Some Physical Properties of Protein Moiety of Alkali-Extracted Tea Polysaccharide Conjugates Were Shielded by Its Polysaccharide
Abstract
:1. Introduction
2. Results and Discussion
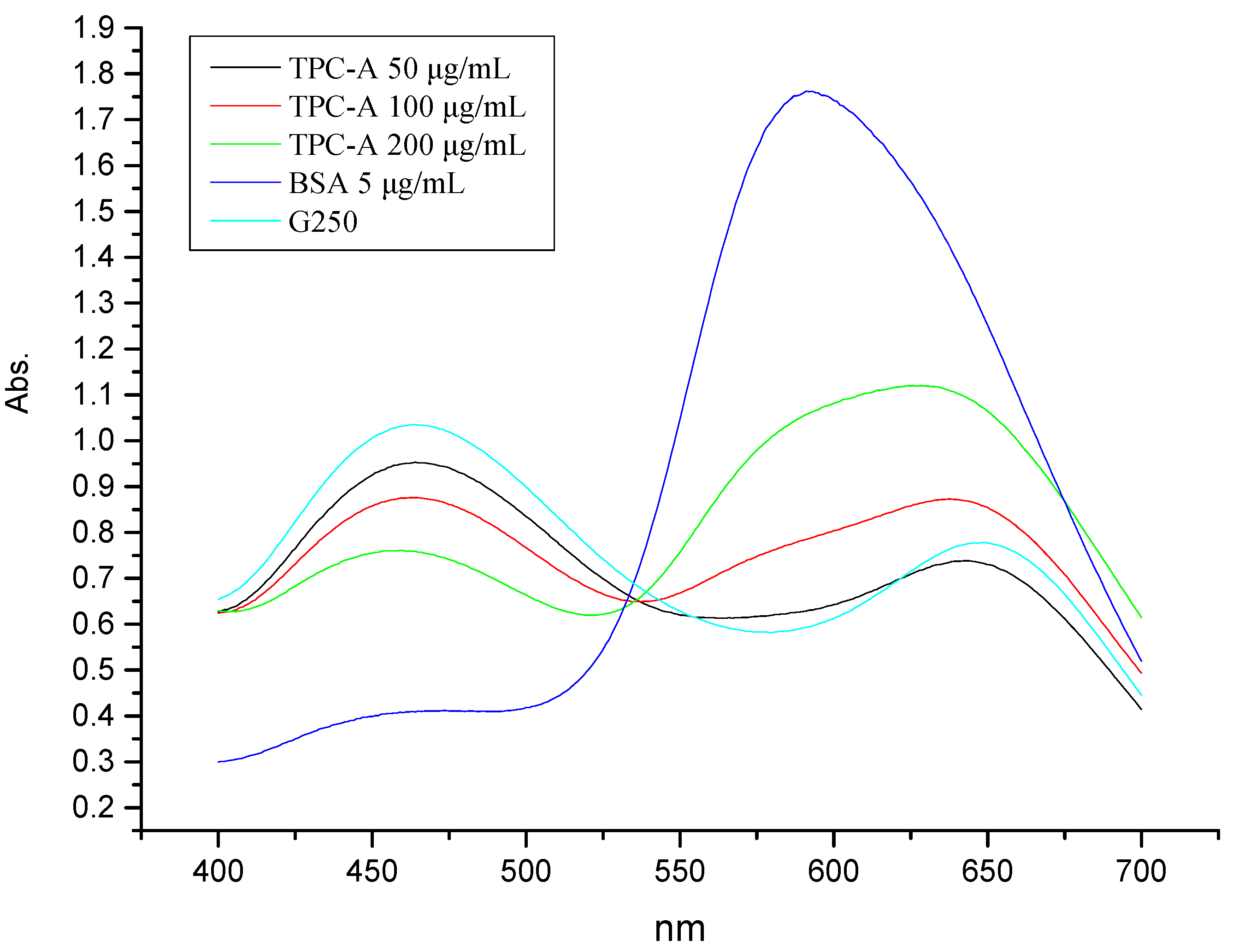
2.1. Coomassie Brilliant Blue Dye Detection of the Protein Portion of TPC-A
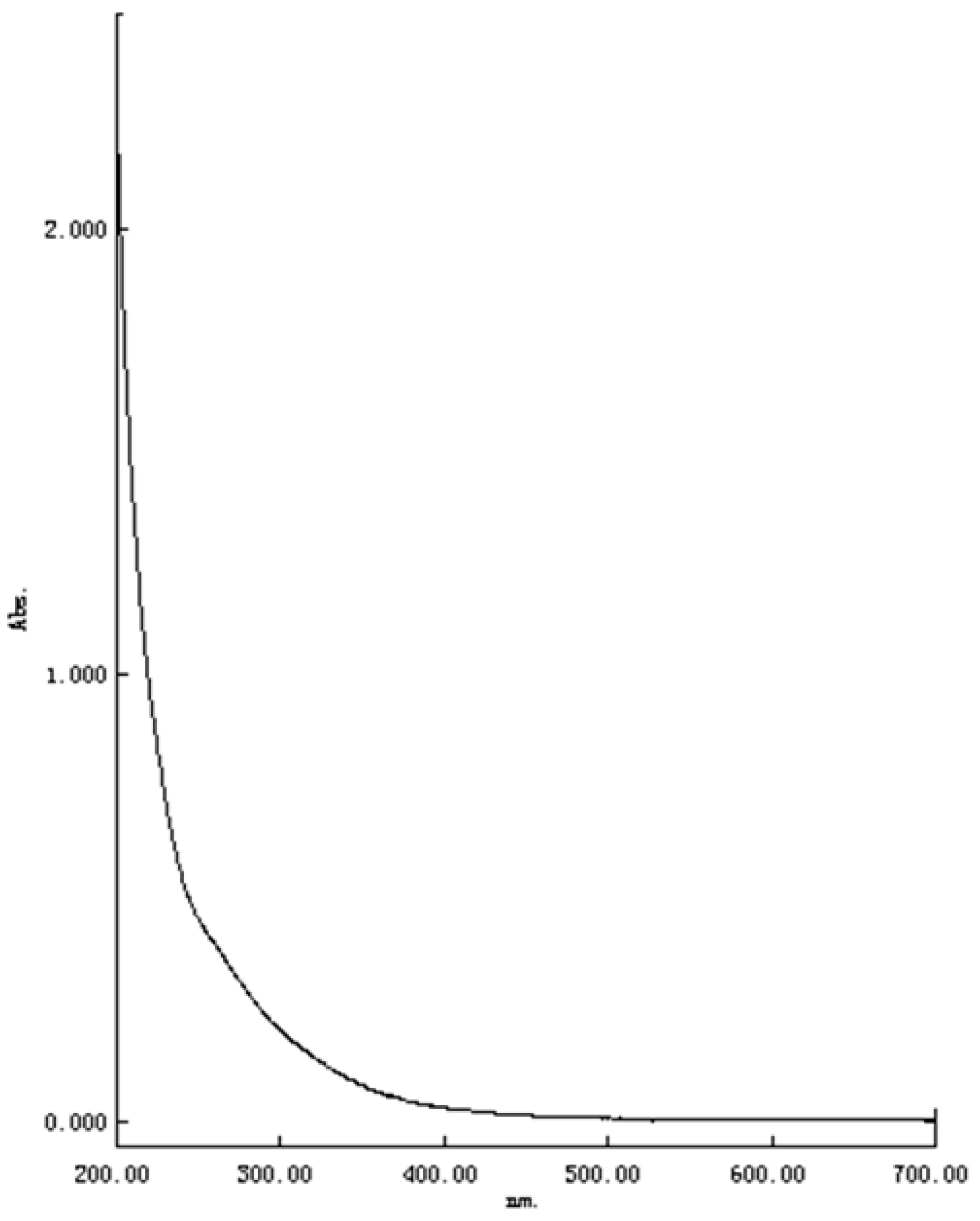
2.2. UV-Vis Spectrum Analysis of TPC-A Aqueous Solution
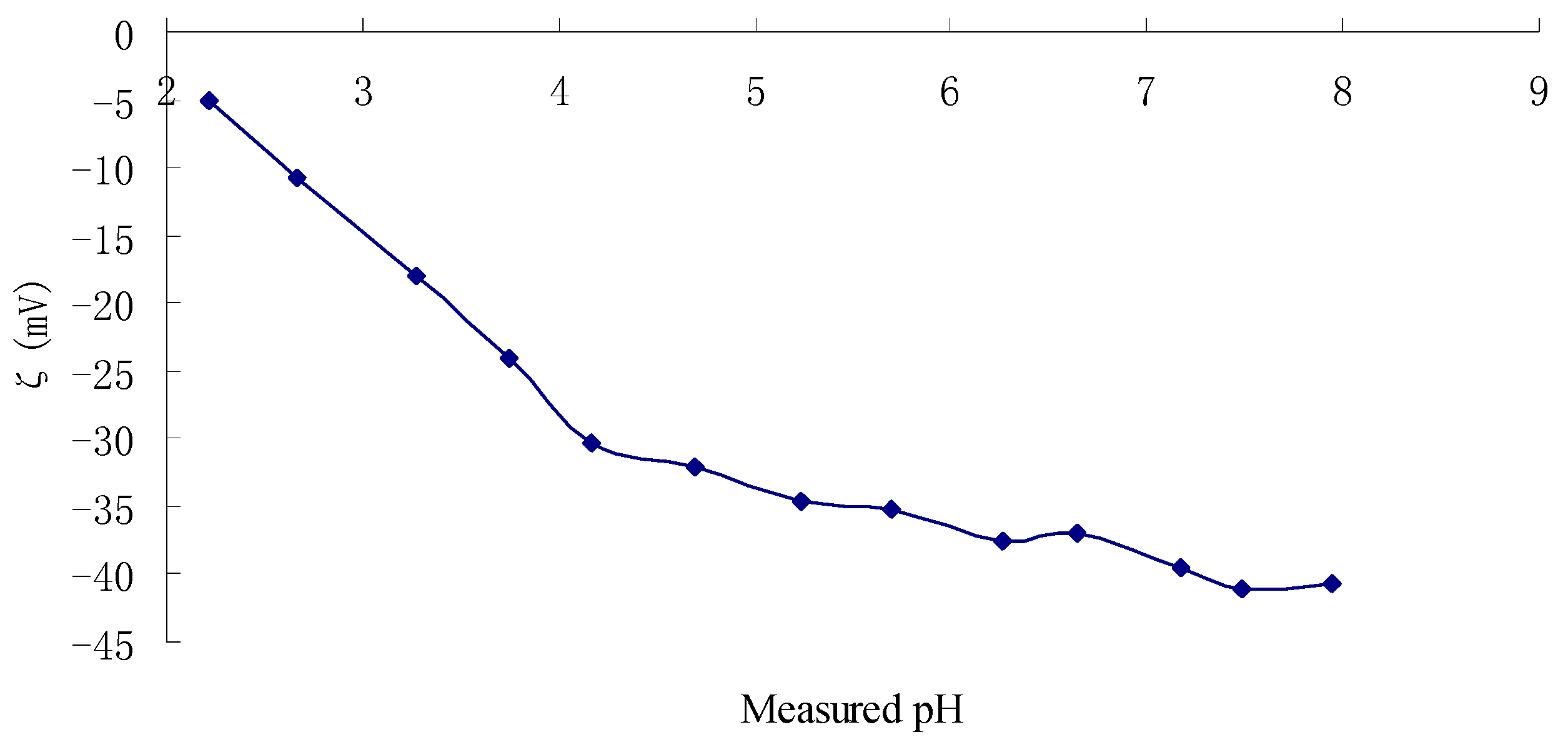
2.3. Zeta Potentials and Stability of TPC-A Aqueous Solution as a Function of pH
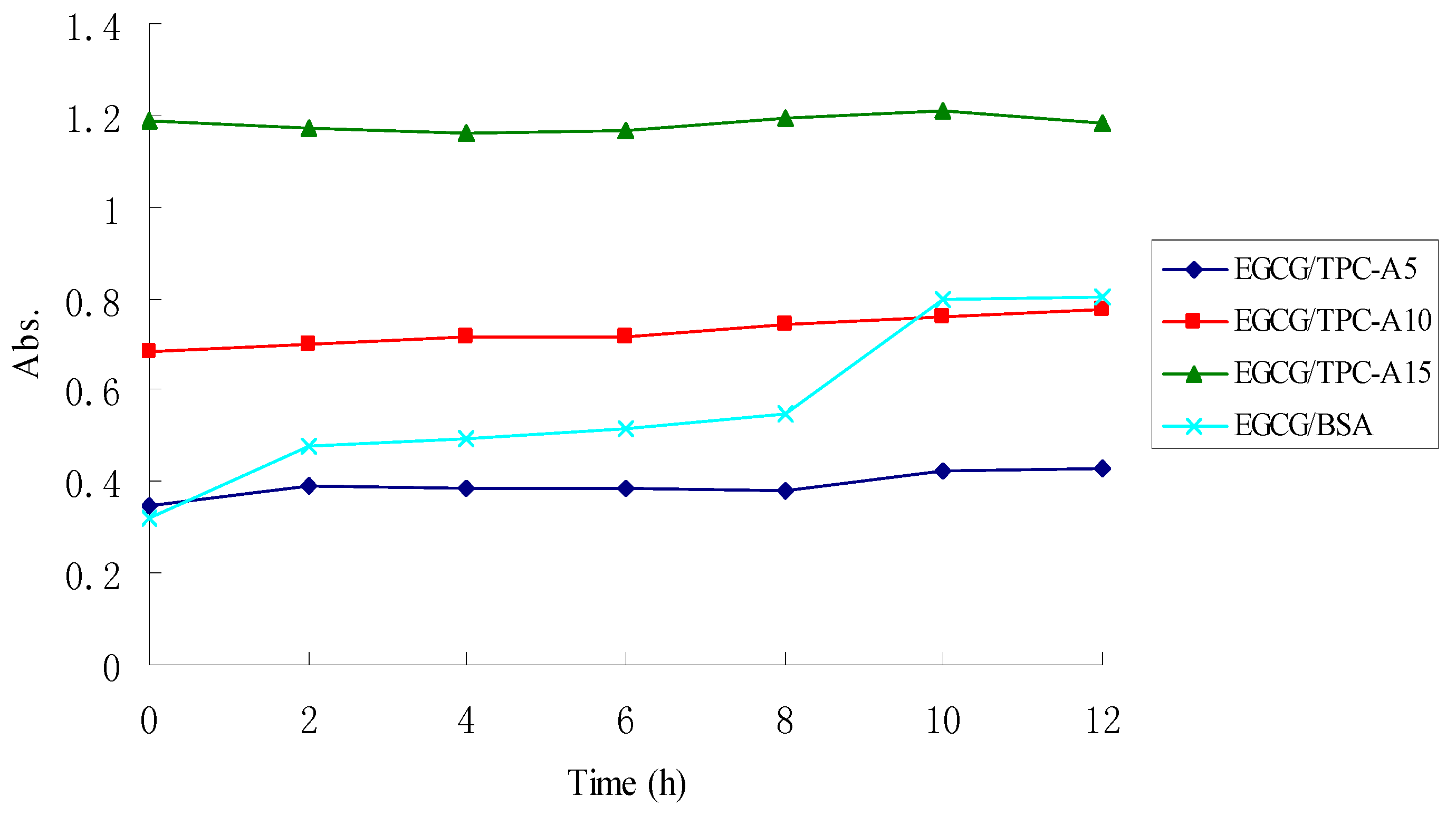
2.4. Stability of TPC-A and EGCG Aqueous Mixtures
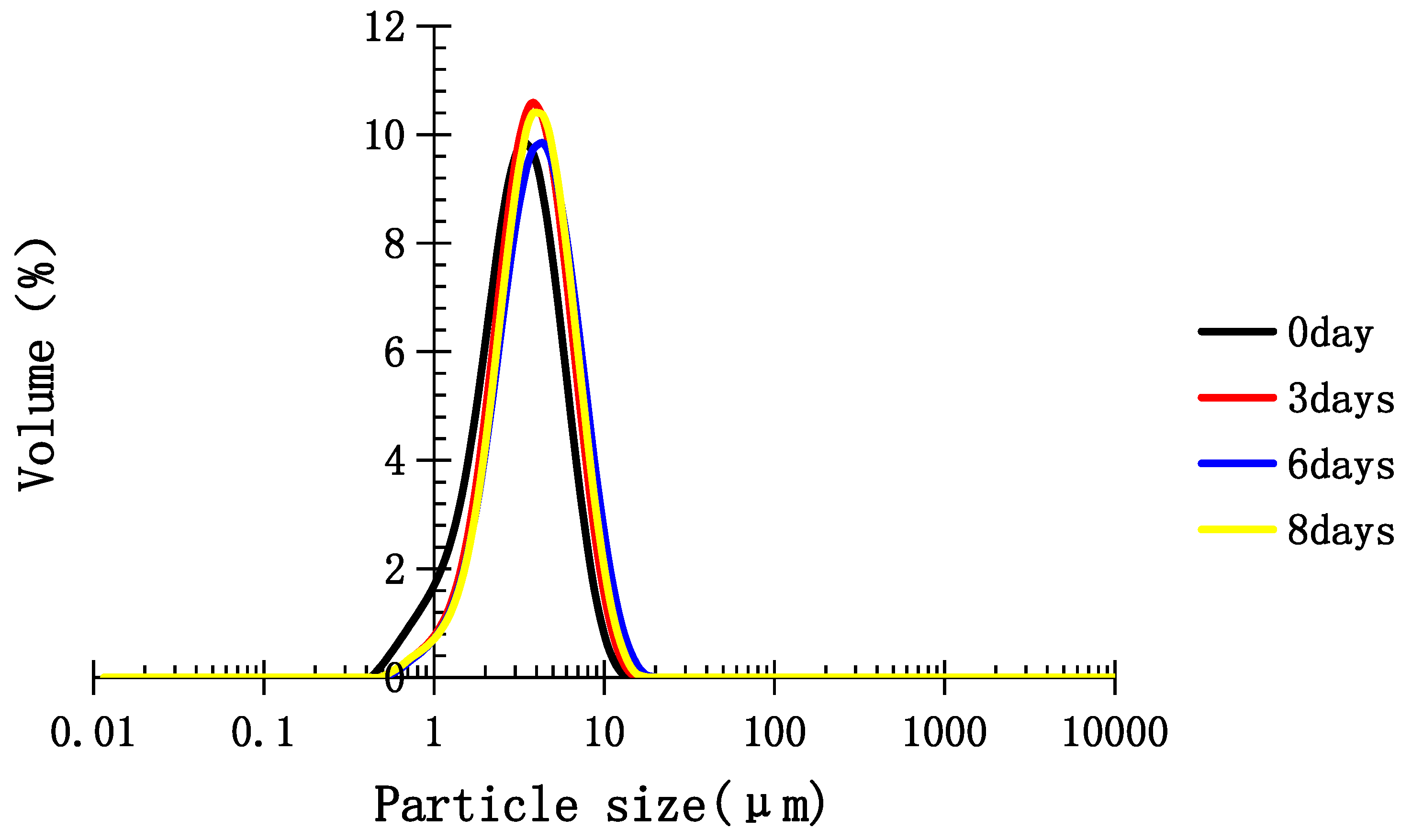
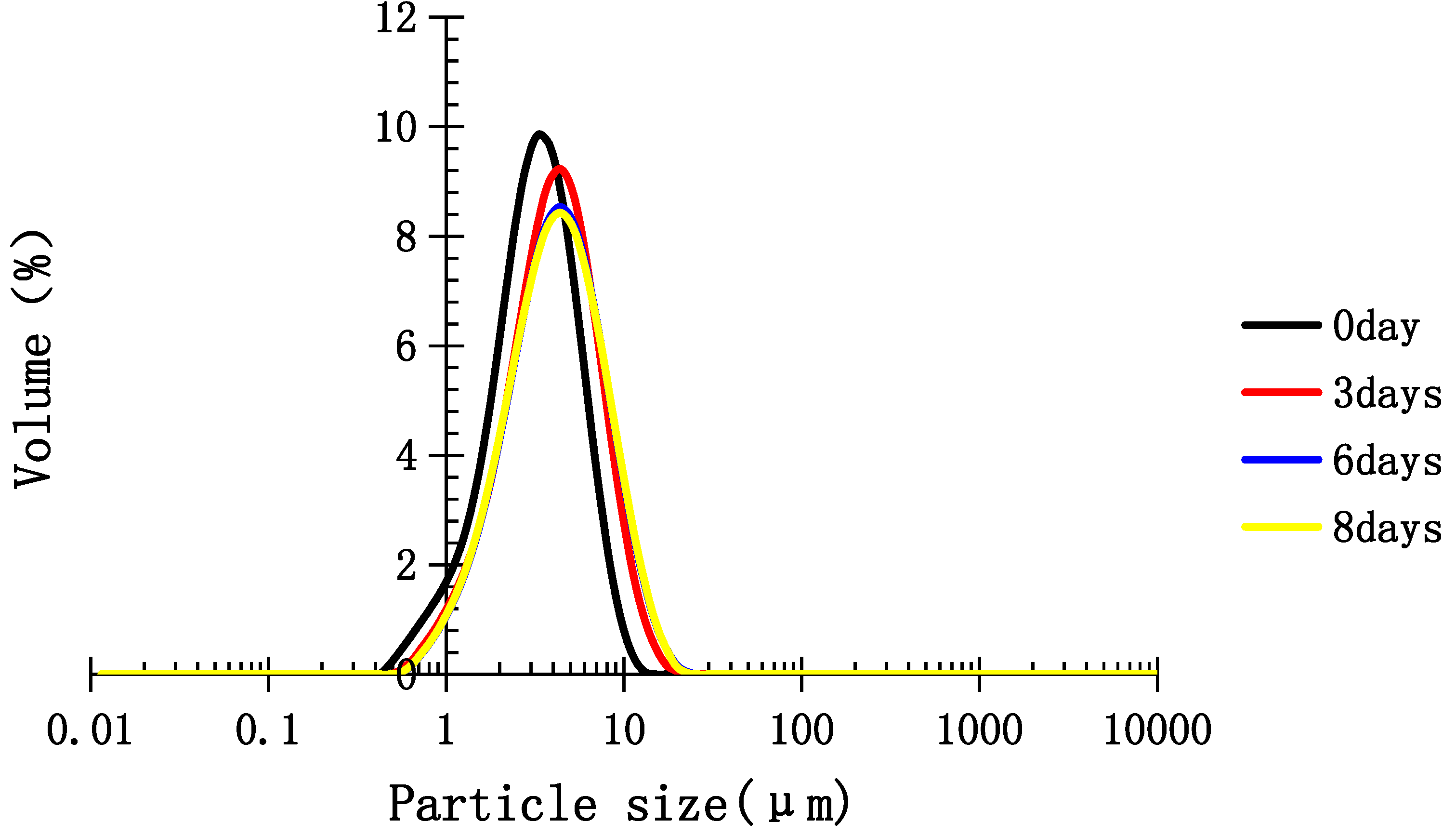
2.5. Stability of Medium-Chain Triglyceride (MCT) Emulsion Stabilized with TPC-A
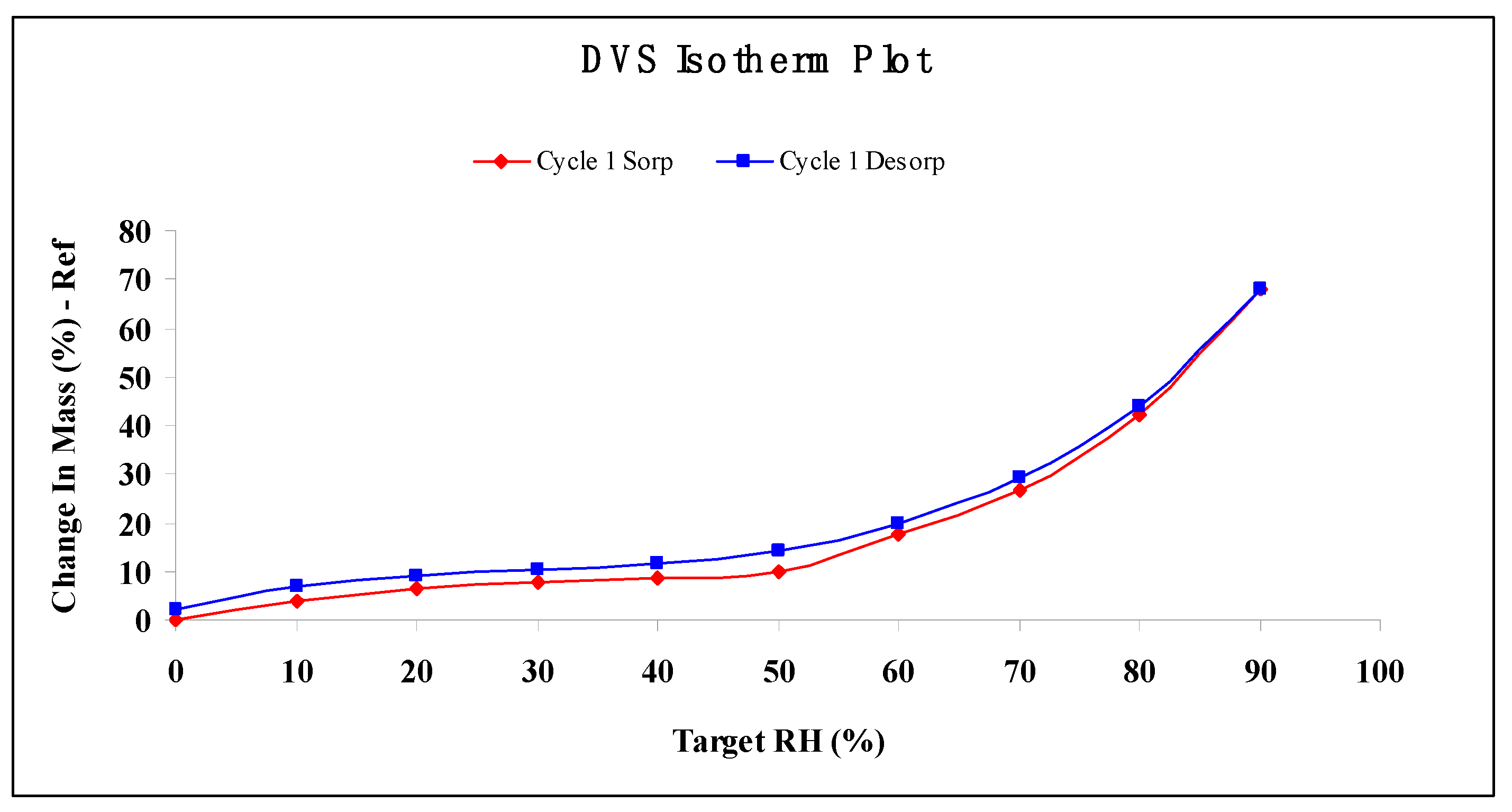
2.6. Water Vapor Sorption Properties
3. Materials and Methods
3.1. Materials and Reagents
3.2. Polysaccharide Conjugates Prepared from Green Tea through Alkaline Extraction Method
3.3. Monosaccharide Compositions
3.4. Amino Acid Compositions Protein Content
3.5. Coomassie Brilliant Blue Dye Detection of Protein in TPC-A
3.6. UV-Vis Spectrum Scan of TPC-A Aqueous Solution
3.7. Determination of Zeta Potentials
3.8. Stability of TPC-A and EGCG Aqueous Mixtures
3.9. Medium-Chain Triglycerides Emulsion Stabilized with TPC-A
3.10. Moisture Absorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.; Ye, Y.; Cheng, H.; Jiang, Y.; Wu, Y. Thermal effects on the stability and antioxidant activity of an acid polysaccharide conjugate derived from green tea. J. Agric. Food Chem. 2009, 57, 5795–5798. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Z.; Ye, Y.; Zhang, R.; Yin, J.; Jiang, Y.; Wan, H. Suppression of diabetes in non-obese diabetic (NOD) mice by oral administration of water-soluble and alkali-soluble polysaccharide conjugates prepared from green tea. Carbohyd. Polym. 2010, 82, 28–33. [Google Scholar] [CrossRef]
- Nie, S.; Xie, M. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z. Isolation of polysaccharides from tea and their hypoglycemic activity. Chin. Tradit. Herb. Drugs 2005, 36, 1453–1457. [Google Scholar]
- Wang, D.; Li, J.; Wang, C.; Zhao, G.; Jin, Y.; Cheng, D.; Ye, S. Study on the component and immune activity of polysaccharides from tea. J. Tea Sci. 2000, 20, 45–50. [Google Scholar]
- Chen, H.; Xie, B. The preventive and curative effects on diabetic mice of tea polysaccharides. Acta Nutr. Sin. 2002, 24, 85–86. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, L. Biochemistry Experiment; Chemical Industry Press: Beijing, China, 2009. [Google Scholar]
- Xu, Z.X.; Wang, K.B. Study on chemistry and bioactivities of tea polysaccharides. J. Tea Sci. 2004, 24, 75–81. [Google Scholar]
- Zhou, P.; Xie, M.Y.; Wang, Y.X. Purification and relative molecular mass determination of tea glycoprotein by high performance liquid chromatography-electrospray ionisation mass spectrometry. Chin. J. Chromatogr. 2004, 22, 27–29. [Google Scholar]
- Ozawa, T.; Lilley, T.; Haslam, E. Polyphenol interactions: Astringency and the loss of astringency in ripening fruit. Phytochemistry 1987, 26, 2937–2942. [Google Scholar] [CrossRef]
- Luck, G.; Liao, H.; Murray, N.; Grimmer, H.; Warminski, E.; Williamson, M.; Lilley, T.; Haslam, E. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Chen, X.; Fang, Y.; Nishinari, K.; We, H.; Sun, C.; Li, J.; Jiang, Y. Physicochemical characteristics of polysaccharide conjugates prepared from fresh tea leaves and their improving impaired glucose tolerance. Carbohyd. Polym. 2014, 112, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Manek, R.; Kolling, W.; Neau, S. Water distribution studies within microcrystalline cellulose and chitosan using differential scanning calorimetry and dynamic vapor sorption analysis. J. Pharm. Sci. 2004, 93, 1766–1779. [Google Scholar] [CrossRef] [PubMed]
- Driemeier, C.; Mendes, F.; Oliveira, M. Dynamic vapor sorption and thermoporometry to probe water in celluloses. Cellulose 2012, 19, 1051–1063. [Google Scholar] [CrossRef]
- Sambrook, J.F.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Al-Assaf, S.; Phillips, G. Characterization and prediction of emulsification performance of Acacia gums. LC GC N. Am. 2008, 26, 196–200. [Google Scholar]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.; Jiang, F. Complexation of bovine serum albumin and sugar beet pectin: Stabilising oil-in-water emulsions. J. Colloid Interface Sci. 2012, 388, 103–111. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Song, W.; Zhao, J.; Zhang, Z.; Zhang, Y. Some Physical Properties of Protein Moiety of Alkali-Extracted Tea Polysaccharide Conjugates Were Shielded by Its Polysaccharide. Molecules 2017, 22, 914. https://doi.org/10.3390/molecules22060914
Chen X, Song W, Zhao J, Zhang Z, Zhang Y. Some Physical Properties of Protein Moiety of Alkali-Extracted Tea Polysaccharide Conjugates Were Shielded by Its Polysaccharide. Molecules. 2017; 22(6):914. https://doi.org/10.3390/molecules22060914
Chicago/Turabian StyleChen, Xiaoqiang, Wei Song, Jin Zhao, Zhifa Zhang, and Yuntian Zhang. 2017. "Some Physical Properties of Protein Moiety of Alkali-Extracted Tea Polysaccharide Conjugates Were Shielded by Its Polysaccharide" Molecules 22, no. 6: 914. https://doi.org/10.3390/molecules22060914




