UV-Curable Aliphatic Silicone Acrylate Organic–Inorganic Hybrid Coatings with Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
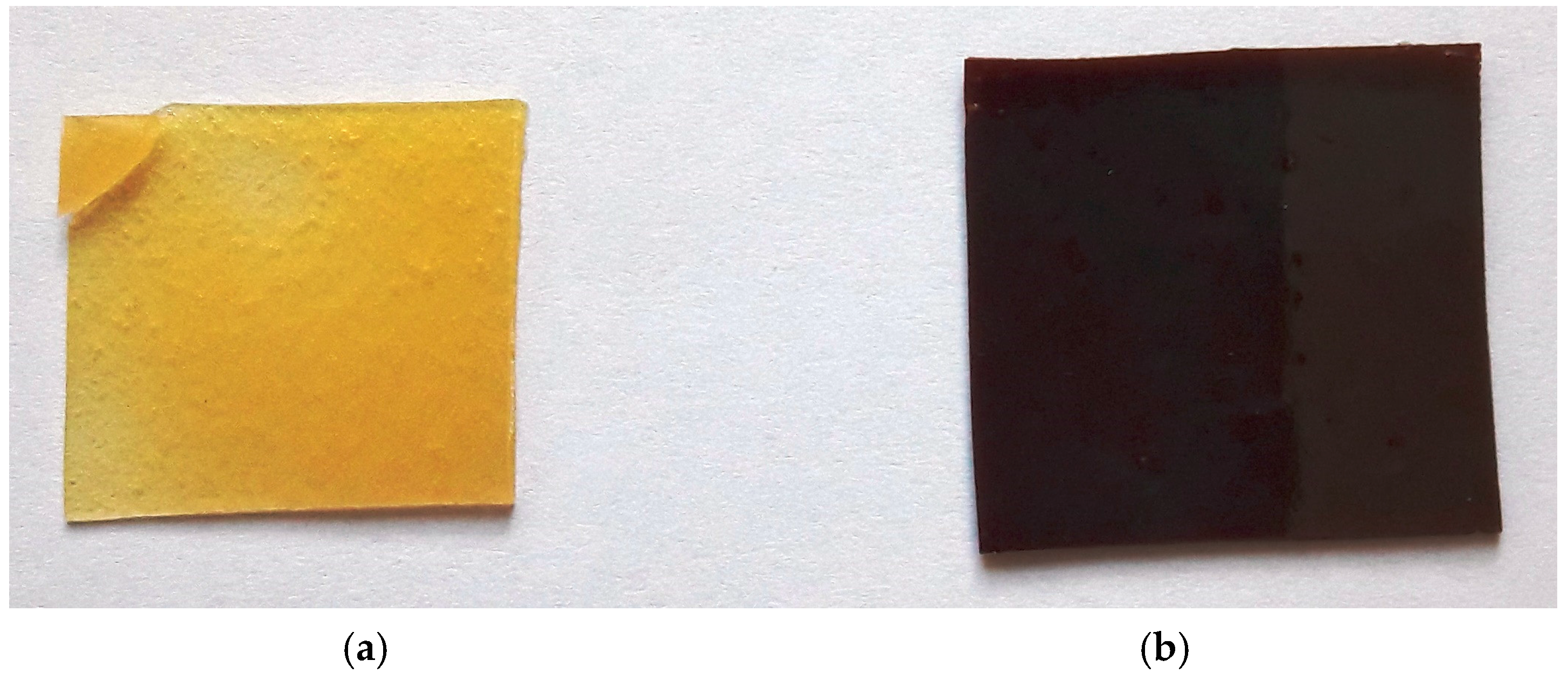
2.1. Structure Analysis
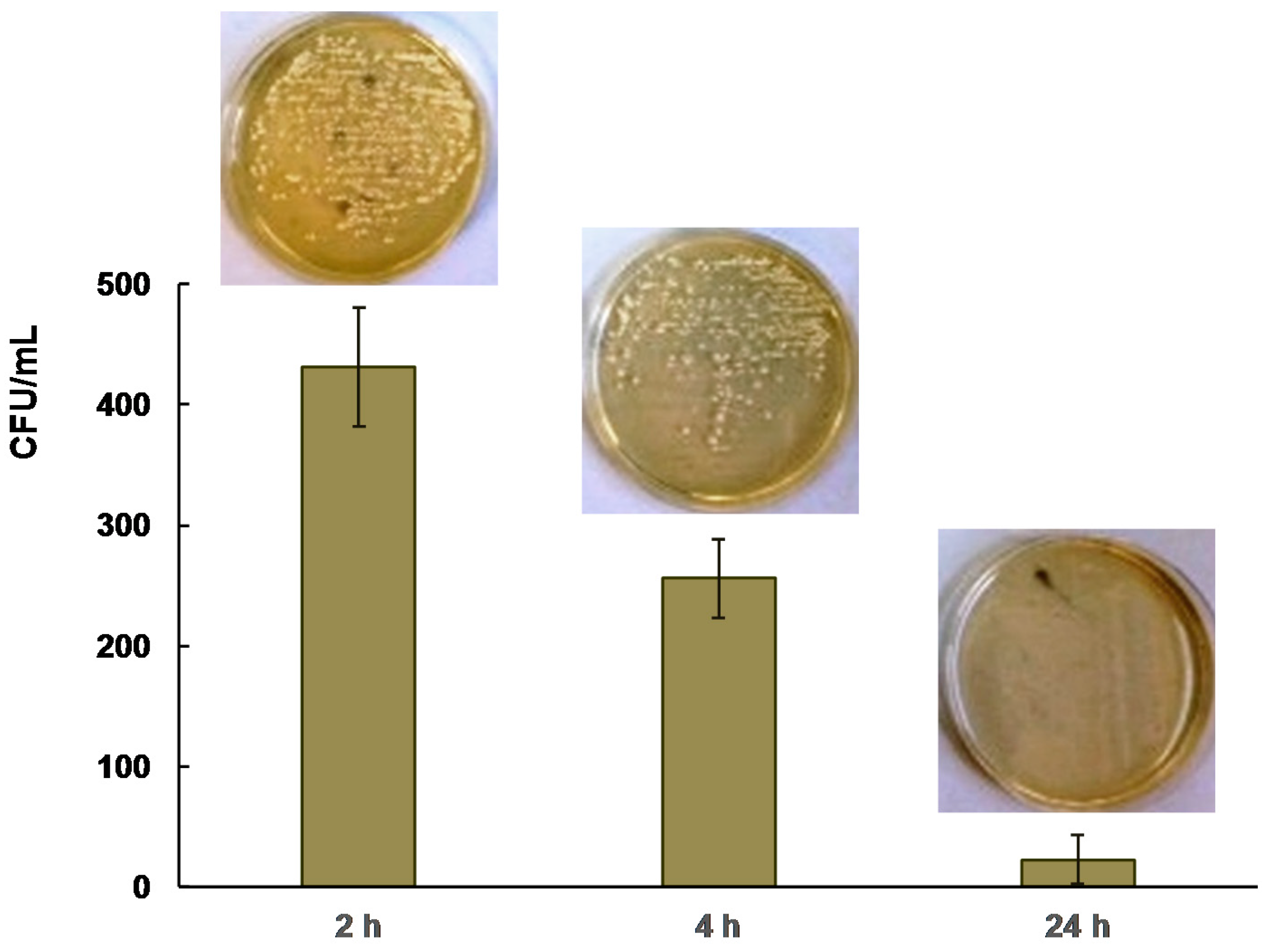
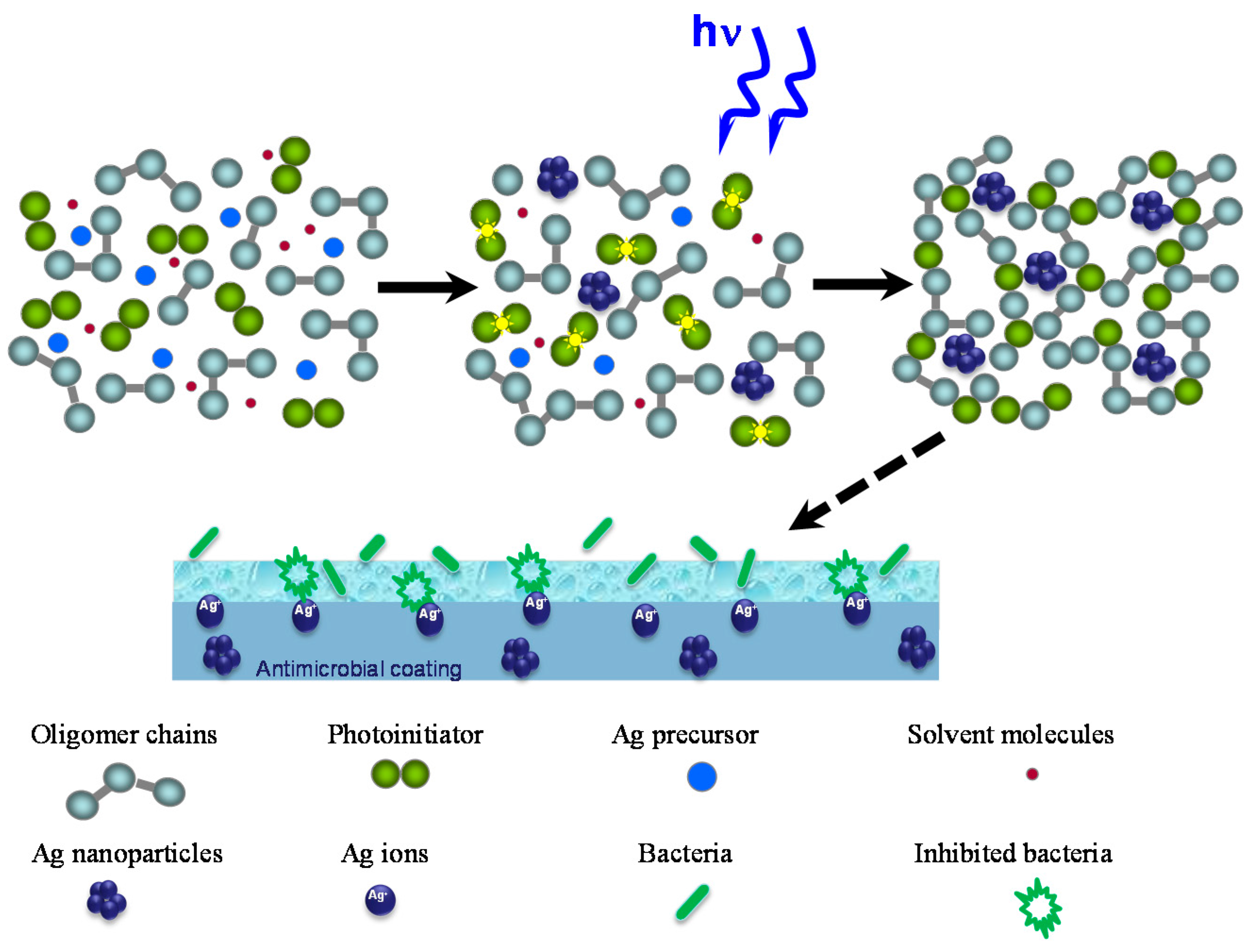
2.2. Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Nanocomposite Preparation
3.3. Nanocomposite Characterization
3.4. Antibacterial Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amerio, E.; Sangermano, M.; Malucelli, G.; Priola, A.; Voit, B. Preparation and characterization of hybrid nanocomposite coatings by photopolymerization and sol–gel process. Polymer 2005, 46, 11241–11246. [Google Scholar] [CrossRef]
- Sanchez, C.; Ribot, F.; Lebeau, B. Molecular design of hybrid organic–inorganic nanocomposites synthesized via sol-gel chemistry. J. Mater. Chem. 1999, 9, 35–44. [Google Scholar] [CrossRef]
- Draxl, C.; Nabok, D.; Hannewald, K. Organic/inorganic hybrid materials: Challenges for ab initio methodology. Acc. Chem. Res. 2014, 18, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Chujo, Y. Organic–inorganic polymer hybrids prepared by the sol-gel method. Compos. Interface 2005, 11, 539–566. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Pyun, J.; Matyjaszewski, K. Synthesis of Nanocomposite organic/inorganic hybrid materials using controlled/“living” radical polymerization. Chem. Mater. 2001, 13, 3436–3448. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Baek, J.B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Merchan, M.; Sedlarikova, J.; Vesel, A.; Sedlarik, V.; Pastorek, M.; Sáha, P. Characterization of antibacterial, mechanical, and structural properties of polyvinyl chloride/silver nitrate composites prepared by thermoplastic compounding. Int. J. Polym. Anal. Charact. 2010, 15, 360–369. [Google Scholar] [CrossRef]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, S.; Zhang, G.; An, D.; Wang, C.; Yang, Q.; Chen, X.; Jing, X.; Wei, Y. A convenient route to polyvinyl pyrrolidone/silver nanocomposite by electrospinning. Nanotechnology 2006, 17, 3304–3307. [Google Scholar] [CrossRef]
- Chahal, R.P.; Mahendia, S.; Tomar, A.; Kumar, S. Effect of ultraviolet irradiation on the optical and structural characteristics of in-situ prepared PVP-Ag nanocomposites. Dig. J. Nanomater. Biostruct. 2011, 6, 301–308. [Google Scholar]
- Liao, W.; Gu, A.; Liang, G.; Yuan, L. New high performance transparent UV-curable poly(methyl methacrylate) grafted ZnO/silicone-acrylate resin composites with simultaneously improved integrated performance. Colloid Surf. A 2012, 396, 74–82. [Google Scholar] [CrossRef]
- Nagel, C.; Heekeren, M.; Frank, A. New silicones structures. Eur. Coat. J. 2010, 4, 33–39. [Google Scholar]
- Kim, H.K.; Ju, H.T.; Hong, J.W. Characterization of UV-cured polyester acrylate films containing acrylate functional polydimethylsiloxane. Eur. Polym. J. 2003, 39, 2235–2241. [Google Scholar] [CrossRef]
- Shah, S.R.; Tatara, A.M.; D’Souza, R.M.; Mikos, A.G.; Kasper, F.K. Evolving strategies for preventing biofilm on implantable materials. Mater. Today 2013, 16, 177–182. [Google Scholar] [CrossRef]
- Dahl, J.A.; Maddux, B.L.; Hutchison, J.E. Toward greener nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Roucoux, A.; Schulz, J.; Patin, H. Reduced transition metal colloids: A novel family of reusable catalysts? Chem. Rev. 2002, 102, 3757–3778. [Google Scholar] [CrossRef] [PubMed]
- Fendler, J.H. Nanoparticles and Nanostructured Films: Preparation, Characterization, and Applications; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Jankauskaitė, V.; Vitkauskienė, A.; Lazauskas, A.; Baltrušaitis, J.; Prosyčevas, I.; Andrulevičius, M. Bactericidal effect of graphene oxide/Cu/Ag nanoderivatives against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus. Int. J. Pharm. 2016, 511, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Duan, S.-S.; Ouyang, Y.-S.; Chen, Y.-B. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals 2011, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Dror-Ehre, A.; Mamane, H.; Belenkova, T.; Markovich, G.; Adin, A. Silver nanoparticle–E. coli colloidal interaction in water and effect on E. coli survival. J. Colloid Interface Sci. 2009, 339, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.-C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; Bispo de Jesus, M.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed: Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, T.; Zhang, X.-Y.; Song, Y.-J.; Li, R.-Z.; Zhu, S.-Q. Optical properties of Ag nanoparticle-polymer composite film based on two-dimensional Au nanoparticle array film. Nanoscale Res. Lett. 2014, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Stalmashonak, A.; Seifert, G.; Abdolvand, A. Optical properties of nanocomposites containing metal nanoparticles. In Ultra-Short Pulsed Laser Engineered Metal-Glass Nanocomposites; Springer: Heidelberg, Germany, 2013; pp. 5–15. [Google Scholar]
- Pacioni, N.L.; Borsarelli, C.D.; Rey, V.; Veglia, A.V. Synthetic routes for the preparation of silver nanoparticles. In Silver Nanoparticle Applications. In the Fabrication and Design of Medical and Biosensing Devices; Emilio, I., Alarcon, E.I., Griffith, M., Udekwu, K.I., Eds.; Springer: Heidelberg, Germany, 2015; pp. 13–46. [Google Scholar]
- Oldenburg, S.J. Silver Nanoparticles: Properties and Applications, Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/technical-documents/articles/materials-science/nanomaterials/silver-nanoparticles.html (accessed on 14 March 2017).
- Park, H.-S.; Kim, M.-S.; Hahm, H.-S.; Kim, S.-K.; Rhee, H.-W. Synthesis of silicone–acrylic resins and their applications to superweatherable coatings. Appl. Polym. Sci. 2001, 81, 1614–1623. [Google Scholar] [CrossRef]
- Oktay, B.; Kayaman-Apohan, N. Polydimethylsiloxane (PDMS)-based antibacterial organic–inorganic hybrid coatings. J. Coat. Technol. Res. 2013, 10, 785–798. [Google Scholar] [CrossRef]
- Zou, M.; Wang, S.; Zhang, Z.; Ge, X. Preparation and characterization of polysiloxane-poly(butyl acrylate-styrene) composite latices and their film properties. Eur. Polym. J. 2005, 41, 2602–2613. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, H. Synthesis and properties of novel silicone-acrylate self-emulsifying emulsion. Asian J. Chem. 2013, 25, 10172–10174. [Google Scholar]
- Wang, H.; Liu, W.; Yan, Z.; Tan, J.; Xia-Hou, G. Synthesis and characterization of UV-curable acrylate films modified by functional methacrylate terminated polysiloxane hybrid oligomers. RSC Adv. 2015, 5, 81838–81846. [Google Scholar] [CrossRef]
- Kasraei, S.; Azarsina, M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz. Oral Res. 2012, 26, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
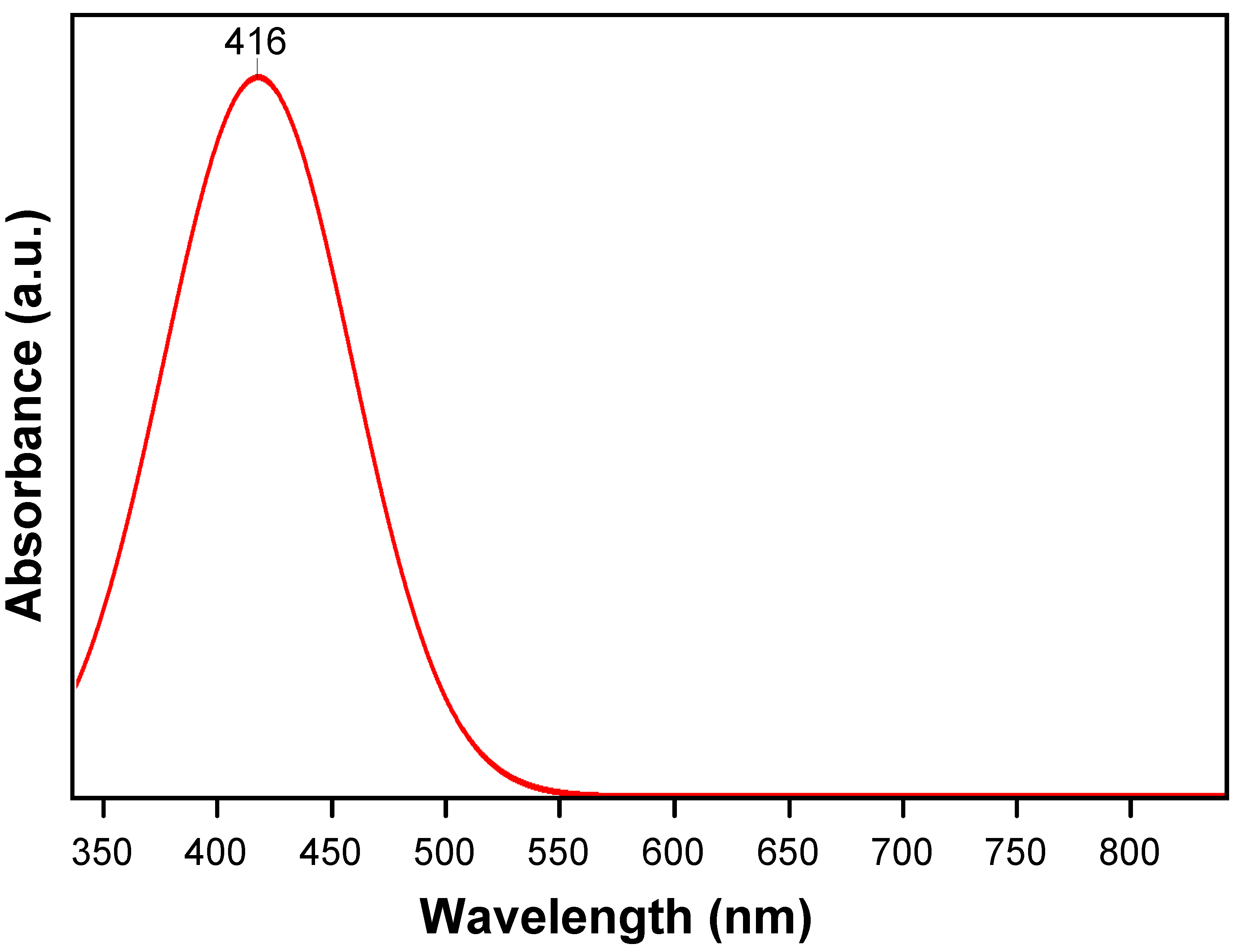

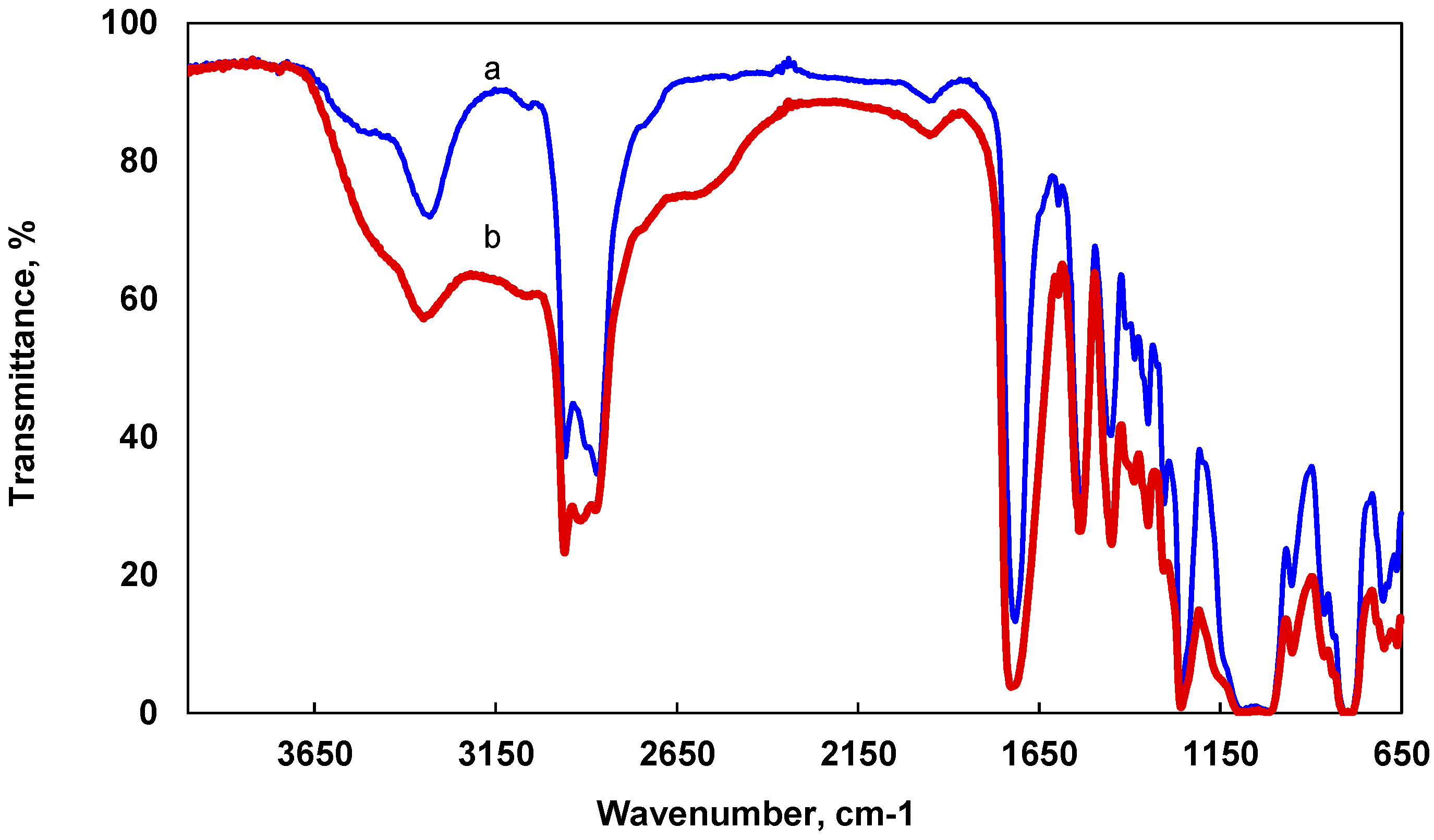

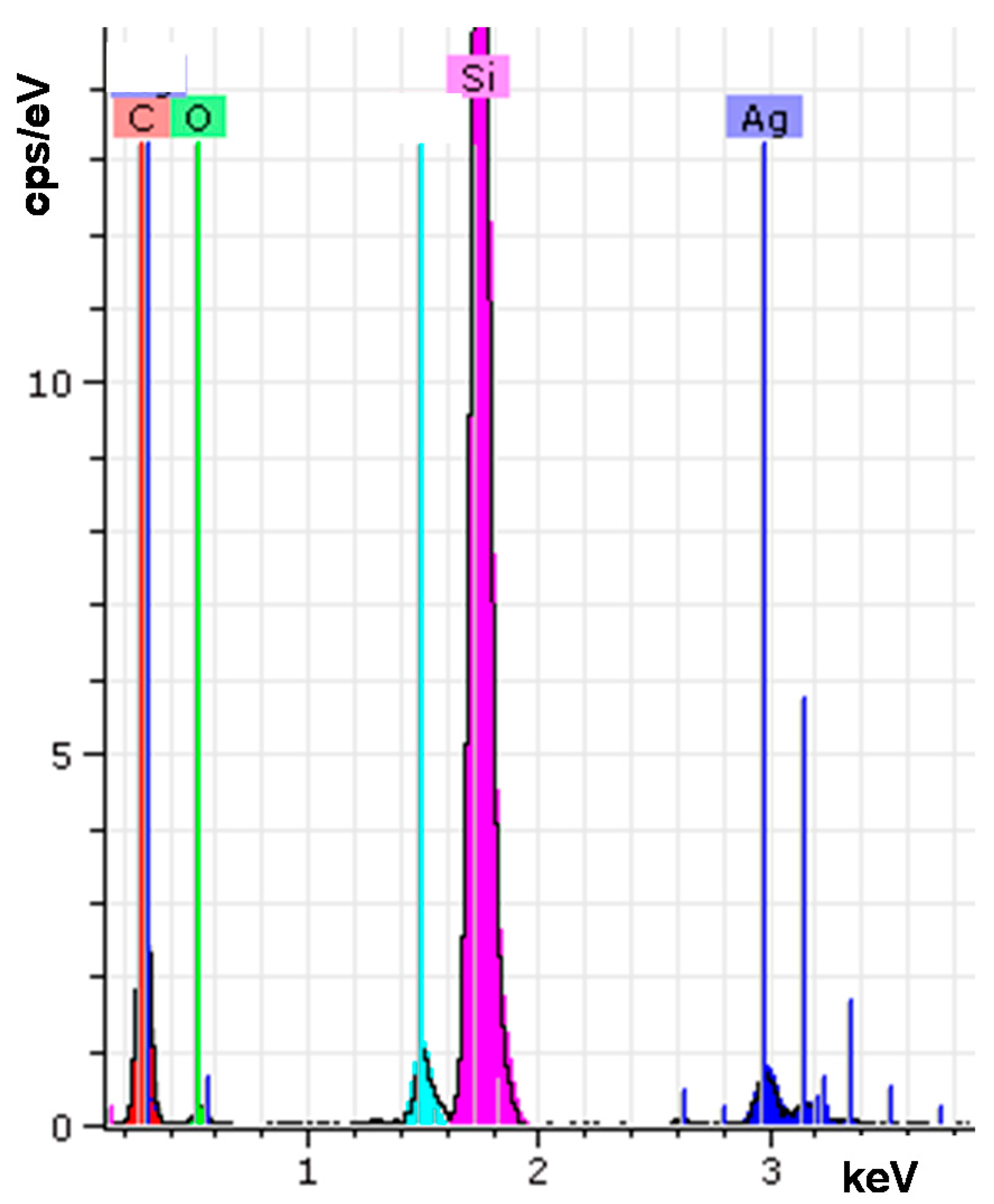
| Element | wt % | at % | Error in % |
|---|---|---|---|
| Carbon K | 41.53 | 61.16 | 6.79 |
| Oxygen K | 10.81 | 11.95 | 3.47 |
| Silver L | 6.87 | 1.13 | 0.19 |
| Silicon K | 38.48 | 24.24 | 1.63 |
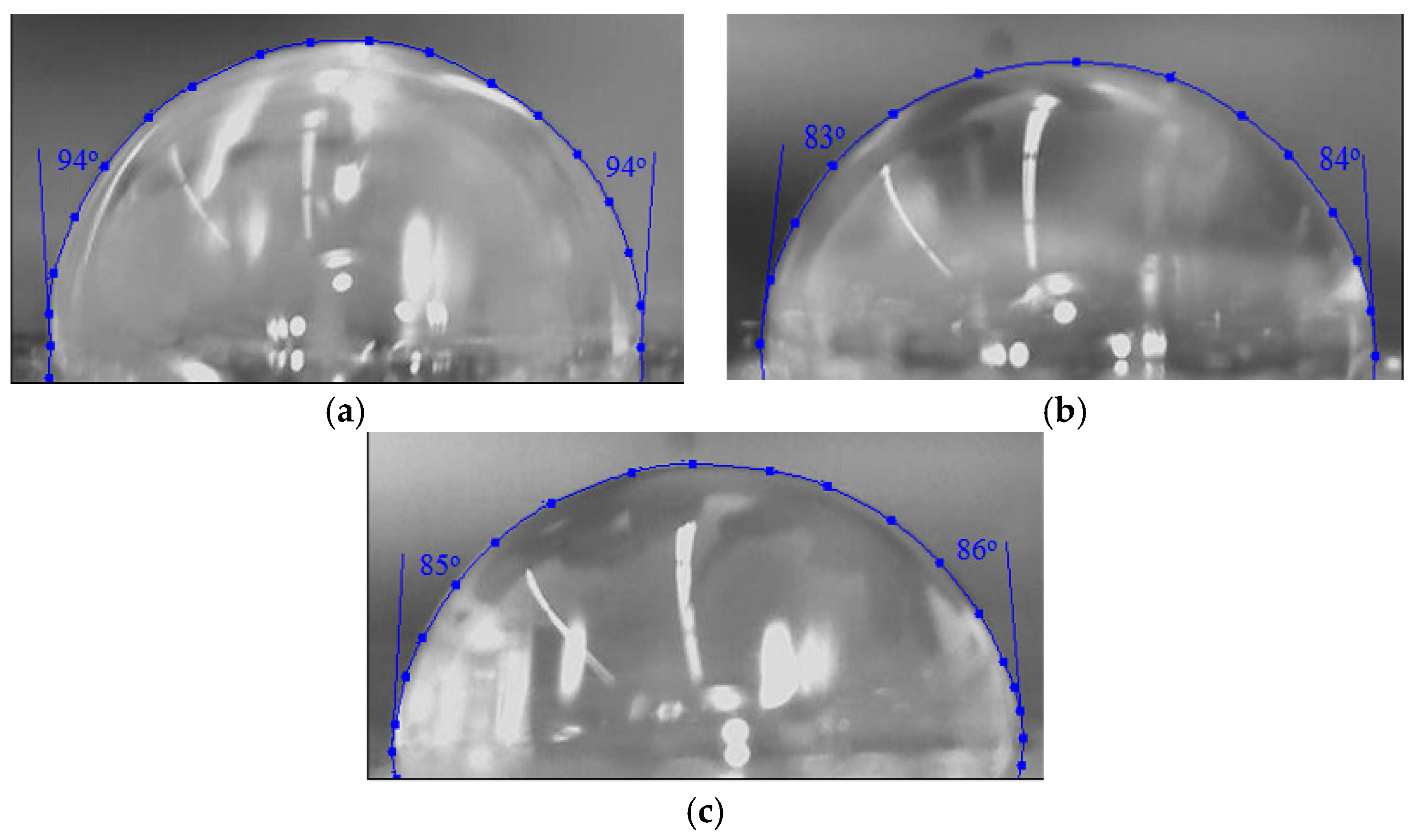
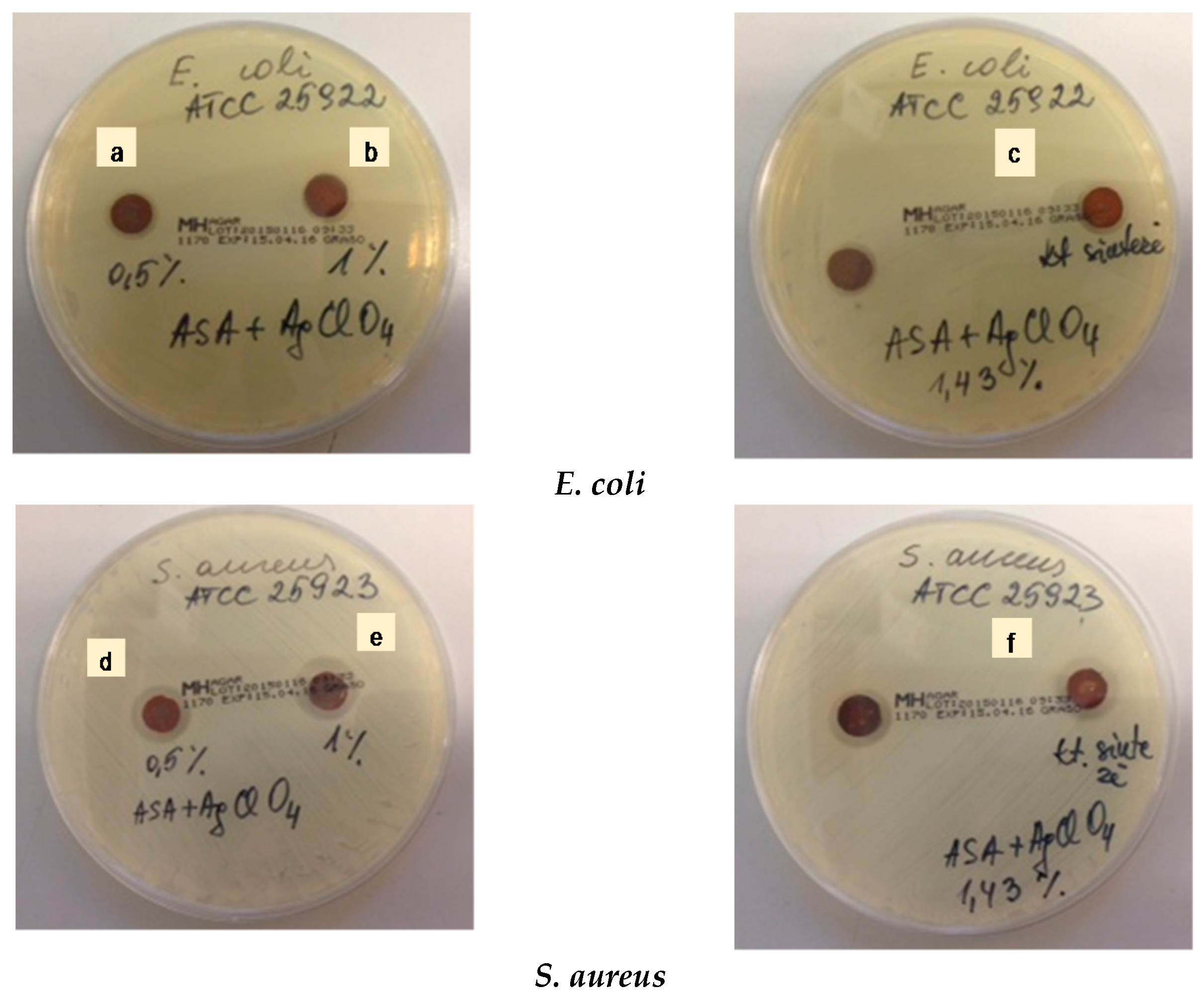
| Silver Nanoparticles Concentration (wt %) | Inhibition Zone * | |
|---|---|---|
| E. coli | S. aureus | |
| 0 | 0 | 0 |
| 0.5 | 1.14–1.27 | 1.61–1.66 |
| 1.0 | 1.15–1.34 | 1.82–1.94 |
| 1.43 | 1.26–1.34 | 1.83–2.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankauskaitė, V.; Lazauskas, A.; Griškonis, E.; Lisauskaitė, A.; Žukienė, K. UV-Curable Aliphatic Silicone Acrylate Organic–Inorganic Hybrid Coatings with Antibacterial Activity. Molecules 2017, 22, 964. https://doi.org/10.3390/molecules22060964
Jankauskaitė V, Lazauskas A, Griškonis E, Lisauskaitė A, Žukienė K. UV-Curable Aliphatic Silicone Acrylate Organic–Inorganic Hybrid Coatings with Antibacterial Activity. Molecules. 2017; 22(6):964. https://doi.org/10.3390/molecules22060964
Chicago/Turabian StyleJankauskaitė, Virginija, Algirdas Lazauskas, Egidijus Griškonis, Aistė Lisauskaitė, and Kristina Žukienė. 2017. "UV-Curable Aliphatic Silicone Acrylate Organic–Inorganic Hybrid Coatings with Antibacterial Activity" Molecules 22, no. 6: 964. https://doi.org/10.3390/molecules22060964





