Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases
Abstract
:1. Introduction
2. Polyphenols as the Main Bioactive Compounds of Chokeberry Fruit
2.1. Phenolic Acids of Chokeberry Fruit
2.2. Flavonoids of Chokeberry Fruit
2.2.1. Anthocyanins
2.2.2. Flavonols and Flavanols, Proanthocyanins
2.2.3. Bioavailability of Black Chokeberry Polyphenols
3. Antioxidant Activity of Chokeberry Fruit
4. Chokeberry Fruit and Its Health-Promoting Activity
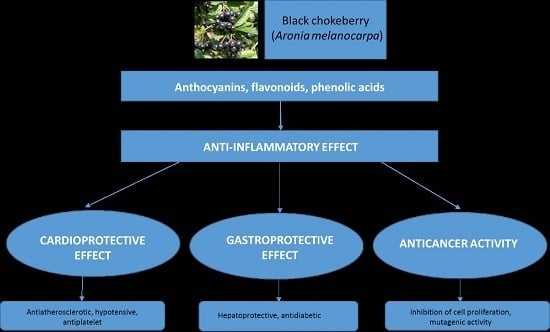
4.1. Anti-Inflammatory Effect of Chokeberry Fruit as a Base in the Prevention of Chronic Diseases
4.2. Gastroprotective and Antidiabetic Effect of Chokeberry Fruit
4.3. Cardioprotective Effect of Chokeberry Fruit
4.4. Anticancer Effect of Chokeberry Fruit
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Jurikova, T.; Pohanka, M.; Skutkova, H.; Baron, M.; Tomaskova, L.; Balla, S.; Klejdus, B.; Pokluda, R.; Mlcek, J.; et al. Evaluation of antioxidant activity, polyphenolic compounds, amino acids and mineral elements of representative genotypes of Lonicera edulis. Molecules 2014, 19, 6504–6523. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlček, J.; Balla, Š.; Szekeres, L.; Žitný, R.; Zitka, O.; Adam, V.; Kizek, R.; et al. Evaluation of polyphenolic profile and nutritional value of non-traditional fruit species in the czech republic-a comparative study. Molecules 2012, 17, 8968–8981. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, Z.; Reznicek, V.; Adam, V.; Zitka, O.; Jurikova, T.; Krska, B.; Matuskovic, J.; Plsek, J.; Saloun, J.; Horna, A.; et al. Use of liquid chromatography with electrochemical detection for the determination of antioxidants in less common fruits. Molecules 2008, 13, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar] [CrossRef] [PubMed]
- Lavola, A.; Karjalainen, R.; Julkunen-Tiitto, R. Bioactive polyphenols in leaves, stems, and berries of saskatoon (Amelanchier alnifolia nutt.) cultivars. J. Agric. Food Chem. 2012, 60, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Kapci, B.; Neradová, E.; Čížková, H.; Voldřich, M.; Rajchl, A.; Capanoglu, E. Investigating the antioxidant potential of chokeberry (Aronia melanocarpa) products. J. Food Nutr. Res. 2013, 52, 219–229. [Google Scholar]
- Vagiri, M.; Jensen, M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.T.; Krbavčić, I.P.; Vujević, P.; Milinović, B.; Jurčević, I.L.; Vahčić, N. Effects of Weather Conditions on Phenolic Content and Antioxidant Capacity in Juice of Chokeberries (Aronia melanocarpa L.). Pol. J. Food Nutr. Sci. 2017, 67, 67–74. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (poly) phenols and cardiovascular health. J. Agric. Food Chem. 2013, 62, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, G.S.; Park, S.; Kim, Y.H.; Kim, M.B.; Lee, W.S.; Shin, S.C. Determination of chokeberry (Aronia melanocarpa) polyphenol components using liquid chromatography–tandem mass spectrometry: Overall contribution to antioxidant activity. Food Chem. 2014, 146, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest date affects Aronia juice polyphenols, sugars, and antioxidant activity, but not anthocyanin stability. Food Chem. 2015, 187, 189–196. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crops Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2013, 62, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Krivak, P. The influence of interactions among phenolic compounds on the antiradical activity of chokeberries (Aronia melanocarpa). Int. J. Food Sci. Nutr. 2011, 62, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and Their Products as a Possible Means for the Prevention and Treatment of Noncommunicable Diseases and Unfavorable Health Effects Due to Exposure to Xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, D.; Podsędek, A.; Kucharska, A.Z.; Redzynia, M.; Opęchowska, M.; Koziołkiewicz, M. Comparison of in vitro anti-lipase and antioxidant activities, and composition of commercial chokeberry juices. Eur. Food Res. Technol. 2016, 242, 505–515. [Google Scholar] [CrossRef]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, 28–34. [Google Scholar]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. Ingestion of Black Chokeberry Fruit Extract Leads to Intestinal and Systemic Changes in a Rat Model of Prediabetes and Hyperlipidemia. Plant. Foods Hum. Nutr. 2008, 63, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Marazova, K.; Krasnaliev, I.; Galunska, B.; Borisova, P.; Belcheva, A. Effect of Aronia melanocarpa fruit juice on indomethacin-induced gastric mucosal damage and oxidative stress in rats. Exp. Toxicol. Pathol. 2005, 56, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Ciocoiu, M.; Badescu, M.; Badulescu, O.; Tutunaru, D.; Badescu, L. Polyphenolic extract association with renin inhibitors in experimental arterial hypertension. J. Biomed. Sci. Eng. 2013, 6, 493–497. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2005, 48, 11–17. [Google Scholar]
- Kowalczyk, E.; Charyk, K.; Fijalkowski, P.; Niedworok, J.; Blaszczyk, J.; Kowalski, J. Protective influence of natural anthocyanins of Aronia melanocarpa on selected parameters of antioxidative status in experimental intoxication with sulphide-2-chloroethyl-3-chloropropyl. Pol. J. Environ. Stud. 2004, 13, 339–341. [Google Scholar]
- Howard, L.R.; Brownmiller, C.; Prior, R.L.; Mauromoustakos, A. Improved Stability of Chokeberry Juice Anthocyanins by β-Cyclodextrin Addition and Refrigeration. J. Agric. Food Chem. 2013, 61, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar]
- Sikora, J.; Broncel, M.; Markowicz, M.; Chałubiński, M.; Wojdan, K.; Mikiciuk-Olasik, E. Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. Eur. J. Nutr. 2012, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, J.; Sadowska, K.; Klóska, L.; Rogowski, L. The effect of plant age and harvest time on the content of chosen components and antioxidative potential of black chokeberry fruit. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Janick, J.; Paull, R.E. Encyclopedia of Fruit and Nuts, 1st ed.; CABI: Wallingford, UK, 2008; pp. 622–624. [Google Scholar]
- Brand, M. Aronia: Native shrubs with untapped potential. Arnoldia 2010, 67, 14–25. [Google Scholar]
- Ochmian, I.; Grajkowski, J.; Smolik, M. Comparison of Some Morphological Features, Quality and Chemical Content of Four Cultivars of Chokeberry Fruits (Aronia melanocarpa). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 253–260. [Google Scholar] [CrossRef]
- González-Molina, E.; Moreno, D.A.; García-Viguera, C. Aronia-enriched lemon juice: A new highly antioxidant beverage. J. Agric. Food Chem. 2008, 56, 11327–11333. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, D.; Ludneva, D. Possibilities for production of nectars and purees from fruits of black chokeberry (Aronia melanocarpa). Acta Horticult. 2009, 825, 595–597. [Google Scholar] [CrossRef]
- Pogorzelski, E.; Wilkowska, A.; Kobus, M. Inhibiting effect of tannin in chokeberry must on the winemaking process. Pol. J. Food Nutr. Sci. 2006, 15, 49–53. [Google Scholar]
- Balcerek, M. Carbonyl compounds in Aronia spirits. Pol. J. Food Nutr. Sci. 2010, 60, 243–249. [Google Scholar]
- Mayer-Miebach, E.; Adamiuk, M.; Behsnilian, D. Stability of chokeberry bioactive polyphenols during juice processing and stabilization of a polyphenol-rich material from the by-product. Agriculture 2012, 2, 244–258. [Google Scholar] [CrossRef]
- Kmiecik, W.; Lisiewska, Z.; Jaworska, G. Effect of Aronia berry honey syrup used for sweetening jams on their quality. Mol. Nutr. Food Res. 2001, 45, 273–279. [Google Scholar] [CrossRef]
- Kraemer-Schafhalter, A.; Fuchs, H.; Pfannhauser, W. Solid-phase extraction (SPE)—A comparison of 16 materials for the purification of anthocyanins from Aronia melanocarpa var Nero. J. Sci. Food Agric. 1998, 78, 435–440. [Google Scholar] [CrossRef]
- Zeković, Z.; Pavlić, B.; Ramić, M.; Vladić, J.; Cvejin, A.; Vidović, S. Optimization of ultrasound-assisted extraction of anthocyanins from Aronia melanocarpa by-product from filter-tea factory. Ann. Fac. Eng. Hunedoara-Int. J. Eng. 2014, 12, 185–188. [Google Scholar]
- D’Alessandro, L.G.; Dimitrov, K.; Vauchel, P.; Nikov, I. Kinetics of ultrasound assisted extraction of anthocyanins from Aronia melanocarpa (black chokeberry) wastes. Chem. Eng. Res. Des. 2014, 92, 1818–1826. [Google Scholar] [CrossRef]
- D’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Jakobek, L.; Drenjančević, M.; Jukić, V.; Šeruga, M. Phenolic acids, flavonols, anthocyanins and antiradical activity of “Nero”,“Viking”,“Galicianka” and wild chokeberries. Sci. Horticult. 2012, 147, 56–63. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agric. Consp. Sci. 2007, 72, 301–306. [Google Scholar]
- Rop, O.; Mlcek, J.; Jurikova, T.; Valsikova, M.; Sochor, J.; Reznicek, V.; Kramarova, D. Phenolic content, antioxidant capacity, radical oxygen species scavenging and lipid peroxidation inhibiting activities of extracts of five black chokeberry (Aronia melanocarpa (michx.) elliot) cultivars. J. Med. Plants Res. 2010, 22, 2432–2437. [Google Scholar]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, 164–169. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Yoon, W.B.; Lee, O.-H.; Cha, S.J.; Dai Kim, J. Radical-scavenging-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in Korea. Food Chem. 2014, 146, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar]
- Wangensteen, H.; Bräunlich, M.; Nikolic, V.; Malterud, K.E.; Slimestad, R.; Barsett, H. Anthocyanins, proanthocyanidins and total phenolics in four cultivars of Aronia: Antioxidant and enzyme inhibitory effects. J. Funct. Foods 2014, 7, 746–752. [Google Scholar] [CrossRef]
- Jeppsson, N.; Johansson, R. Changes in fruit quality in black chokeberry (Aronia melanocarpa) during maturation. J. Hortic. Sci. Biotechnol. 2000, 75, 34–67. [Google Scholar] [CrossRef]
- Misiak, M.; Irzyniec, Z. Effect of the temperature and storage time on polyphenol content and antioxidant properties of freeze-dried chokeberries. Zesz. Nauk. Pol. Łódź Chem. Spoż Biotechnol. 2009, 73, 73–81. [Google Scholar]
- Oszmiański, J.; Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Sainova, I.V.; Pavlova, V.G.; Alexieva, B.S.; Valkova, I.P.; Ilieva, I.N.; Nikolova, E.B. Attenuation of cellular oxidative stress by natural products and plant extracts after chemotherapeutic exposure. J. Biosci. Biotechnol. 2015, 1–7. [Google Scholar]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, L.G.; Vauchel, P.; Przybylski, R.; Chataigné, G.; Nikov, I.; Dimitrov, K. Integrated process extraction–adsorption for selective recovery of antioxidant phenolics from Aronia melanocarpa berries. Sep. Purif. Technol. 2013, 120, 92–101. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Lech, K. The influence of different the drying methods on chemical composition and antioxidant activity in chokeberries. LWT-Food Sci. Technol. 2016, 66, 484–489. [Google Scholar] [CrossRef]
- Šavikin, K.; Zdunić, G.; Janković, T.; Gođevac, D.; Stanojković, T.; Pljevljakušić, D. Berry fruit teas: Phenolic composition and cytotoxic activity. Food Res. Int. 2014, 62, 677–683. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Hirth, M.; Preiß, R.; Mayer-Miebach, E.; Schuchmann, H.P. Influence of HTST extrusion cooking process parameters on the stability of anthocyanins, procyanidins and hydroxycinnamic acids as the main bioactive chokeberry polyphenols. LWT-Food Sci. Technol. 2015, 62, 511–516. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmiański, J.; Skupień, K. Chemical composition, phenolics, and firmness of small black fruits. J. Appl. Bot. Food Qual. 2009, 83, 64–69. [Google Scholar]
- Hakkinen, S.; Heinonen, M.; Karenlampi, S.; Mykkanen, H.; Ruuskanen, J.; Torronen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Kovačević, D.B.; Kljusurić, J.G.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Cretu, G.C.; Morlock, G.E. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem. 2014, 146, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Comp. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Baloghova, M.; Paulovicsova, B.; Turianica, I. Biological characteristics of selected berries. Acta Hort. Regio 2009, 12, 49–52. [Google Scholar]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Park, H.-M.; Hong, J.-H. Physiological activities of Aronia melanocarpa extracts on extraction solvents. Korean J. Food Preserv. 2014, 21, 718–726. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Gilewicz-Lukasik, B.; Koter, S.; Kurzawa, J. Concentration of anthocyanins by the membrane filtration. Sep. Purif. Technol. 2007, 57, 418–424. [Google Scholar] [CrossRef]
- Yong, L.H. Effect of Storage Conditions on the Stability of the Extracts from Aronia melanocarpa Elliot. Res. J. Biotechnol. 2016, 11, 38–45. [Google Scholar]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Niedworok, J.; Brzozowski, F. Badania nad biologicznymi i fitoterapeutycznymi właściwościami antocyjanin aronii czarnoowocowej E. Post. Fitot. 2001, 5, 20–24. [Google Scholar]
- Sueiro, L.; Yousef, G.G.; Seigler, D.; De Mejia, E.G.; Grace, M.H.; Lila, M.A. Chemopreventive Potential of Flavonoid Extracts from Plantation-Bred and Wild Aronia melanocarpa (Black Chokeberry) Fruits. J. Food Sci. 2006, 71, 480–488. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and In vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; R´em´esy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [PubMed]
- Talavera, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Rem´esy, C. Anthocyanins and efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [PubMed]
- Kay, C.D.; Mazza, G.; Holub, B.J. Anthocyanins exist in the circulation primarily as metabolites in adult men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [PubMed]
- Felgines, C.; Texier, O.; Garcin, P.; Besson, C.; Lamaison, J.L.; Scalbert, A. Tissue distribution of anthocyanins in rats fed a blackberry anthocyanin-enriched diet. Mol. Nutr. Food Res. 2009, 53, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Wolforth, J.; McNish, R.; Kaufman, P.B.; Bolling, S.F. Tissue bioavailability of anthocyanins from whole tart cherry in healthy rats. Food Chem. 2015, 171, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lala, N.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia. melanocarpa) juice and dietary-relevant dose of anthocyanins in humans. J. Agric. Food Chem. 2010, 58, 12130–12136. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Anighoro, A.; Quartieri, A.; Amaretti, A.; Tomas-Barber´an, F.A.; Rastelli, G.; Rossi, M. Role of bifidobacteria in the hydrolysis of chlorogenic acid. Microbiologyopen 2015, 4, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barber´an, F.; Garcia-Villabla, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Liu, J.; Ren, X.; Zhang, J.; Zhou, S.; Xu, X.P. Pharmacokinetics and excretion of chlorogenic acid in beagle dogs. J. Pharm. Sci. 2008, 63, 520–524.
- Sochor, J.; Ryvolova, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully automated spectrometric protocols for determination of antioxidant activity: Advantages and disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, M.; Olas, B.; Wachowicz, B.; Glowacki, R.; Bald, E.; Czernek, U.; Szydlowska-Pazera, K.; Potemski, P.; Piekarski, J.; Jeziorski, A.; et al. Effects of the commercial extract of Aronia on oxidative stress in blood platelets isolated from breast cancer patients after the surgery and various phases of the chemotherapy. Fitoterapia 2012, 83, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, J.; Babicz, K.; Olas, B.; Stochmal, A.; Oleszek, W. Aronia melanocarpa extract suppresses the biotoxicity of homocysteine and its metabolite on the hemostatic activity of fibrinogen and plasma. Nutrition 2012, 28, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Muszalska, A.; Kopka, J.; Kontek, B. Polyphenols from berries of Aronia melanocarpa reduce the plasma lipid peroxidation induced by ziprasidone. Schizoph. Res. Treat. 2014, 2014, 13–18. [Google Scholar]
- Tolić, M.-T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic content, antioxidant capacity and quality of chokeberry (Aronia melanocarpa) products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rugină, D.; Sconţa, Z.; Leopold, L.; Pintea, A.; Bunea, A.; Socaciu, C. Antioxidant activities of chokeberry extracts and the cytotoxic action of their anthocyanin fraction on hela human cervical tumor cells. J. Med. Food 2012, 15, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Bomser, J.A.; Schwartz, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J. Agric. Food Chem. 2008, 56, 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.; Bräunlich, M.; Austarheim, I.; Wangensteen, H.; Malterud, K.E.; Slimestad, R.; Barsett, H. Immunomodulating activity of Aronia melanocarpa polyphenols. Int. J. Mol. Sci. 2014, 15, 11626–11636. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mojsoska, B. The immunomodulation effect of Aronia extract lacks association with its antioxidant anthocyanins. J. Med. Food 2013, 16, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Nowicka, G.; Sygitowicz, G.; Jarosz, M. Anthocyanin-rich aronox extract from Aronia melanocarpa protects against 7beta-hydroxycholesterol-induced apoptosis of endothelial cells. Ann. Nutr. Metab. 2008, 53, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-κB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.A.; Taheri, R.; Brand, M.H.; Draghi, A.; Sylvester, F.A.; Bolling, B.W. Anti-inflammatory activity of Aronia berry extracts in murine splenocytes. J. Funct. Foods 2014, 8, 68–75. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [PubMed]
- Liepiņa, I.; Nikolajeva, V.; Jākobsone, I. Antimicrobial activity of extracts from fruits of Aronia melanocarpa and Sorbus aucuparia. Environ. Exp. Biol. 2013, 11, 195–199. [Google Scholar]
- Bräunlich, M.; Økstad, O.; Slimestad, R.; Wangensteen, H.; Malterud, K.; Barsett, H. Effects of Aronia melanocarpa constituents on biofilm formation of Escherichia coli and Bacillus cereus. Molecules 2013, 18, 14989–14999. [Google Scholar] [CrossRef] [PubMed]
- Handeland, M.; Grude, N.; Torp, T.; Slimestad, R. Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term-a pilot study. Nutr. Res. 2014, 34, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.-W.; Bae, J.-Y.; Heo, J.; Kim, D.; Han, S.-Z.; Park, M.-S.; et al. Aronia melanocarpa and its components demonstrate antiviral activity against influenza viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of cAMP-PKA-dependent signalling pathway. Free Radic. Biol. Med. 2012, 52, 314–327. [Google Scholar]
- De Lira Mota, K.S.; Nunes Dias, G.E.; Ferreira Pinto, M.E.; Luiz-Ferreira, A.; Monteiro Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with Gastroprotective Activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef] [PubMed]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkowicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa l.). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Qin, B.; Anderson, R.A. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Med. 2002, 44, 20–23. [Google Scholar]
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Meth. Find. Exp. Clin. Pharm. 2007, 29, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Broncel, M.; Mikiciuk-Olasik, E. Aronia melanocarpa elliot reduces the activity of angiotensin i-converting enzyme-in vitro and ex vivo studies. Oxid. Med. Cell. Longev. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dragan, S.; Andrica, F.; Serban, M.-C.; Timar, R. Polyphenols-rich natural products for treatment of diabetes. Curr. Med. Chem. 2015, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Skoczyñska, A.; Jêdrychowska, I.; Porêba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Kowalczyk, E.; Fijalkowski, P.; Kura, M.; Krzesinski, P.; Blaszczyk, J.; Kowalski, J.; Smigielski, J.; Rutkowski, M.; Kopff, M. The influence of anthocyanins from Aronia melanocarpa on selected parameters of oxidative stress and microelements contents in men with hypercholesterolemia. Pol. Merkur Lekarski 2005, 19, 651–653. [Google Scholar] [PubMed]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Bryk, D.; Małecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Aronia melanocarpa fruit extract exhibits anti-inflammatory activity in human aortic endothelial cells. Eur. J. Nutr. 2012, 51, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnaliev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant Foods Hum. Nutr. 2007, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Konić-Ristić, A.; Šavikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of polyphenol-rich chokeberry juice on antioxidant/pro-oxidant status in healthy subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Bobrowski, M.; Borowiecka, M.; Podsędek, A.; Golański, J.; Nowak, P. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia 2011, 82, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Wachowicz, B.; Nowak, P.; Kedzierska, M.; Tomczak, A.; Stochma, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J. Studies on antioxidant properties of polyphenol-rich extract from berries of Aronia melanocarpa in blood platelets. Acta Physiol. Pol. 2008, 59, 823–835. [Google Scholar]
- Ryszawa, N.; Kawczynska-Drozdz, A.; Pryjma, J.; Czesnikiewicz-Guzik, M.; Adamek-Guzik, T.; Naruszewicz, M.; Korbut, R.; Guzik, T.J. Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. J. Physiol. Pharmacol. 2006, 57, 611–626. [Google Scholar] [PubMed]
- Daskalova, E.; Delchev, S.; Peeva, Y.; Vladimirova-Kitova, L.; Kratchanova, M.; Kratchanov, C.; Denev, P. Antiatherogenic and cardioprotective effects of black chokeberry (Aronia melanocarpa) juice in aging rats. Evid. Based Complement. Alternat. Med. 2015, 2015, 717439. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Burt, T.D. Phenolic acids contained in anthocyanin enriched extracts from elderberry, bilberry and chokeberry possess endothelium dependent and independent vasorelaxation properties in porcine coronary arteries. FASEB J. 2007, 21, A366. [Google Scholar]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Auger, C.; Kurita, I.; Anselm, E.; Rivoarilala, L.O.; Lee, H.J.; Lee, K.W.; Schini-Kerth, V.B. Aronia. melanocarpa juice, a rich source of polyphenols, induces endothelium-dependent relaxations in porcine coronary arteries via the redox-sensitive activation of endothelial nitric oxide synthase. Nitr. Oxide. 2013, 35, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ciocoiu, M.; Badescu, L.; Miron, A.; Badescu, M. The involvement of a polyphenol-rich extract of black chokeberry in oxidative stress on experimental arterial hypertension. Evid.-Based Complet. Altern. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ga̧siorowski, K.; Szyba, K.; Brokos, B.; Oszmiański, J. Antimutagenic activity of anthocyanins isolated from Aronia melanocarpa fruits. Cancer Lett. 1997, 119, 37–46. [Google Scholar] [CrossRef]
- Olas, B.; Kedzierska, M.; Wachowicz, B.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J.; Glowacki, R. Effect of Aronia on thiol levels in plasma of breast cancer patients. Open Life Sci. 2010, 5, 38–46. [Google Scholar] [CrossRef]
- Sharif, T.; Stambouli, M.; Burrus, B.; Emhemmed, F.; Dandache, I.; Auger, C.; Etienne-Selloum, N.; Schini-Kerth, V.B.; Fuhrmann, G. The polyphenolic-rich Aronia melanocarpa juice kills teratocarcinomal cancer stem-like cells, but not their differentiated counterparts. J. Funct. Foods 2013, 5, 1244–1252. [Google Scholar] [CrossRef]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa e. Induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 5, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Mallery, S.R.; Stoner, G.D. Black raspberries in cancer clinical trials: Past, present and future. J. Berry Res. 2016, 6, 251–261. [Google Scholar] [CrossRef] [PubMed]
| Effect | Mechanism of Action | Study Model | Sources |
|---|---|---|---|
| Anti-inflammatory | Inhibition of release IL-6,IL-8,IL-10 TNF-α Activation NF-κB, cytokines, PGE2 | Human monocytes, mouse macrophage cells, murine splenocytes | [102,104,105] |
| Inhibition on nitric oxide production | Mouse macrophage | [102] | |
| Antidiabetic | Reduction of gastric mucosal demage | Wister rat model | [22] |
| Inhibition of α-glucosidase, lipase and amylase | In vitro model studies | [115,117] | |
| Modulation of multiple pathways associated with insulin signaling | Rats on fructose-rich diet, in vivo, in vitro studies | [118,119] | |
| Restoring of beta cells integrity | In vivo models | [116,120,121] | |
| Cardioprotective | Lipid metabolism–decrease of cholesterol synthesis | Caco-2-cells | [125] |
| -Inhibition of LDL oxidation, lipid peroxidation | Patients with metabolic syndrome | [20] | |
| -Increase activity of glutathione peroxidase and catalase, reduction of LDL cholesterol -Inhibition of platelet aggregation, protein carbonylation and thiol oxidation | Mens with hypercholesterolemia | [128] | |
| Anticoagulant, antithrombotic -Decrease in fibrin polymerization | Human plasma | [23,25,123] | |
| -Inhibition of platelet aggregation, protein carbonylation and thiol oxidation | Human plasma, purified fibrinogen | [95,120,129,130,131] | |
| Decrease blood pressure | |||
| -inhibition of angiotensis I-converting enzyme ACE | Patients with metabolic syndrome | [135] | |
| Protection of coronary arteries | |||
| Coronary vasoactive, vasoprotective effect | Caco-2-cells | ||
| Decrease oxidative stress—stimulation of endothelial formation of NO | Porcine coronary arteries | ||
| Anticancer | Inhibition of oxidation | Caco-2-cells line | [138] |
| Reduction of oxidative stress | Human granulocytes, HeLa cervical tumor line, murine leukemia cells, brest cancer patients | [94,98,130,139,141] | |
| Induction of apoptosis | Lymphoblastic leukemia Jurkat cell line | [141] | |
| Blockage at G1/G0 and G2/M phases of cell cycle | Human colon cancer | [142] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. https://doi.org/10.3390/molecules22060944
Jurikova T, Mlcek J, Skrovankova S, Sumczynski D, Sochor J, Hlavacova I, Snopek L, Orsavova J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules. 2017; 22(6):944. https://doi.org/10.3390/molecules22060944
Chicago/Turabian StyleJurikova, Tunde, Jiri Mlcek, Sona Skrovankova, Daniela Sumczynski, Jiri Sochor, Irena Hlavacova, Lukas Snopek, and Jana Orsavova. 2017. "Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases" Molecules 22, no. 6: 944. https://doi.org/10.3390/molecules22060944