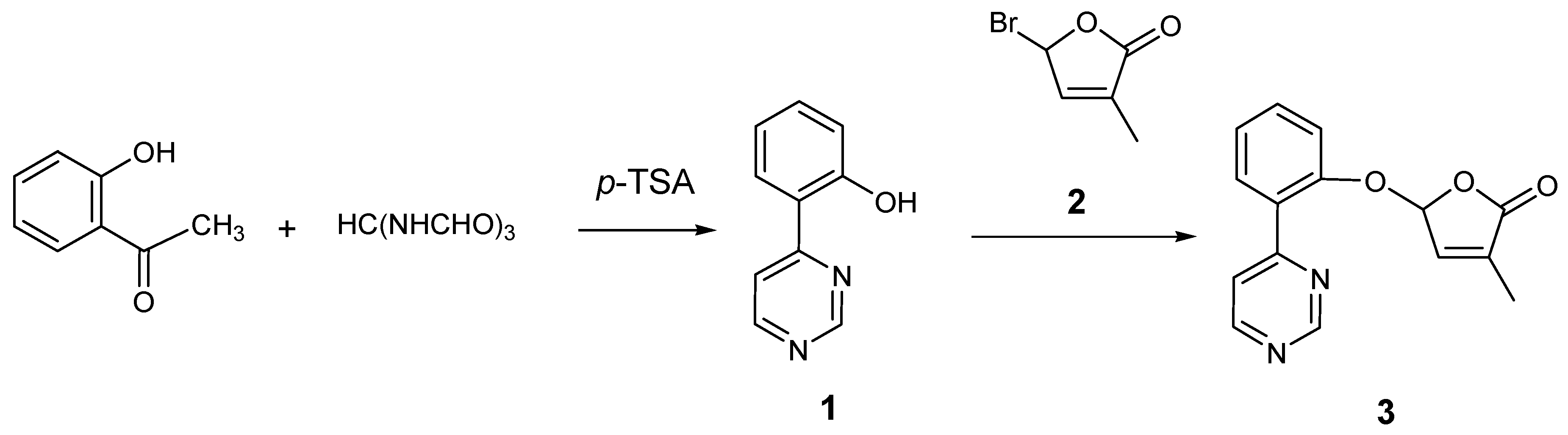
3.2. Preparation of 4-(2-Hydroxyphenyl)pyrimidine (1)
3.3. Typical Procedure for the synthesis of SL mimics
3.4. Product Characterization
3.5. Biological Activity Tests
3.5.1. Germination Bioassay
Preparation of Seeds
Preparation of Test Solutions
Bioassay
Calculation of Logistic Dose–Response Curves.
3.5.2. Test on Plant Pathogenic Fungi
Biological Material
Preparation of Test Solutions
Bioassay
Statistical Analysis
3.6. Chemical Stability Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matusova, R.; Rani, K.; Verstappen, F.W.A.; Franssen, M.C.R.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; van Dijk, A.D.; Scaffidi, A.; Flematti, G.R.; Hofmann, M.; Charnikhova, T.; Verstappen, F.; Hepworth, J.; van der Krol, S.; Leyser, O.; et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014, 10, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Delaux, P.-M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Séjalon-Delmas, N.; Combier, J.-P.; Bécard, G.; Belausov, E.; et al. Strigolactones affect lateral root formation and root-hair elongation in arabidopsis. Planta 2011, 233, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M. Strigolactones as part of the plant defence system. Trends Plant Sci. 2016, 21, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging roles of strigolactones in plant responses to stress and development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A.; Pozo, M.J.; García-Garrido, J.M. Strigolactones: A cry for help in the rhizosphere. Botany 2011, 89, 513–522. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospíšil, T.; Ćavar Zeljković, S. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Xie, X. Structural diversity of strigolactones and their distribution in the plant kingdom. J. Pestic. Sci. 2016, 41, 175–180. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (striga lutea lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones: Structures and biological activities. Pest. Manag. Sci. 2009, 65, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K. Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Matsuzaki, K.-I.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in am fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Becard, G. Rhizosphere communication of plants, parasitic plants and am fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; García-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Yoneyama, K.; Hugill, C.; Quittenden, L.J.; Reid, J.B. Strigolactones—Internal and external signals in plant symbioses? Plant Signal. Behav. 2013, 8, e23168. [Google Scholar] [CrossRef] [PubMed]
- Peláez-Vico, M.A.; Bernabéu-Roda, L.; Kohlen, W.; Soto, M.J.; López-Ráez, J.A. Strigolactones in the rhizobium-legume symbiosis: Stimulatory effect on bacterial surface motility and down-regulation of their levels in nodulated plants. Plant Sci. 2016, 245, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Dor, E.; Joel, D.M.; Kapulnik, Y.; Koltai, H.; Hershenhorn, J. The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta 2011, 234, 419. [Google Scholar] [CrossRef] [PubMed]
- Belmondo, S.; Marschall, R.; Tudzynski, P.; López Ráez, J.A.; Artuso, E.; Prandi, C.; Lanfranco, L. Identification of genes involved in fungal responses to strigolactones using mutants from fungal pathogens. Curr. Genet. 2017, 63, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Foo, E.; Blake, S.N.; Fisher, B.J.; Smith, J.A.; Reid, J.B. The role of strigolactones during plant interactions with the pathogenic fungus fusarium oxysporum. Planta 2016, 243, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H. Strigolactones are regulators of root development. New Phytol. 2011, 190, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Boyer, F.-D.; Saint Germain, A.D.; Pillot, J.-P.; Pouvreau, J.-B.; Chen, V.X.; Ramos, S.; Stevenin, A.; Simier, P.; Delavault, P.; Beau, J.-M.; et al. Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: Molecule design for shoot branching. Plant Physiol. 2012, 159, 1524–1544. [Google Scholar] [CrossRef] [PubMed]
- Boyer, F.-D.; Saint Germain, A.D.; Pouvreau, J.-B.; Clavé, G.; Pillot, J.-P.; Roux, A.; Rasmussen, A.; Depuydt, S.; Lauressergues, D.; Frei dit Frey, N.; et al. New strigolactone analogs as plant hormones with low activities in the rhizosphere. Mol. Plant 2014, 7, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Dor, E.; Hershenhorn, J.; Joel, D.M.; Weininger, S.; Lekalla, S.; Shealtiel, H.; Bhattacharya, C.; Eliahu, E.; Resnick, N.; et al. Strigolactones’ effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J. Plant Growth Regul. 2010, 29, 129–136. [Google Scholar] [CrossRef]
- Arite, T.; Kameoka, H.; Kyozuka, J. Strigolactone positively controls crown root elongation in rice. J. Plant Growth Regul. 2012, 31, 165–172. [Google Scholar] [CrossRef]
- Lockhart, J. Shape-shifters: How strigolactone signaling helps shape the shoot. Plant Cell 2016, 28, 1506–1507. [Google Scholar] [PubMed]
- Screpanti, C.; Fonné-Pfister, R.; Lumbroso, A.; Rendine, S.; Lachia, M.; de Mesmaeker, A. Strigolactone derivatives for potential crop enhancement applications. Bioorg. Med. Chem. Lett. 2016, 26, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. Characterization of strigolactones exuded by asteraceae plants. Plant Growth Regul. 2011, 65, 495–504. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospíšil, T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant 2013, 6, 38–62. [Google Scholar] [CrossRef] [PubMed]
- Magnus, E.M.; Zwanenburg, B. Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogs. J. Agric. Food Chem. 1992, 40, 1066–1070. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Tadokoro, E.; Matsuura, M.; Iwasaki, K.; Sugimoto, Y.; Miyake, H.; Takikawa, H.; Sasaki, M. Synthesis and seed germination stimulating activity of some imino analogs of strigolactones. Biosci. Biotechnol. Biochem. 2007, 71, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Mwakaboko, A.S.; Zwanenburg, B. Single step synthesis of strigolactone analogues from cyclic keto enols, germination stimulants for seeds of parasitic weeds. Bioorg. Med. Chem. 2011, 19, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Mwakaboko, A.S.; Zwanenburg, B. Strigolactone analogs derived from ketones using a working model for germination stimulants as a blueprint. Plant Cell Physiol. 2011, 52, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Gowada, G.; Hassanali, A.; Knox, J.; Monaco, S.; Razavi, Z.; Rosebery, G. The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1 1981, 1734–1743. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Dommerholt, F.J.; de Jong, R.L.P.; Zwanenburg, B. Improved synthesis of strigol analog GR24 and evaluation of the biological activity of its diastereomers. J. Agric. Food Chem. 1992, 40, 1230–1235. [Google Scholar] [CrossRef]
- Fukui, K.; Ito, S.; Asami, T. Selective mimics of strigolactone actions and their potential use for controlling damage caused by root parasitic weeds. Mol. Plant 2013, 6, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Ito, S.; Ueno, K.; Yamaguchi, S.; Kyozuka, J.; Asami, T. New branching inhibitors and their potential as strigolactone mimics in rice. Bioorg. Med. Chem. Lett. 2011, 21, 4905–4908. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Mwakaboko, A.S. Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds. Bioorg. Med. Chem. 2011, 19, 7394–7400. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Nayak, S.K.; Charnikhova, T.V.; Bouwmeester, H.J. New strigolactone mimics: Structure–activity relationship and mode of action as germinating stimulants for parasitic weeds. Bioorg. Med. Chem. Lett. 2013, 23, 5182–5186. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Ann. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Soudek, P.; Vanek, T. Triazolide strigolactone mimics influence root development in arabidopsis. J. Nat. Prod. 2017, 80, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.D.S.; Clavé, G.; Badet-Denisot, M.-A.; Pillot, J.-P.; Cornu, D.; Le Caer, J.-P.; Burger, M.; Pelissier, F.; Retailleau, P.; Turnbull, C.; et al. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016, 12, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, K.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Wang, F.; Ming, Z.; Du, X. ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Res. 2017, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vurro, M.; Prandi, C.; Baroccio, F. Strigolactones: How far is their commercial use for agricultural purposes? Pest. Manag. Sci. 2016, 72, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, E.; Georgescu, F.; Popa, M.M.; Draghici, C.; Tarko, L.; Dumitrascu, F. Efficient one-pot, three-component synthesis of a library of pyrrolo[1,2-c]pyrimidine derivatives. ACS Comb. Sci. 2012, 14, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vlad, P.F.; Ciocarlan, A.; Edu, C.; Aricu, A.; Biriiac, A.; Coltsa, M.; D’Ambrosio, M.; Deleanu, C.; Nicolescu, A.; Shova, S.; et al. Regio- and stereoselective synthesis of (+)-6-ketoeuryfuran, (+)-6-ketowinterin and (-)-7-ketoeuryfuran from accessible labdane diterpenoids (+)-larixol and (-)-sclareol. Tetrahedron 2013, 69, 918–926. [Google Scholar] [CrossRef]
- Oancea, A.; Georgescu, E.; Georgescu, F.; Nicolescu, A.; Oprita, E.I.; Tudora, C.; Vladulescu, L.; Vladulescu, M.-C.; Oancea, F.; Deleanu, C. Isoxazole derivatives as new nitric oxide elicitors in plants. Beil. J. Org. Chem. 2017, 13, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Bredereck, H.; Gompper, R.; Geiger, B. Säureamid-reaktionen, xxii. Synthese von pyrimidinen mittels tris-formamino-methans. Chem. Ber. 1960, 93, 1402–1406. [Google Scholar] [CrossRef]
- Hoffmann, O.L.; Smith, A.E. A new group of plant growth regulators. Science 1949, 109, 588. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Hoffmann, O.L. Plant Growth Regulants and Phytocides. U.S. Patent 2,556,664, 1951. [Google Scholar]
- Thomas, T.H. Gibberellin-like stimulation of celery (Apium graveolens L.) seed germination by n-substituted phthalimides. Sci. Hortic. 1984, 23, 113–117. [Google Scholar] [CrossRef]
- Gott, K.A.; Thomas, T.H. Comparative effects of gibberellins and an n-substituted phthalimide on seed germination and extension growth of celery (Apium graveolens L.). Plant Growth Regul. 1986, 4, 273–279. [Google Scholar] [CrossRef]
- Cala, A.; Ghooray, K.; Fernández-Aparicio, M.; Molinillo, J.M.G.; Galindo, J.C.G.; Rubiales, D.; Francisco, A.; Macías, F.A. Phthalimide-derived strigolactone mimics as germinating agents for seeds of parasitic weeds. Pest. Manag. Sci. 2016, 27, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Matusova, R.; van Mourik, T.; Bouwmeester, H.J. Changes in the sensitivity of parasitic weed seeds to germination stimulants. Seed Sci. Res. 2004, 14, 335–344. [Google Scholar]
- MacAlpine, G.; Raphael, R.; Shaw, A.; Taylor, A.; Wild, H. Synthesis of the germination stimulant (±)-strigol. J. Chem. Soc. Perkin Trans. 1 1976, 410–416. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available. |




| Tested Fungi | Water Control | Acetone Control | GR24 | 3 | 5 | 7 |
|---|---|---|---|---|---|---|
| Fusarium graminearum (DSM 4527) | 1.67 (a) | 1.33 (a) | 5.83 (c) | 4.50 (bc) | 5.16 (c) | 2.83 (ab) |
| Sclerotinia sclerotiorum (DSM 1946) | 2.83 (a) | 3.33 (a) | 20.66 (c) | 13.83 (b) | 21.33 (c) | 12.33 (b) |
| Rhizoctonia solanii (DSM 22842) | 4.50 (a) | 3.67 (a) | 6.83 (ab) | 6.67 (ab) | 10.67 (b) | 7.83 (ab) |
| Macrophomina phaseolina (DSM 62744) | 17.83 (a) | 12.33 (ab) | 3.60 (c) | 5.33 (c) | 6.67 (bc) | 7.33 (bc) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oancea, F.; Georgescu, E.; Matusova, R.; Georgescu, F.; Nicolescu, A.; Raut, I.; Jecu, M.-L.; Vladulescu, M.-C.; Vladulescu, L.; Deleanu, C. New Strigolactone Mimics as Exogenous Signals for Rhizosphere Organisms. Molecules 2017, 22, 961. https://doi.org/10.3390/molecules22060961
Oancea F, Georgescu E, Matusova R, Georgescu F, Nicolescu A, Raut I, Jecu M-L, Vladulescu M-C, Vladulescu L, Deleanu C. New Strigolactone Mimics as Exogenous Signals for Rhizosphere Organisms. Molecules. 2017; 22(6):961. https://doi.org/10.3390/molecules22060961
Chicago/Turabian StyleOancea, Florin, Emilian Georgescu, Radoslava Matusova, Florentina Georgescu, Alina Nicolescu, Iuliana Raut, Maria-Luiza Jecu, Marius-Constantin Vladulescu, Lucian Vladulescu, and Calin Deleanu. 2017. "New Strigolactone Mimics as Exogenous Signals for Rhizosphere Organisms" Molecules 22, no. 6: 961. https://doi.org/10.3390/molecules22060961