Highly Stereoselective Synthesis of a Compound Collection Based on the Bicyclic Scaffolds of Natural Products
Abstract
:1. Introduction
2. Results and Discussion
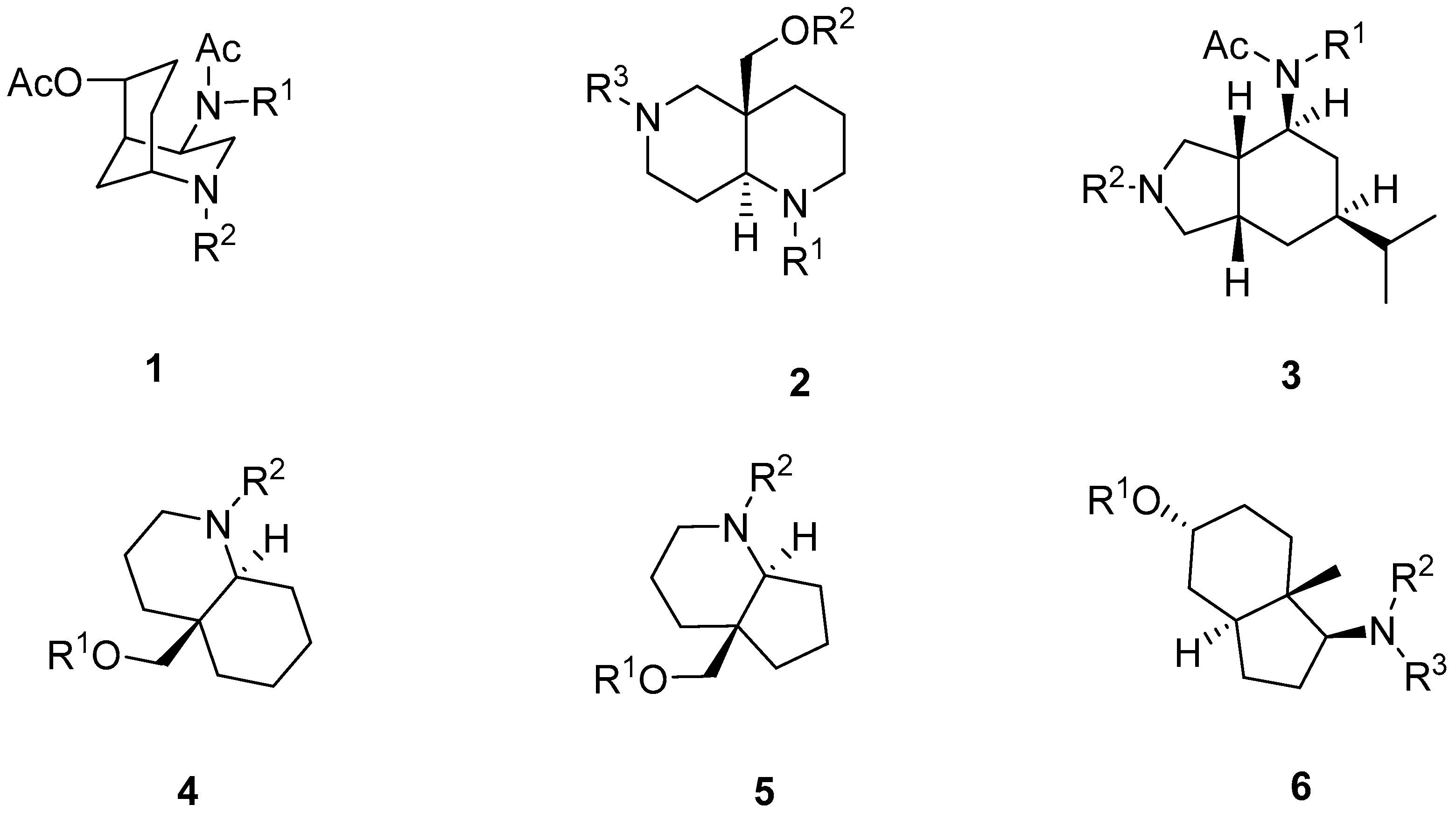
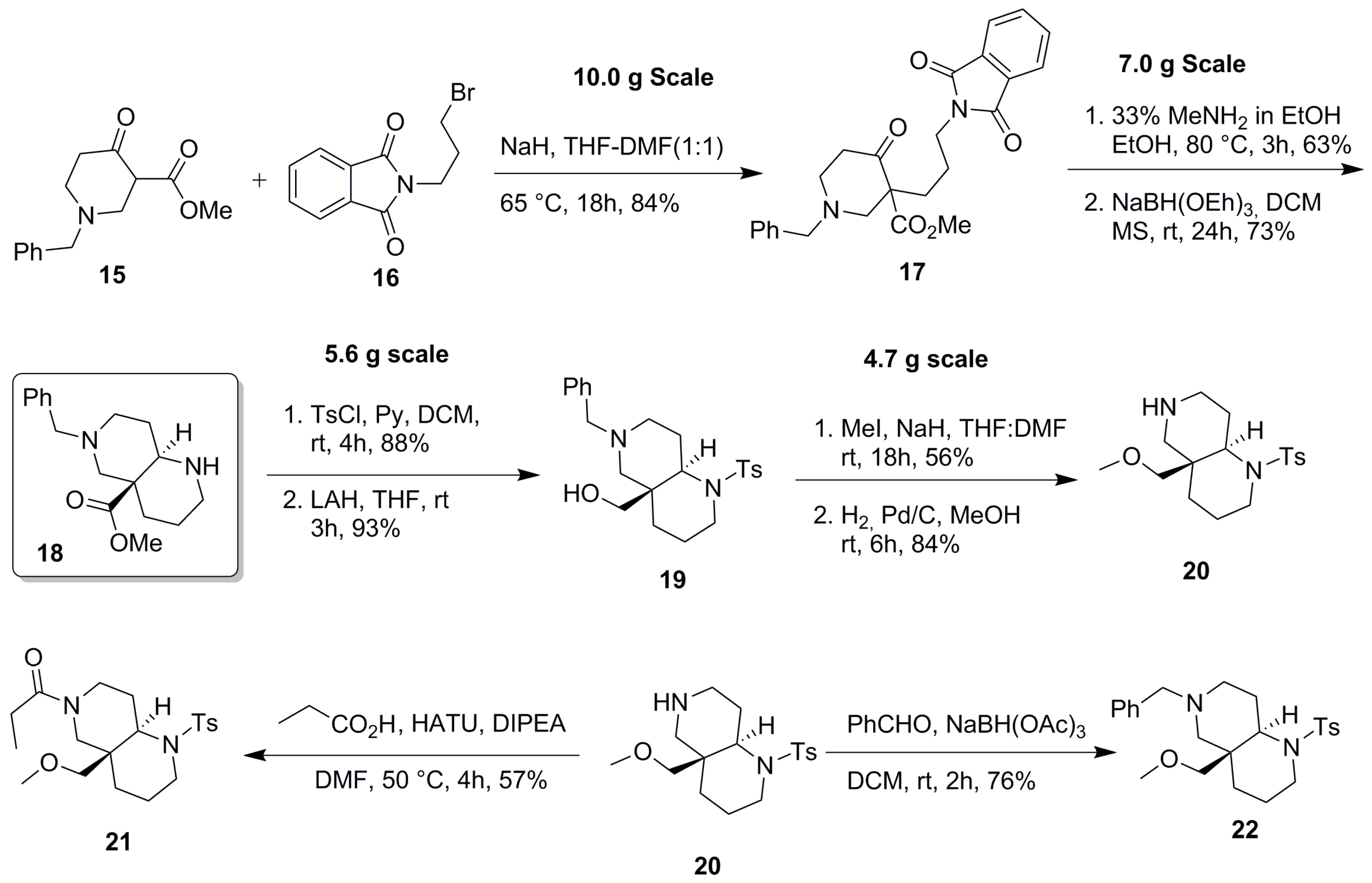
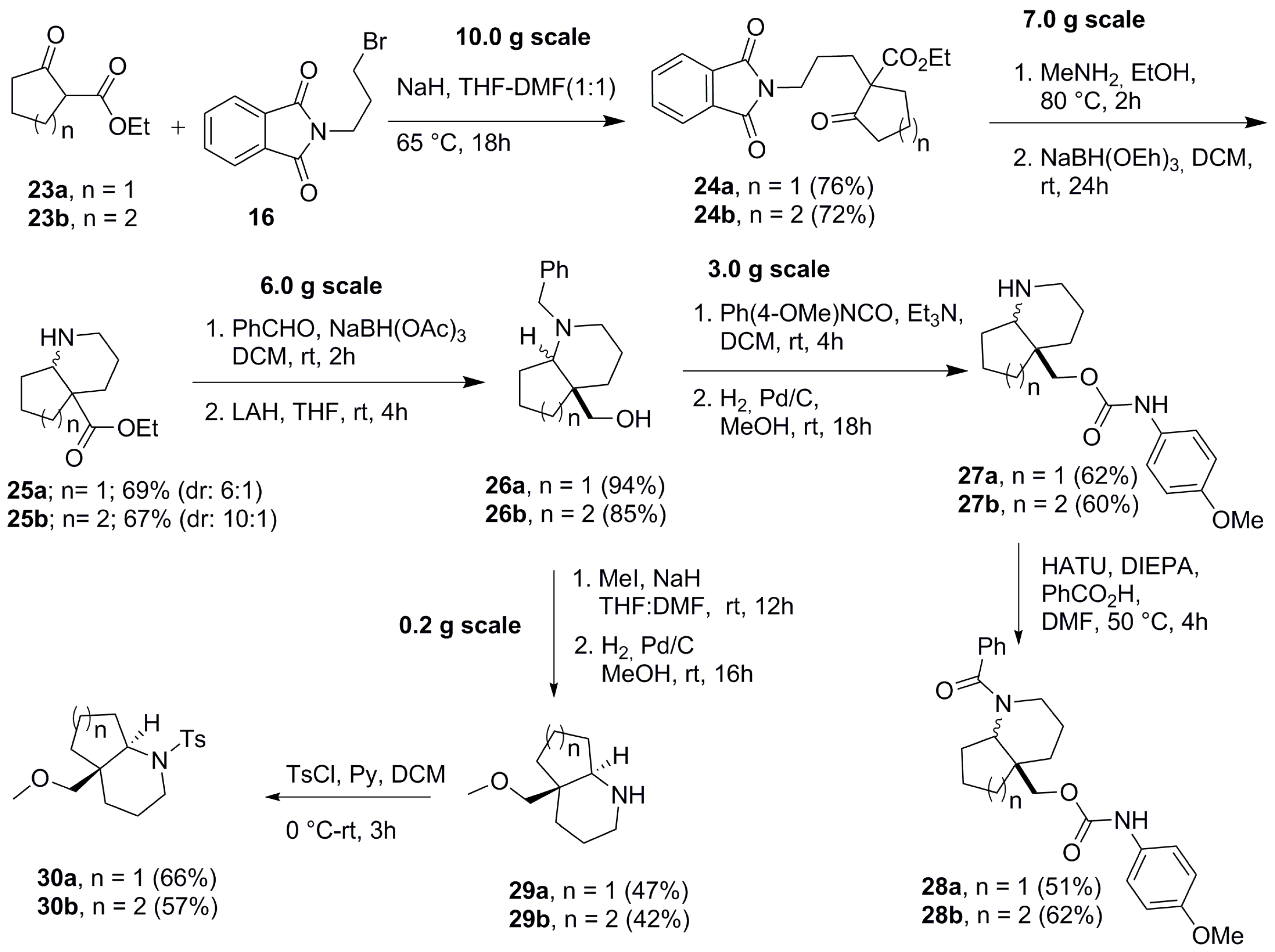
2.1. Synthesis of Aza-Bicyclic Scaffolds
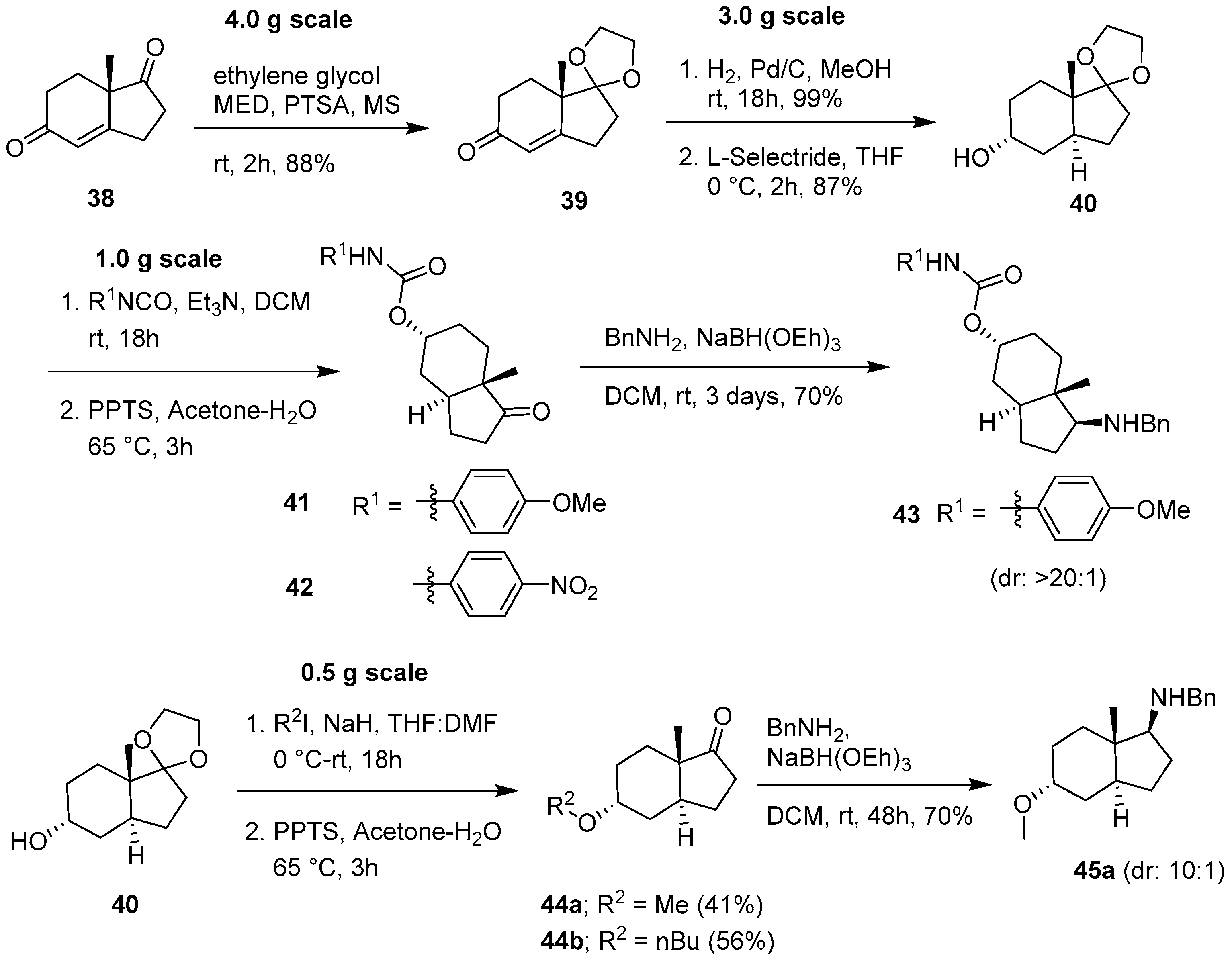
2.2. Synthesis Optimization of a Carbo-Bicyclic Scaffold
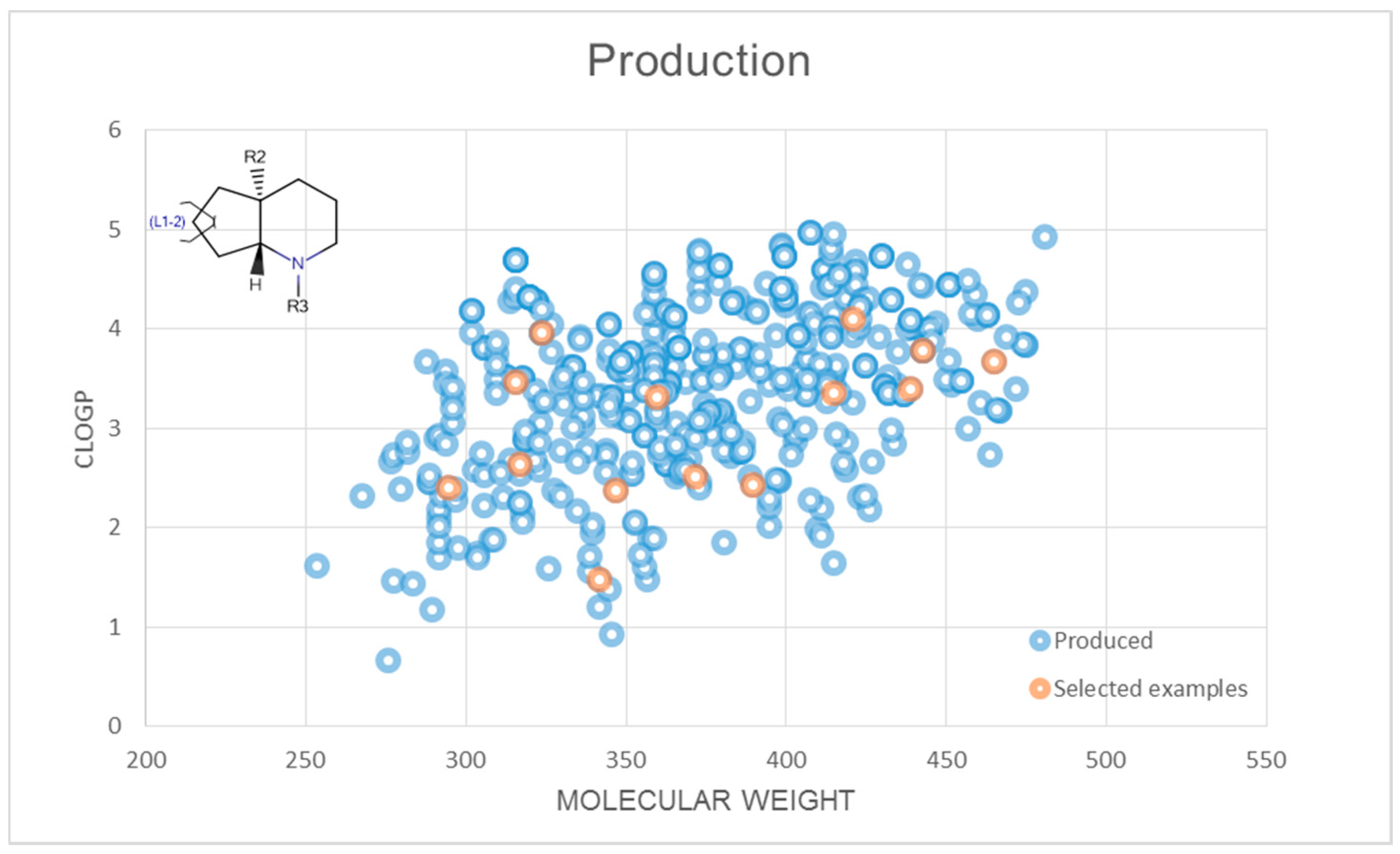
2.3. Representative Compound Collection Synthesis around Bicyclic Scaffolds
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butler, M.S. Natural products to drugs: Natural product derived compounds in clinical trials. Nat. Prod. Rep. 2005, 22, 162–195. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Villar, H.O.; Hansen, M.R. Design of chemical libraries for screening. Expert Opin. Drug Discov. 2009, 4, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Clark, R.L.; Mackay, S.P.; Johnston, B.F. Current strategies for drug discovery through natural products. Expert Opin. Drug Discov. 2010, 5, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Danishefsky, S. On the potential of natural products in the discovery of pharma leads: A case for reassessment. Nat. Prod. Rep. 2010, 27, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.G.; Liu, N.; Hu, D.D.; Dong, G.Q.; Miao, Z.Y.; Yao, J.Z.; He, H.Y.; Jiang, Y.Y.; Zhang, W.N.; Wang, Y.; et al. The discovery of novel antifungal scaffolds by structural simplification of the natural product sampangine. Chem. Commun. 2015, 51, 14648–14651. [Google Scholar] [CrossRef] [PubMed]
- Van Hattum, H.; Waldmann, H. Biology-oriented synthesis: Harnessing the power of evolution. J. Am. Chem. Soc. 2014, 136, 11853–11859. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.E. Natural products as chemical probes. ACS Chem. Biol. 2010, 5, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Bon, R.S.; Waldmann, H. Bioactivity-guided navigation of chemical space. Acc. Chem. Res. 2010, 43, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Waldmann, H. Synthesis of natural product inspired compound collections. Angew. Chem. Int. Ed. 2009, 48, 3224–3242. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castro, M.; Zimmermann, S.; Sankar, M.G.; Kumar, K. Scaffold diversity synthesis and its application in probe and drug discovery. Angew. Chem. Int. Ed. 2016, 55, 7586–7605. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. European lead factory opens for business. Nat. Rev. Drug Disc. 2013, 12, 173–175. [Google Scholar] [CrossRef] [PubMed]
- The European Lead Factory. Available online: https://www.europeanleadfactory.eu/ (accessed on 17 May 2017).
- Karawajczyk, A.; Giordanetto, F.; Benningshof, J.; Hamza, D.; Kalliokoski, T.; Pouwer, K.; Morgentin, R.; Nelson, A.; Muller, G.; Piechot, A.; et al. Expansion of chemical space for collaborative lead generation and drug discovery: The european lead factory perspective. Drug Discov. Today 2015, 20, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Karawajczyk, A.; Orrling, K.M.; de Vlieger, J.S.B.; Rijnders, T.; Tzalis, D. The european lead factory: A blueprint for public–private partnerships in early drug discovery. Front Med. (Lausanne) 2016, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Besnard, J.; Jones, P.S.; Hopkins, A.L.; Pannifer, A.D. The joint european compound library: Boosting precompetitive research. Drug Discov. Today 2015, 20, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Madhavachary, R.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Domling, A. De novo assembly of highly substituted morpholines and piperazines. Org. Lett. 2017, 19, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.T.; Packard, E.; Nortcliffe, A.; Lewis, W.; Hamza, D.; Jones, G.; Moody, C.J. Synthesis of epibatidine analogues by pyrrole diels-alder reactions: Rapid access to azabicyclo[2.2.1]heptane and 3,8-diazabicyclo[3.2.1]octane scaffolds for library synthesis. Eur. J. Org. Chem. 2017, 138–148. [Google Scholar] [CrossRef]
- Wu, P.; Petersen, M.A.; Cohrt, A.E.; Petersen, R.; Morgentin, R.; Lemoine, H.; Roche, C.; Willaume, A.; Clausen, M.H.; Nielsen, T.E. A metal-catalyzed enyne-cyclization step for the synthesis of bi- and tricyclic scaffolds amenable to molecular library production. Org. Biomol. Chem. 2016, 14, 6947–6950. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.D.; Craven, P.G.E.; Lilburn, M.; Pahl, A.; Marsden, S.P.; Nelson, A. A biosynthesis-inspired approach to over twenty diverse natural product-like scaffolds. Chem. Commun. 2016, 52, 9837–9840. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Empson, C.J.; Craven, P.; Owen, Z.; Doveston, R.G.; Churcher, I.; Marsden, S.P.; Nelson, A. A divergent synthetic approach to diverse molecular scaffolds: Assessment of lead-likeness using llama, an open-access computational tool. Chem. Commun. 2016, 52, 7209–7212. [Google Scholar] [CrossRef] [PubMed]
- Chandgude, A.L.; Domling, A. Unconventional passerini reaction toward alpha-aminoxy-amides. Org. Lett. 2016, 18, 6396–6399. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, E.M.M.; Kurpiewska, K.; Kalinowska-Tluscik, J.; Domling, A. Artificial macrocycles by ugi reaction and passerini ring closure. J. Org. Chem. 2016, 81, 8789–8795. [Google Scholar] [CrossRef] [PubMed]
- Craven, P.; Aimon, A.; Dow, M.; Fleury-Bregeot, N.; Guilleux, R.; Morgentin, R.; Roche, D.; Kalliokoski, T.; Foster, R.; Marsden, S.P.; et al. Design, synthesis and decoration of molecular scaffolds for exploitation in the production of alkaloid-like libraries. Bioorg. Med. Chem. 2015, 23, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Colomer, I.; Adeniji, O.; Burslem, G.M.; Craven, P.; Rasmussen, M.O.; Willaume, A.; Kalliokoski, T.; Foster, R.; Marsden, S.P.; Nelson, A. Aminomethylhydroxylation of alkenes: Exploitation in the synthesis of scaffolds for small molecule libraries. Bioorg. Med. Chem. 2015, 23, 2736–2740. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.G.; Mantilli, L.; Bull, J.; Giordanetto, F.; Bauer, J.O.; Strohmann, C.; Waldmann, H.; Kumar, K. Stereoselective synthesis of a natural product inspired tetrahydroindolo[2,3-a]-quinolizine compound library. Bioorg. Med. Chem. 2015, 23, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Duckert, H.; Pries, V.; Khedkar, V.; Menninger, S.; Bruss, H.; Bird, A.W.; Maliga, Z.; Brockmeyer, A.; Janning, P.; Hyman, A.; et al. Natural product-inspired cascade synthesis yields modulators of centrosome integrity. Nat. Chem. Biol. 2012, 8, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Dakas, P.Y.; Parga, J.A.; Hoing, S.; Scholer, H.R.; Sterneckert, J.; Kumar, K.; Waldmann, H. Discovery of neuritogenic compound classes inspired by natural products. Angew. Chem. Int. Ed. 2013, 52, 9576–9581. [Google Scholar] [CrossRef] [PubMed]
- Eschenbrenner-Lux, V.; Kuchler, P.; Ziegler, S.; Kumar, K.; Waldmann, H. An enantioselective inverse-electron-demand imino diels-alder reaction. Angew. Chem. Int. Ed. 2014, 53, 2134–2137. [Google Scholar] [CrossRef] [PubMed]
- Schroder, P.; Forster, T.; Kleine, S.; Becker, C.; Richters, A.; Ziegler, S.; Rauh, D.; Kumar, K.; Waldmann, H. Neuritogenic militarinone-inspired 4-hydroxypyridones target the stress pathway kinase map4k4. Angew. Chem. Int. Ed. 2015, 54, 12398–12403. [Google Scholar] [CrossRef] [PubMed]
- Svenda, J.; Sheremet, M.; Kremer, L.; Maier, L.; Bauer, J.O.; Strohmann, C.; Ziegler, S.; Kumar, K.; Waldmann, H. Biology-oriented synthesis of a withanolide-inspired compound collection reveals novel modulators of hedgehog signaling. Angew. Chem. Int. Ed. 2015, 54, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Schroder, P.; Bauer, J.O.; Strohmann, C.; Kumar, K.; Waldmann, H. Synthesis of an iridoid-inspired compound collection and discovery of autophagy inhibitors. J. Org. Chem. 2016, 81, 10242–10255. [Google Scholar] [CrossRef] [PubMed]
- Laraia, L.; Ohsawa, K.; Konstantinidis, G.; Robke, L.; Wu, Y.W.; Kumar, K.; Waldmann, H. Discovery of novel cinchona-alkaloid-inspired oxazatwistane autophagy inhibitors. Angew. Chem. Int. Ed. 2017, 56, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Patil, S.; Golz, C.; Strohmann, C.; Ziegler, S.; Kumar, K.; Waldmann, H. A ligand-directed divergent catalytic approach to establish structural and functional scaffold diversity. Nat. Commun. 2017, 8, 14043. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F. Escape from flatland 2: Complexity and promiscuity. Medchemcomm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Schuffenhauer, A.; Brown, N.; Selzer, P.; Ertl, P.; Jacoby, E. Relationships between molecular complexity, biological activity, and structural diversity. J. Chem. Inf. Model. 2006, 46, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Selzer, P.; Roth, H.M.; Ertl, P.; Schuffenhauer, A. Complex molecules: Do they add value? Curr. Opin. Chem. Biol. 2005, 9, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Tsujii, E.; Abe, F.; Nakanishi, T.; Yamashita, M.; Shigematsu, N.; Izumi, S.; Okuhara, M. Fr901483, a novel immunosuppressant isolated from cladobotryum sp no 11231—Taxonomy of the producing organism, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 1996, 49, 37–44. [Google Scholar] [CrossRef]
- Diaba, F.; Bonjoch, J. Asymmetric synthesis of 2-azabicyclo[3.3.1]nonanes by a microwave-assisted organocatalysed tandem desymmetrisation and intramolecular aldolisation. Org. Biomol. Chem. 2009, 7, 2517–2519. [Google Scholar] [CrossRef] [PubMed]
- Comins, D.L.; Hong, H. Chiral dihydropyridones as synthetic intermediates—Asymmetric-synthesis of (+)-elaeokanine-a and (+)-elaeokanine-c. J. Am. Chem. Soc. 1991, 113, 6672–6673. [Google Scholar] [CrossRef]
- McGill, J.M.; LaBell, E.S.; Williams, M. Hydride reagents for stereoselective reductive amination. An improved preparation of 3-endo-tropanamine. Tetrahedron Lett. 1996, 37, 3977–3980. [Google Scholar] [CrossRef]
- Ishida, H.; Kimura, S.; Kogure, N.; Kitajima, M.; Takayama, H. The first asymmetric total synthesis of lycoposerramine-r. Org. Biomol. Chem. 2015, 13, 7762–7771. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Kimura, S.; Kogure, N.; Kitajima, M.; Takayama, H. Total synthesis of (+/−)-lycoposerramine-r, a novel skeletal type of lycopodium alkaloid. Tetrahedron 2015, 71, 51–56. [Google Scholar] [CrossRef]
- Takayama, H.; Katakawa, K.; Kitajima, M.; Yamaguchi, K.; Aimi, N. Seven new lycopodium alkaloids, lycoposerramines-c, -d, -e, -p, -q, -s, and -u, from lycopodium serratum thunb. Tetrahedron Lett. 2002, 43, 8307–8311. [Google Scholar] [CrossRef]
- Takayama, H.; Katakawa, K.; Kitajima, M.; Seki, H.; Yamaguchi, K.; Aimi, N. A new type of lycopodium alkaloid, lycoposerramine-a, from lycopodium serratum thunb (vol 3, pg 4167, 2001). Org. Lett. 2002, 4, 1243. [Google Scholar] [CrossRef]
- Shimada, N.; Abe, Y.; Yokoshima, S.; Fukuyama, T. Total synthesis of (−)-lycoposerramine-s. Angew. Chem. Int. Ed. 2012, 51, 11824–11826. [Google Scholar] [CrossRef] [PubMed]
- Srihari, P.; Yaragorla, S.R.; Basu, D.; Chandrasekhar, S. Tris(pentafluorophenyl)borane-catalyzed synthesis of n-benzyl pyrrolidines. Synth. Stuttg. 2006, 2646–2648. [Google Scholar] [CrossRef]
- Quintard, A.; Rodriguez, J. Synergistic cu-amine catalysis for the enantioselective synthesis of chiral cyclohexenones. Chem. Commun. 2015, 51, 9523–9526. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, T. Price-focused analysis of commercially available building blocks for combinatorial library synthesis. ACS Comb. Sci. 2015, 17, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Allu, T.K.; Fara, D.C.; Rad, R.F.; Ostopovici, L.; Bologa, C.G. Lead-like, drug-like or “pub-like”: How different are they? J. Comp. Mol. Des. 2007, 21, 113–119. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



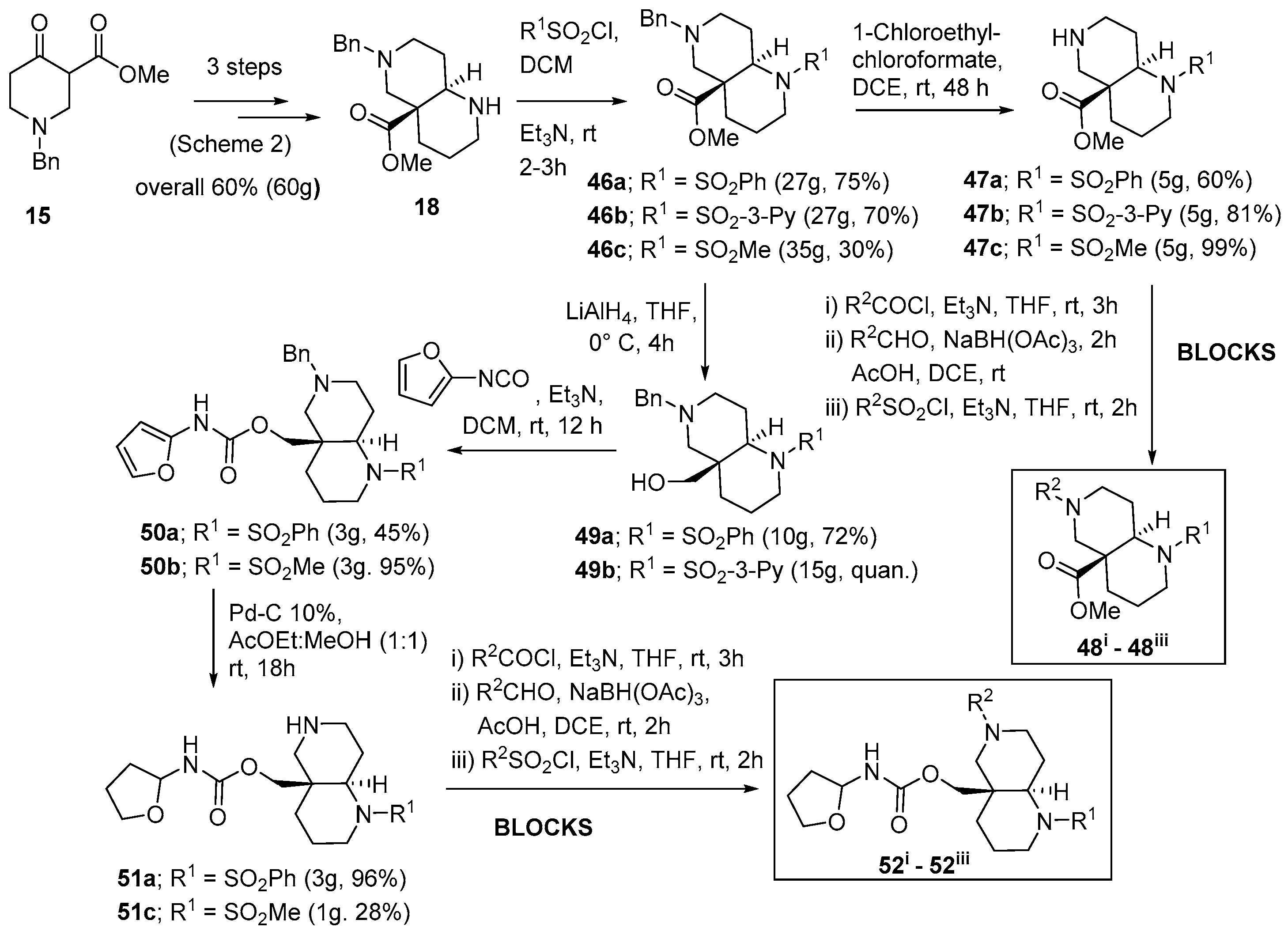


| Entry | Product | R1 | Reagent | Reaction Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 47a | SO2Ph | Pd/C 10% (0.3 Eqv.), H2 (1 atm) | 96 | 0 |
| 2 | 47a | SO2Ph | 1-Cholorethylchloroformate (2.5 Eqv.) | 96 | 58 |
| 3 | 47b | SO2-3-Py | 1-Cholorethylchloroformate (2.5 Eqv.) | 48 | 81 |
| 4 | 47c | SO2Me | Pd/C 10% (0.3 Eqv.), H2 (1 atm) | 48 | 0 |
| 5 | 47c | SO2Me | 1-Cholorethylchloroformate (2.5 Eqv.) | 48 | 99 |
| 6 | 51a | SO2-Ph | Pd/C 10% (0.3 Eqv.), H2 (1 atm) | 48 | 96 |
| 7 | 51c | SO2Me | Pd/C 10% (0.3 Eqv.), H2 (1 atm) | 96 | 28 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annamalai, M.; Hristeva, S.; Bielska, M.; Ortega, R.; Kumar, K. Highly Stereoselective Synthesis of a Compound Collection Based on the Bicyclic Scaffolds of Natural Products. Molecules 2017, 22, 827. https://doi.org/10.3390/molecules22050827
Annamalai M, Hristeva S, Bielska M, Ortega R, Kumar K. Highly Stereoselective Synthesis of a Compound Collection Based on the Bicyclic Scaffolds of Natural Products. Molecules. 2017; 22(5):827. https://doi.org/10.3390/molecules22050827
Chicago/Turabian StyleAnnamalai, Murali, Stanimira Hristeva, Martyna Bielska, Raquel Ortega, and Kamal Kumar. 2017. "Highly Stereoselective Synthesis of a Compound Collection Based on the Bicyclic Scaffolds of Natural Products" Molecules 22, no. 5: 827. https://doi.org/10.3390/molecules22050827
APA StyleAnnamalai, M., Hristeva, S., Bielska, M., Ortega, R., & Kumar, K. (2017). Highly Stereoselective Synthesis of a Compound Collection Based on the Bicyclic Scaffolds of Natural Products. Molecules, 22(5), 827. https://doi.org/10.3390/molecules22050827







