Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition of LPPMs
2.2. Maillard-Type Characteristics of LPPMs during Dry-Heating
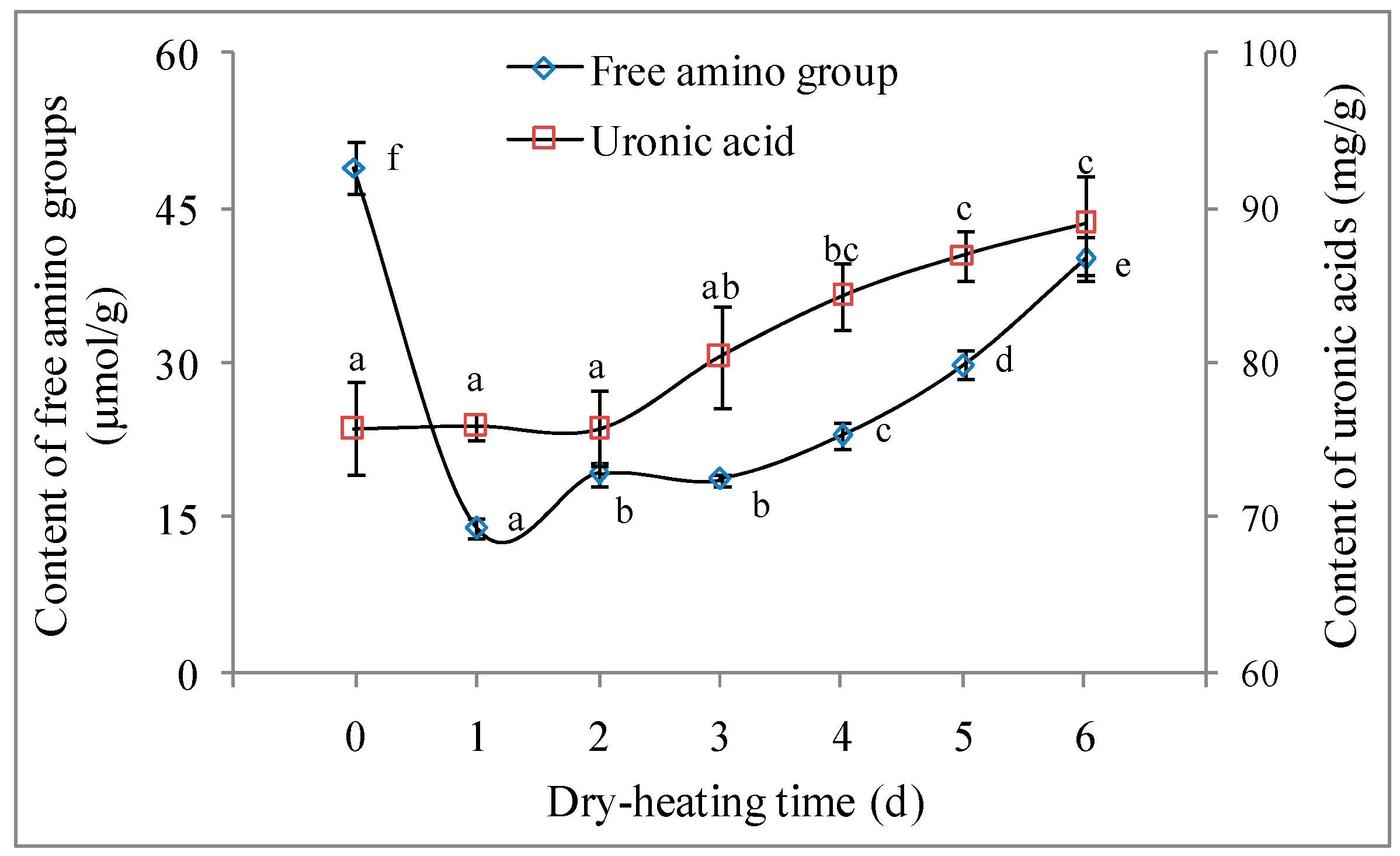
2.2.1. Free Amino Group Content and Uronic Acid Content
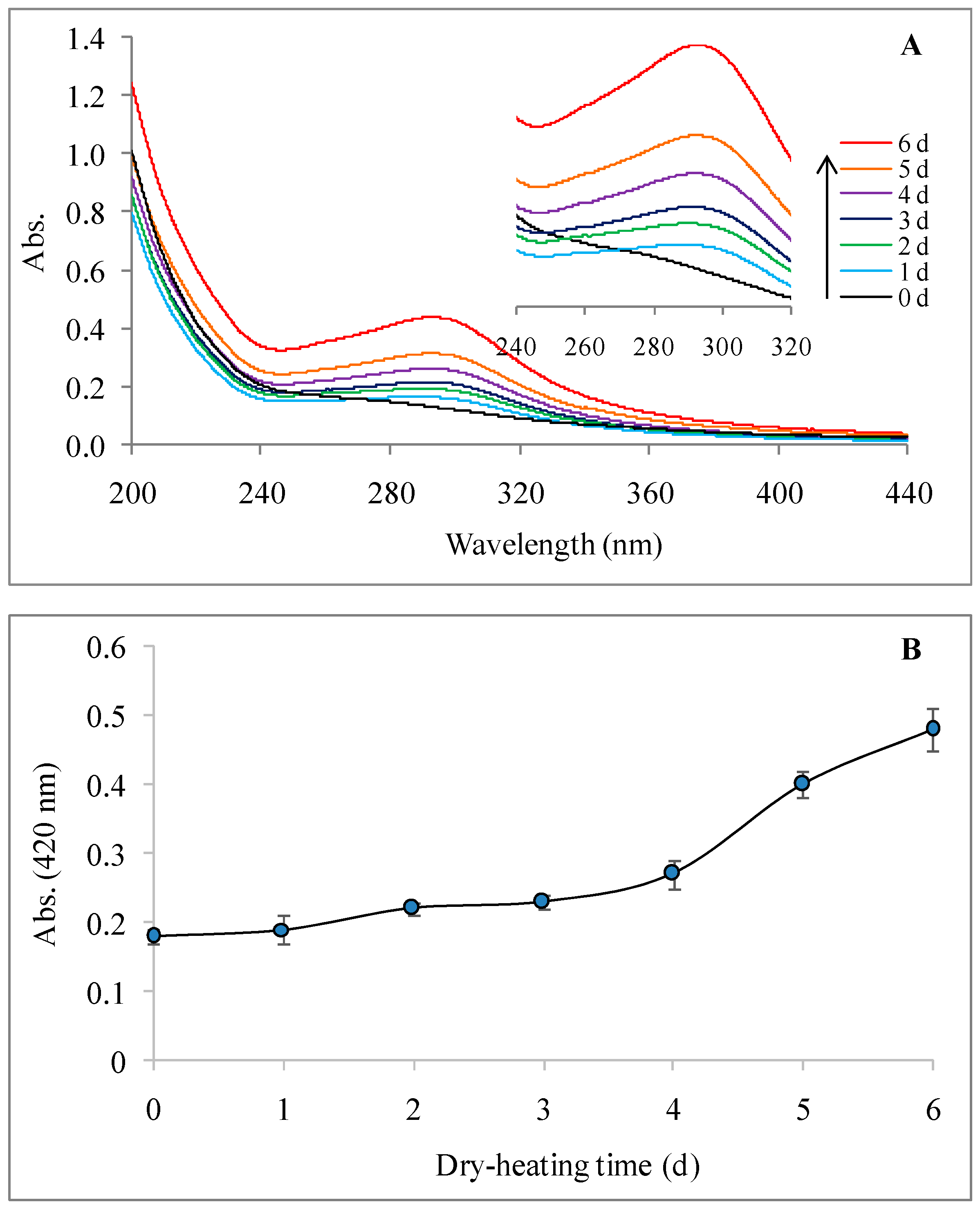
2.2.2. UV-Visible Spectrum
2.2.3. FTIR Spectrum
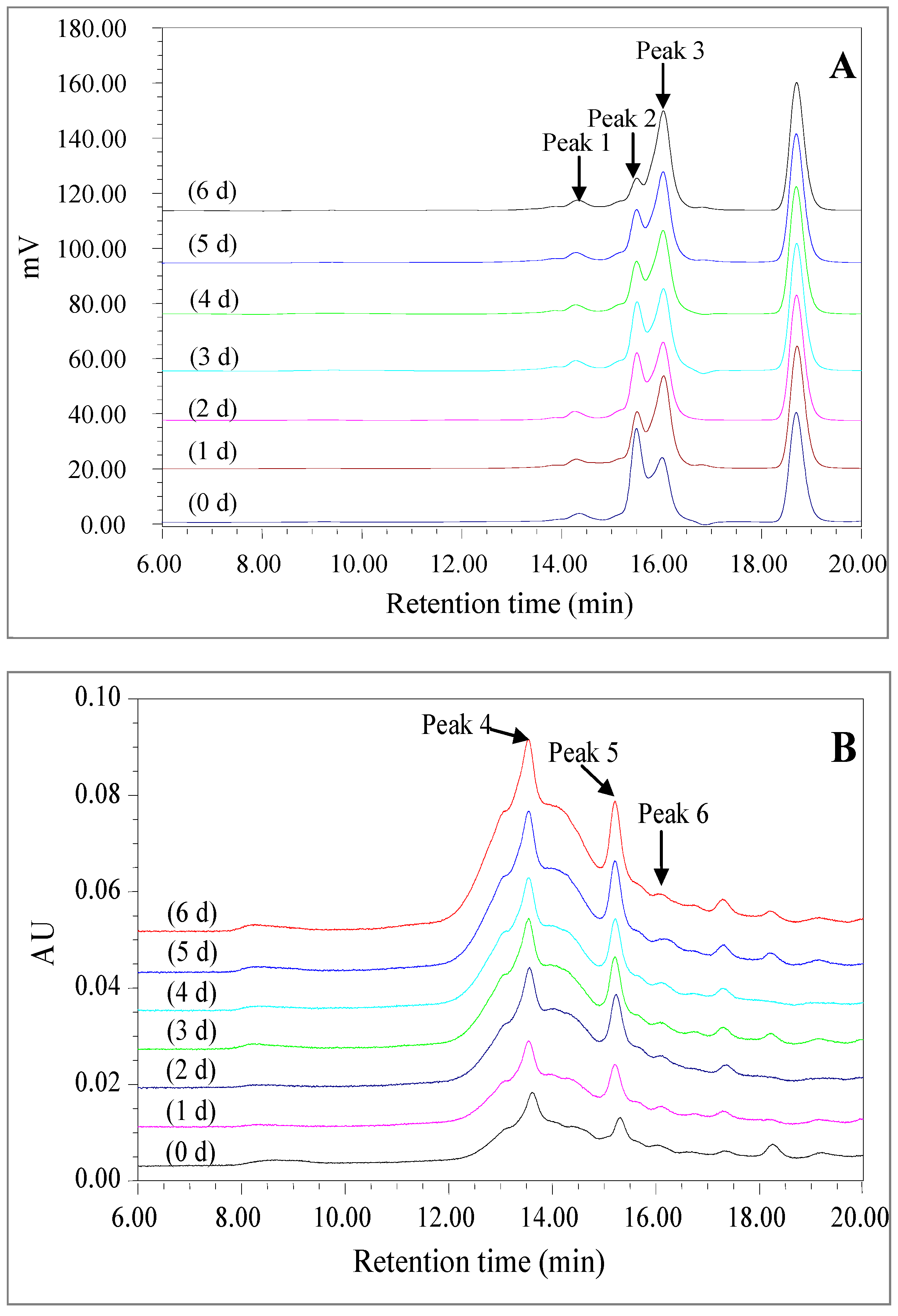
2.2.4. High Performance Size Exclusion Chromatogram
2.3. Effects of Dry-Heating on the Activities of LPPMs
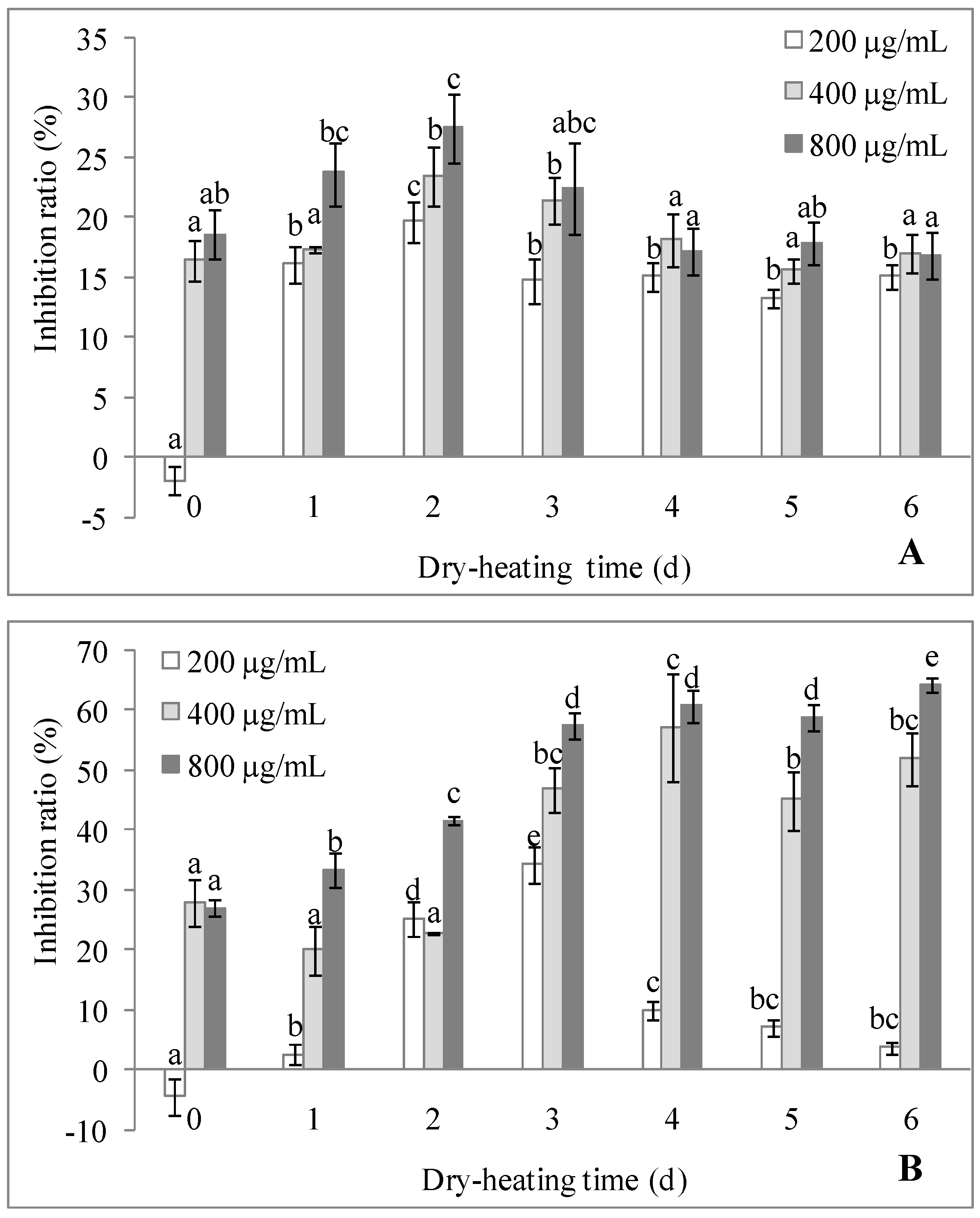
2.3.1. Antioxidant Activity
2.3.2. Antitumor Activity
2.3.3. Immunostimulating Activity
3. Materials and Methods
3.1. Materials, Chemicals and Cells
3.2. Preparation of LPPMs
3.3. Dry-Heating Treatment of LPPMs
3.4. Composition Analysis
3.5. UV-Visible Spectrophotometry
3.6. FTIR Analysis
3.7. HPSEC Analysis
3.8. Antioxidant Activity Evaluation
3.9. Antitumor Activity Evaluation
3.10. Immunostimulating Activity Evaluation
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, Y.; Zhang, Z.; Joyce, D.C.; Ketsa, S. Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biol. Technol. 2002, 26, 241–252. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Shi, J.; Chen, F.; Ashraf, M. Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit—A review. Food Res. Int. 2011, 44, 1837–1842. [Google Scholar] [CrossRef]
- Tippayawong, N.; Tantakitti, C.; Thavornun, S. Energy efficiency improvements in longan drying practice. Energy 2008, 33, 1137–1143. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Meng, F.-Y.; He, Z.; Ning, Y.-L.; Li, X.-H.; Song, H.; Wang, J.; Zhou, R. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Huang, F.; Zhang, M.-W.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; He, J.-R. Solution properties and in vitro anti-tumor activities of polysaccharides from longan pulp. Molecules 2013, 18, 11601–11613. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wang, H.; Zhang, R.; Min, T.; Huang, F.; Liu, L.; Zhang, M. Characterization of polysaccharide from longan pulp as the macrophage stimulator. RSC Adv. 2015, 5, 97163–97170. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q.; He, Y.; He, X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int. J. Biol. Macromol. 2010, 47, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Sun, J.; Huang, F.; Yi, Y.; Wang, H.-X.; Wang, L.-M. Effects of ultrasonic treatment on the physicochemical and immunomodulatory properties of polysaccharides from longan pulp. Mod. Food Sci. Technol. 2016, 32, 124–131. [Google Scholar]
- De Oliveira, F.C.; Coimbra, J.S.D.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Shin, W.-S. Characterisation of bovine serum albumin–fucoidan conjugates prepared via the Maillard reaction. Food Chem. 2015, 173, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kasran, M.; Cui, S.W.; Goff, H.D. Covalent attachment of fenugreek gum to soy whey protein isolate through natural Maillard reaction for improved emulsion stability. Food Hydrocoll. 2013, 30, 552–558. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S. Preparation of chemically modified canola protein isolate with gum arabic by means of Maillard reaction under wet-heating conditions. Carbohydr. Polym. 2017, 155, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Jiménez-Castaño, L.; Villamiel, M.; Martín-Álvarez, P.J.; Olano, A.; López-Fandiño, R. Effect of the dry-heating conditions on the glycosylation of β-lactoglobulin with dextran through the Maillard reaction. Food Hydrocoll. 2005, 19, 831–837. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant activities of different fractions of polysaccharide conjugates from green tea (Camellia Sinensis). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Chen, Z.S.; Tan, B.K.H.; Chan, S.H. Activation of t lymphocytes by polysaccharide–protein complex from Lycium barbarum L. Int. Immunopharmacol. 2008, 8, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Surenjav, U.; Zhang, L.; Xu, X.; Zhang, X.; Zeng, F. Effects of molecular structure on antitumor activities of (1→3)-β-d-glucans from different Lentinus edodes. Carbohydr. Polym. 2006, 63, 97–104. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.-E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2016, 239, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, X.; Jin, Y.; Ding, F.; Du, Y. Controllable antioxidative xylan–chitosan Maillard reaction products used for lipid food storage. Carbohydr. Polym. 2013, 91, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ju, Y.; Li, J.; Yu, M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yang, W.; Mariga, A.M.; Fang, Y.; Ma, N.; Pei, F.; Hu, Q. Purification, characterization and antitumor activity of polysaccharides from Pleurotus eryngii residue. Carbohydr. Polym. 2014, 114, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The Maillard Reaction of Xylo-Oligosaccharides and the Biological Activity of Its Derivatives; Shanghai Ocean University: Shanghai, China, 2015. [Google Scholar]
- Melo, E.P.; Aires-Barros, M.R.; Costa, S.M.B.; Cabral, J.M.S. Thermal unfolding of proteins at high pH range studied by UV absorbance. J. Biochem. Biophys. Methods 1997, 34, 45–59. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tschiape, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Wu, S.; Hu, J.; Wei, L.; Du, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014, 148, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, B.; Han, J.; Sun, C.; Li, P. Structure and antioxidant activity of whey protein isolate conjugated with glucose via the Maillard reaction under dry-heating conditions. Food Struct. 2014, 1, 145–154. [Google Scholar] [CrossRef]
- Gu, F.-L.; Kim, J.M.; Abbas, S.; Zhang, X.-M.; Xia, S.-Q.; Chen, Z.-X. Structure and antioxidant activity of high molecular weight maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Li, W.; Dong, F.; Guo, Z. Synthesis and antioxidant property of novel 1,2,3-triazole-linked starch derivatives via ‘click chemistry’. Int. J. Biol. Macromol. 2016, 82, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, B.; Özçiçek Pekmez, N.; Kılıç, E. Electrochemical synthesis of bilayer coatings of poly(N-methylaniline) and polypyrrole on mild steel and their corrosion protection performances. Electrochim. Acta 2011, 56, 9277–9286. [Google Scholar] [CrossRef]
- Ke, C.; Qiao, D.; Gan, D.; Sun, Y.; Ye, H.; Zeng, X. Antioxidant acitivity in vitro and in vivo of the capsule polysaccharides from Streptococcus equi subsp. Zooepidemicus. Carbohydr. Polym. 2009, 75, 677–682. [Google Scholar] [CrossRef]
- Joubran, Y.; Mackie, A.; Lesmes, U. Impact of the Maillard reaction on the antioxidant capacity of bovine lactoferrin. Food Chem. 2013, 141, 3796–3802. [Google Scholar] [CrossRef] [PubMed]
- Capek, P.; Machová, E.; Turjan, J. Scavenging and antioxidant activities of immunomodulating polysaccharides isolated from Salvia officinalis L. Int. J. Biol. Macromol. 2009, 44, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Q.; Ding, S.; Fan, L. Antioxidant activities of five polysaccharides from Inonotus obliquus. Int. J. Biol. Macromol. 2012, 50, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-W.; Zhou, D.-Y.; Li, T.; Yan, S.; Yang, J.-F.; Li, D.-M.; Dong, X.-P.; Murata, Y. Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis discus hannai ino). Food Chem. 2010, 121, 712–718. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.-I.; Saito, N.; Shimazu, Y.; Ozawa, T. A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Arch. Biochem. Biophys. 1996, 333, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.K.; Liu, C.; Zhu, L.F.; Hui, Y.Z.; Yu, B.; Fung, K.P. Chemical and biological characterization of a polysaccharide biological response modifier from Aloe vera L. var. chinensis (Haw.) Berg. Glycobiology 2004, 14, 501–510. [Google Scholar] [PubMed]
- Zhang, X.; Qi, C.; Guo, Y.; Zhou, W.; Zhang, Y. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Pebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Xiong, S.L.; Li, A.; Huang, N.; Lu, F.; Hou, D. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohydr. Polym. 2011, 86, 1273–1280. [Google Scholar] [CrossRef]
- Jia, X.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.E.; Du, L.; Yuan, M. Extraction, purification and characterization of polysaccharides from Hawk tea. Carbohydr. Polym. 2014, 99, 319–324. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the polysaccharide-protein mixtures from longan pulp are available from the authors. |


| Dry-Heating Time (Day) | IC50 Value of DPPH Radical Scavenging (mg/mL) | IC25 Value of Hydroxyl Radical Scavenging (mg/mL) | FRAP Total Antioxidant Capacity (mmol/g) |
|---|---|---|---|
| 0 | 1.398 ± 0.102 e | 2.449 ± 0.092 d | 0.038 ± 0.001 a |
| 1 | 0.966 ± 0.012 d | 1.095 ± 0.035 ab | 0.066 ± 0.002 b |
| 2 | 0.881 ± 0.014 c | 1.015 ± 0.065 a | 0.074 ± 0.002 c |
| 3 | 0.551 ± 0.016 b | 1.125 ± 0.075 ab | 0.087 ± 0.001 d |
| 4 | 0.556 ± 0.011 b | 1.285 ± 0.055 c | 0.107 ± 0.002 e |
| 5 | 0.380 ± 0.020 a | 1.215 ± 0.045 bc | 0.119 ± 0.002 f |
| 6 | 0.326 ± 0.008a | 1.333 ± 0.024 c | 0.146 ± 0.002 g |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.-M.; Yi, Y.; Wang, H.-X.; Huang, F. Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities. Molecules 2017, 22, 938. https://doi.org/10.3390/molecules22060938
Han M-M, Yi Y, Wang H-X, Huang F. Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities. Molecules. 2017; 22(6):938. https://doi.org/10.3390/molecules22060938
Chicago/Turabian StyleHan, Miao-Miao, Yang Yi, Hong-Xun Wang, and Fei Huang. 2017. "Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities" Molecules 22, no. 6: 938. https://doi.org/10.3390/molecules22060938
APA StyleHan, M.-M., Yi, Y., Wang, H.-X., & Huang, F. (2017). Investigation of the Maillard Reaction between Polysaccharides and Proteins from Longan Pulp and the Improvement in Activities. Molecules, 22(6), 938. https://doi.org/10.3390/molecules22060938





