Bioactive Natural Product and Superacid Chemistry for Lead Compound Identification: A Case Study of Selective hCA III and L-Type Ca2+ Current Inhibitors for Hypotensive Agent Discovery
Abstract
:1. Introduction
2. Results
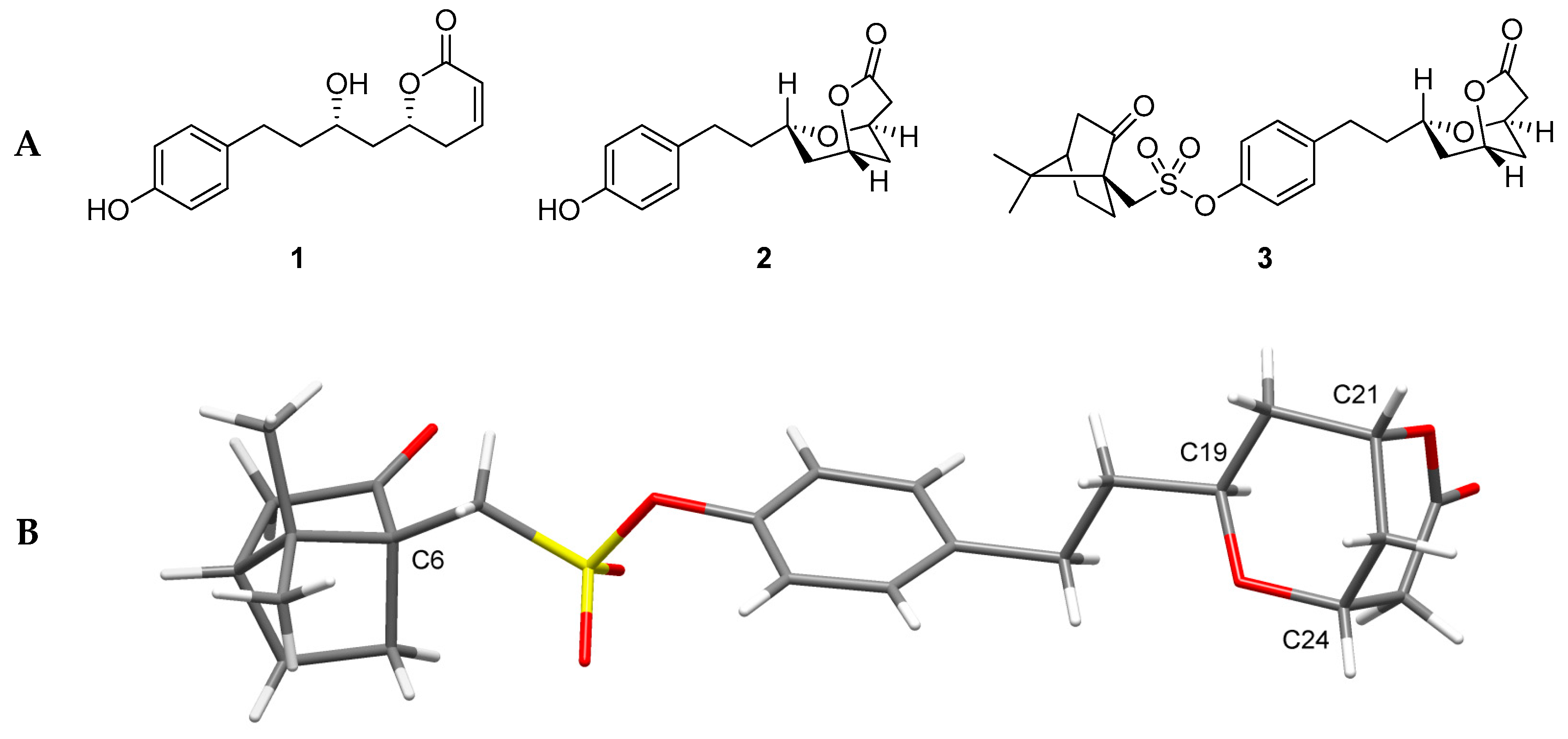
2.1. Structure Elucidation of a Dihydropyranone from Tapinanthus dodoneifolius
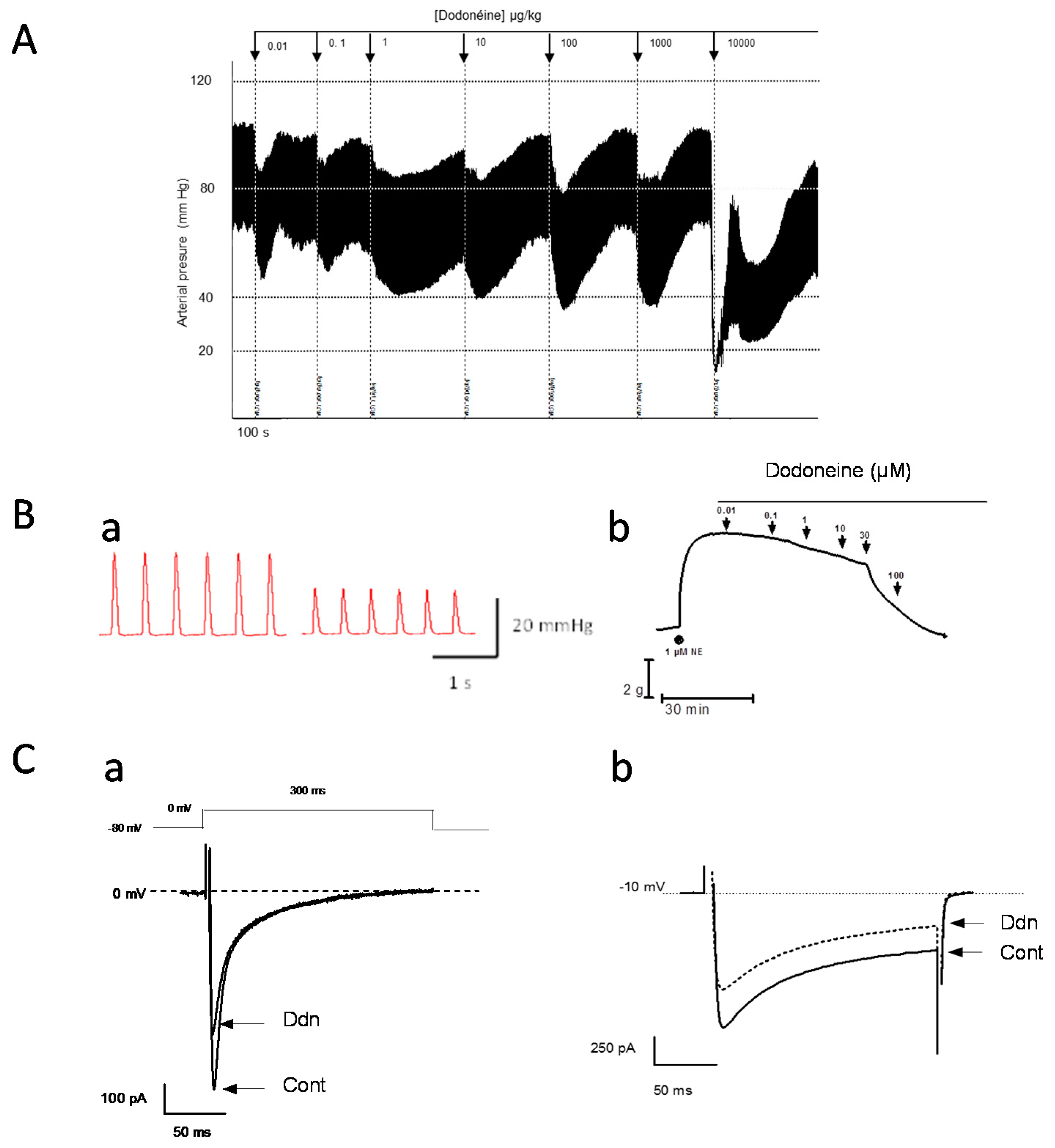
2.2. Hypotensive Effect of Dodoneine
2.3. Hypotensive Properties of Dodoneine are Likely Associated with a Negative Inotropic Effect and L-Type Calcium Current Inhibitor
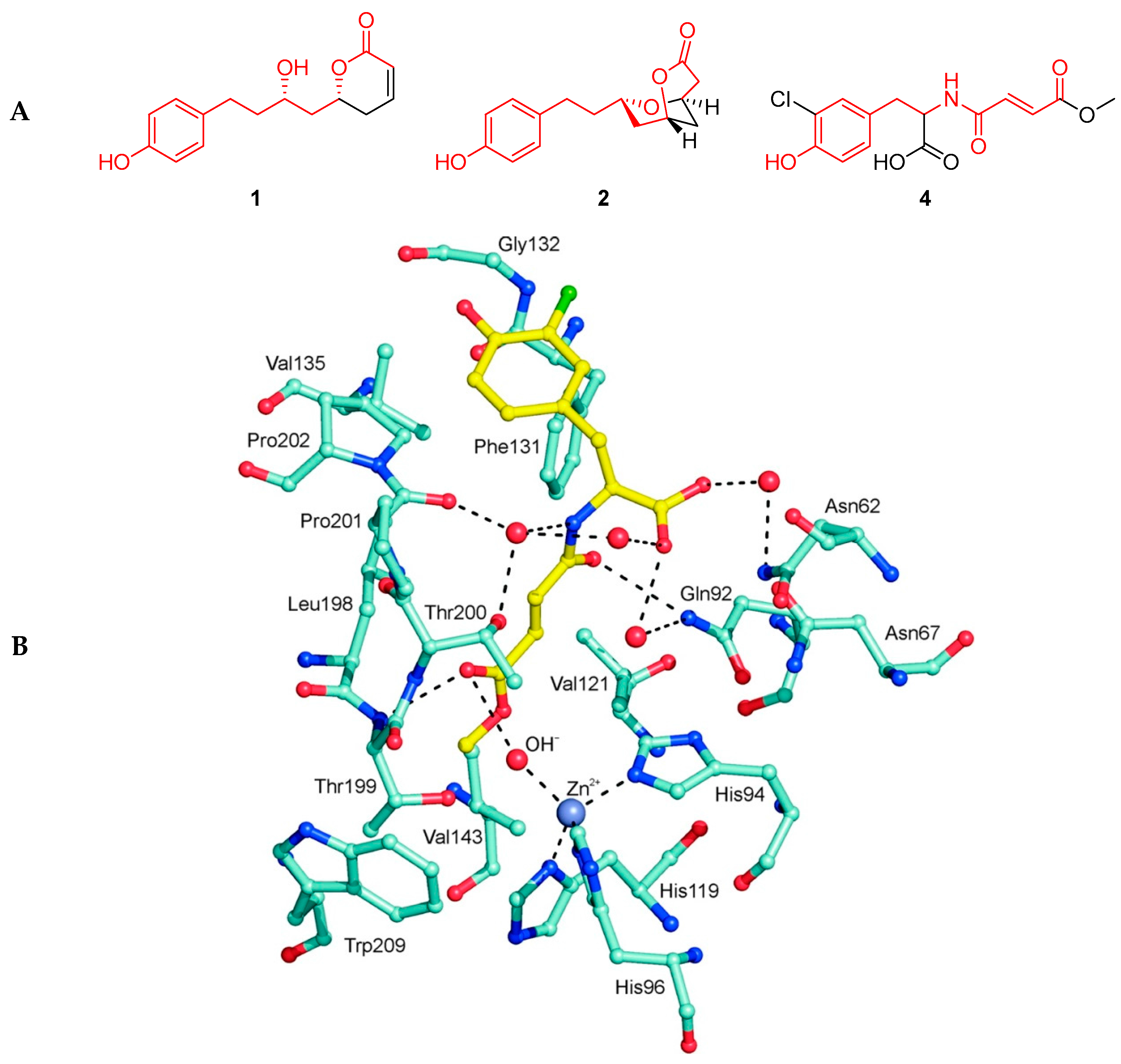
2.4. Dodoneine and Its Analogues Also Inhibit Human Carbonic Anhydrases
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 2009, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Scannel, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef]
- Walsh, G. Pharmaceutical Biotechnology; Wiley: Chichester, UK, 2007. [Google Scholar]
- Ziegler, S.; Pries, V.; Hedberg, C.; Waldmann, H. Target identification for small bioactive molecules: Finding the needle in the haystack. Angew. Chem. Int. Ed. 2013, 52, 2744–2792. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E.; Bayly, A.R.; Skidmore, J. Small molecules, big targets: Drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016, 15, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.L.; Smothers, J.; Srinivasan, R.; Hoos, A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 2015, 14, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.J.; Nelson, A.; Marsden, S.P. Evaluating New Chemistry to Drive Molecular Discovery: Fit for Purpose? Angew. Chem. Int. Ed. 2016, 55, 13650–13657. [Google Scholar] [CrossRef] [PubMed]
- Abet, V.; Mariani, A.; Truscott, F.R.; Britton, S.; Rodriguez, R. Biased and Unbiased Strategies to Identify Biologically Active Small Molecules. Bioorg. Med. Chem. 2014, 22, 4474–4489. [Google Scholar] [CrossRef] [PubMed]
- Cusack, K.P.; Koolman, H.F.; Lange, U.E.W.; Peltier, H.M.; Piel, I.; Vasudevan, A. Emerging Technologies for Metabolite Generation and Structural Diversification. Bioorg. Med. Chem. Lett. 2013, 23, 5471–5483. [Google Scholar] [CrossRef] [PubMed]
- Hert, J.; Irwin, J.J.; Laggner, C.; Keiser, M.J.; Shoichet, B.K. Quantifying Biogenic Bias in Screening Libraries. Nat. Chem. Biol. 2009, 5, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Boström, J. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem. 2016, 59, 4443–4458. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 235, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.U.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Robertson, A.A.B.; Cooper, M.A. Natural products and natural products derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Drewry, D.H.; Macarron, R. Enhancements of Screening Collections to Address Areas of Unmet Medical Need: An Industry Perspective. Curr. Opin. Chem. Biol. 2010, 14, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sukuru, S.C.; Jenkins, J.L.; Scheiber, J.; Bender, A.; Mikhailov, D.; Davies, J.W.; Glick, M. Plate-based Diversity Selection Based on Empirical HTS Data to Enhance the Number of Hits and their Chemical Diversity. J. Biomol. Screen. 2009, 14, 690–699. [Google Scholar] [CrossRef] [PubMed]
- DeCorte, B.L. Underexplored Opportunities for Natural Products in Drug Discovery. J. Med. Chem. 2016, 59, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Robles, O.; Romo, D. Chemo- and site-selective derivatizations of natural products enabling biological studies. Nat. Prod. Rep. 2014, 31, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Petr Vachalb, P.; Krskab, S.W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef] [PubMed]
- Durak, L.J.; Payne, J.T.; Lewis, J.C. Late-Stage Diversification of Biologically Active Molecules via Chemoenzymatic C–H Functionalization. ACS Catal. 2016, 6, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Prakash, G.K.S.; Molnar, A.; Sommer, J. Superacids, 2nd ed.; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Olah, G.A.; Klumpp, D. Superelectrophiles and Their Chemistry; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Jacquesy, J.C. Organic Synthesis in Superacids. In Carbocation Chemistry; Olah, G.A., Ed.; Wiley: New York, NY, USA, 2004; pp. 359–376. [Google Scholar]
- Jacquesy, J.C. Natural Product Chemistry in Superacids. In Stable Carbocation Chemistry; Olah, G.A., Ed.; Wiley: New York, NY, USA, 1997; pp. 549–574. [Google Scholar]
- Fahy, J.; Duflos, A.; Ribert, J.-P.; Jacquesy, J.-C.; Berrier, C.; Jouannetaud, M.-P.; Zunino, F. Vinca Alkaloids in Superacidic Media: A Method for Creating a New Family of Antitumor Derivatives. J. Am. Chem. Soc. 1997, 119, 8576–8577. [Google Scholar] [CrossRef]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Munter, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Damasceno, A. Hypertension in developing countries. Lancet 2012, 380, 611–619. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Traoré, A.; Somé, N.; Lompo, M.; Guissou, P.I.; Schott, C.; Bucher, B.; Andriantsitohaina, R. Cardiovascular properties of aqueous extract from Tapinanthus dodoneifolius DC Danser. Afr. J. Trad. Complement. Altern. Med. 2005, 2, 25–30. [Google Scholar] [CrossRef]
- Ouedraogo, M.; Carreyre, H.; Vandebrouck, C.; Bescond, J.; Raymond, G.; Guissou, I.-P.; Cognard, C.; Becq, F.; Potreau, D.; Cousson, A.; et al. Structure Elucidation of a Dihydropyranone from Tapinanthus dodoneifolius. J. Nat. Prod. 2007, 70, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, M.; Ruiz, M.; Vardelle, E.; Carreyre, H.; Coustard, J.M.; Potreau, D.; Sawadogo, L.L.; Cognard, C.; Becq, F.; Vandebrouck, C.; et al. From the vasodilator and hypotensive effects of an extract fraction from Agelanthus dodoneifolius (DC) Danser (Loranthaceae) to the active coumpound dodoneine. J. Ethnopharmacol. 2011, 133, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Carré, G.; Carreyre, H.; Ouedraogo, M.; Becq, F.; Bois, P.; Thibaudeau, S.; Vandebrouck, C.; Bescond, J. The hypotensive agent dodoneine inhibits L-type calcium current with negative inotropic effect on rat heart. Eur. J. Pharmacol. 2014, 728, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D.; et al. Resistant hypertension: Diagnosis, evaluation and treatment: A scientific statement from the American Heart Association Professional Educational Committee of the Council for High Blood Pressure Research. Circulation 2008, 117, e510–e526. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.H. Ion channels and the control of blood pressure. Br. J. Clin. Pharmacol. 2000, 49, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Carré, G.; Ouedraogo, M.; Magaud, C.; Carreyre, C.; Becq, F.; Bois, P.; Supuran, C.T.; Thibaudeau, S.; Vandebrouck, C.; Bescond, J. Vasorelaxation induced by dodoneine is mediated by calcium channel blockade and carbonic anhydrase inhibition on vascular smooth muscle cells. J. Ethnopharmacol. 2015, 169, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Yusuf, S.; Kubler, W. Current status of safety and efficacy of calcium channel blockers in cardiovascular diseases: A critical analysis based on 100 studies. Prog. Cardiovasc. Dis. 2000, 43, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Swenson, E.R. New Insights into Carbonic Anhydrase Inhibition, Vasodilation, and Treatment of Hypertensive-Related Diseases. Curr. Hypertens. Rep. 2014, 16, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Pickkers, P.; Garcha, R.S.; Schachter, M.; Smits, P.; Hugues, A.D. Inhibition of Carbonic Anhydrase Accounts for the Direct Vascular Effects of Hydrochlorothiazide. Hypertension 1999, 33, 1043–1048. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Johnson, D.E.; Sowah, D.; Soliman, D.; Light, P.E.; Xia, Y.; Karmazyn, M.; Casey, J.R. Carbonic anhydrase inhibition prevents and reverts cardiomyocyte hypertrophy. J. Physiol. 2007, 579, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.; Kaufman, G.K.; Urbach, A.R.; Gitlin, I.; Gudiksen, K.L.; Weibel, D.B.; Whitesides, G.M. Carbonic Anhydrase as a Model for Biophysical and Physical-Organic Studies of Proteins and Protein−Ligand. Chem. Rev. 2008, 108, 946–1051. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Dis. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic Anhydrases as Drug Targets: General Presentation. In Drug Design of Zinc-Enzyme; Supuran, C.T., Winum, J.Y., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 15–38. [Google Scholar]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple Binding Modes of Inhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-Zinc Mediated Inhibition of Carbonic Anhydrases: Coumarins Are a New Class of Suicide Inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the Mechanism of Carbonic Anhydrase Inhibition with Coumarins and Thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Maresca, A.; Scozzafava, A.; Supuran, C.T. Novel Coumarins and 2-Thioxo-Coumarins as Inhibitors of the Tumor-Associated Carbonic Anhydrases IX and XII. Bioorg. Med. Chem. 2012, 20, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Tars, K.; Vullo, D.; Kazaks, A.; Leitans, J.; Lends, A.; Grandane, A.; Zalubovskis, R.; Scozzafava, A.; Supuran, C.T. Sulfocoumarins (1,2-Benzoxathiine-2,2-dioxides): A Class of Potent and Isoform-Selective Inhibitors of Tumor-Associated Carbonic Anhydrases. J. Med. Chem. 2013, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Durdagi, S.; Sentürk, M.; Ekinci, D.; Balaydin, H.T.; Innocenti, A.; Scozzafava, A.; Supuran, C.T. Kinetic and Docking Studies of Phenol-Based Inhibitors of Carbonic Anhydrase Isoforms I, II, IX and XII Evidence a New Binding Mode Within the Enzyme Active Site. Bioorg. Med. Chem. 2011, 19, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Maresca, A.; Scozzafava, A.; Supuran, C.T. 5- and 6-Membered (Thio)Lactones Are Prodrug Type Carbonic Anhydrase Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Ludwig, P.A.; Christianson, D.W. Two-Site Binding of Phenol in the Active Site of Human Carbonic Anhydrase II: Structural Implications for Substrate Association. J. Am. Chem. Soc. 1994, 116, 3659–3660. [Google Scholar] [CrossRef]
- Davis, R.A.; Hohmann, A.; Osman, A.; Hall, R.A.; Mühlschlegel, F.A.; Vullo, D.; Innocenti, A.; Supuran, C.T.; Poulsen, S.A. Natural product-based phenols as novel probes for mycobacterial and fungal carbonic anhydrases. J. Med. Chem. 2001, 54, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- Berg, J.T.; Ramanathan, S.; Gabrielli, M.G.; Swenson, E.R. Carbonic Anhydrase in Mammalian Vascular Smooth Muscle. J. Histichem. Cytochem. 2004, 52, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Gouverneur, V.; Seppelt, K. Introduction: Fluorine chemistry. Chem. Rev. 2015, 115, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Thibaudeau, S.; Carreyre, H.; Mingot, A. Synthesis of fluorinated nitrogen-containing compounds through superelectrophilic activation in superacid HF/SbF5. In Modern Synthesis Process and Reactivity of Fluorinated Compounds; Groult, H., Leroux, F., Tressaud, A., Eds.; Elsevier: Paris, France, 2017; pp. 349–388. [Google Scholar] [CrossRef]
- Bonazaba Milandou, L.J.C.; Carreyre, H.; Alazet, S.; Greco, G.; Martin-Mingot, A.; Nkounkou Loumpangou, C.; Ouamba, J.-M.; Bouazza, F.; Billard, T.; et al. Superacid-catalyzed trifluoromethylthiolation of aromatic amines. Angew. Chem. Int. Ed. 2017, 56, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Métayer, B.; Mingot, A.; Vullo, D.; Supuran, C.T.; Thibaudeau, S. New superacid synthesized (fluorinated) tertiary benzenesulfonamides acting as selective hCA IX inhibitors: Toward a new mode of carbonic anhydrase inhibition by sulfonamides. Chem. Commun. 2013, 49, 6015–6017. [Google Scholar] [CrossRef] [PubMed]
- Métayer, B.; Compain, G.; Jouvin, K.; Martin-Mingot, A.; Bachmann, C.; Marrot, J.; Evano, G.; Thibaudeau, S. Chemo- and stereoselective synthesis of fluorinated enamides from ynamides in HF/pyridine: Second-generation approach to potent urea bioisosteres. J. Org. Chem. 2015, 80, 3397–3410. [Google Scholar] [CrossRef] [PubMed]
- Le Darz, A.; Mingot, A.; Bouazza, F.; Castelli, U.; Karam, O.; Tanc, M.; Supuran, C.T.; Thibaudeau, S. Fluorinated pyrrolidines and piperidines incorporating tertiary benzenesulfonamide moieties are selective carbonic anhydrase II inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 5, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Carreyre, H.; Coustard, J-M.; Carré, G.; Vandebrouck, C.; Bescond, J.; Ouedraogo, M.; Marrot, J.; Vullo, D.; Supuran, C.T.; Thibaudeau, S. Natural product hybrid and its superacid synthesized analogues: Dodoneine and its derivatives show selective inhibition of carbonic anhydrase isoforms I, III, XIII and XIV. Bioorg. Med. Chem. 2013, 21, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Compound | Ki (µm) a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hCA I | hCA II | hCA III | hCA IV | hCA Va | hCA Vb | hCA VI | hCA VII | hCA IX | hCA XII | mCAc XIII | hCA XIV | |
| 1 | 5.48 | - b | 10.35 | 9.61 | - b | - b | - b | - b | - b | - b | 9.27 | 9.34 |
| 2 | - b | - b | 10.80 | - b | - b | - b | - b | - b | - b | - b | 0.91 | - b |
| 5 | 0.13 | 36.9 | - b | 5.36 | 7.13 | 1.36 | - b | 24.9 | 3.57 | 1.48 | 0.96 | 2.44 |
| 6 | - b | - b | 5.13 | - b | - b | - b | - b | - b | - b | - b | 0.34 | - b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreyre, H.; Carré, G.; Ouedraogo, M.; Vandebrouck, C.; Bescond, J.; Supuran, C.T.; Thibaudeau, S. Bioactive Natural Product and Superacid Chemistry for Lead Compound Identification: A Case Study of Selective hCA III and L-Type Ca2+ Current Inhibitors for Hypotensive Agent Discovery. Molecules 2017, 22, 915. https://doi.org/10.3390/molecules22060915
Carreyre H, Carré G, Ouedraogo M, Vandebrouck C, Bescond J, Supuran CT, Thibaudeau S. Bioactive Natural Product and Superacid Chemistry for Lead Compound Identification: A Case Study of Selective hCA III and L-Type Ca2+ Current Inhibitors for Hypotensive Agent Discovery. Molecules. 2017; 22(6):915. https://doi.org/10.3390/molecules22060915
Chicago/Turabian StyleCarreyre, Hélène, Grégoire Carré, Maurice Ouedraogo, Clarisse Vandebrouck, Jocelyn Bescond, Claudiu T. Supuran, and Sébastien Thibaudeau. 2017. "Bioactive Natural Product and Superacid Chemistry for Lead Compound Identification: A Case Study of Selective hCA III and L-Type Ca2+ Current Inhibitors for Hypotensive Agent Discovery" Molecules 22, no. 6: 915. https://doi.org/10.3390/molecules22060915







