Polyamines and α-Carbonic Anhydrases
Abstract
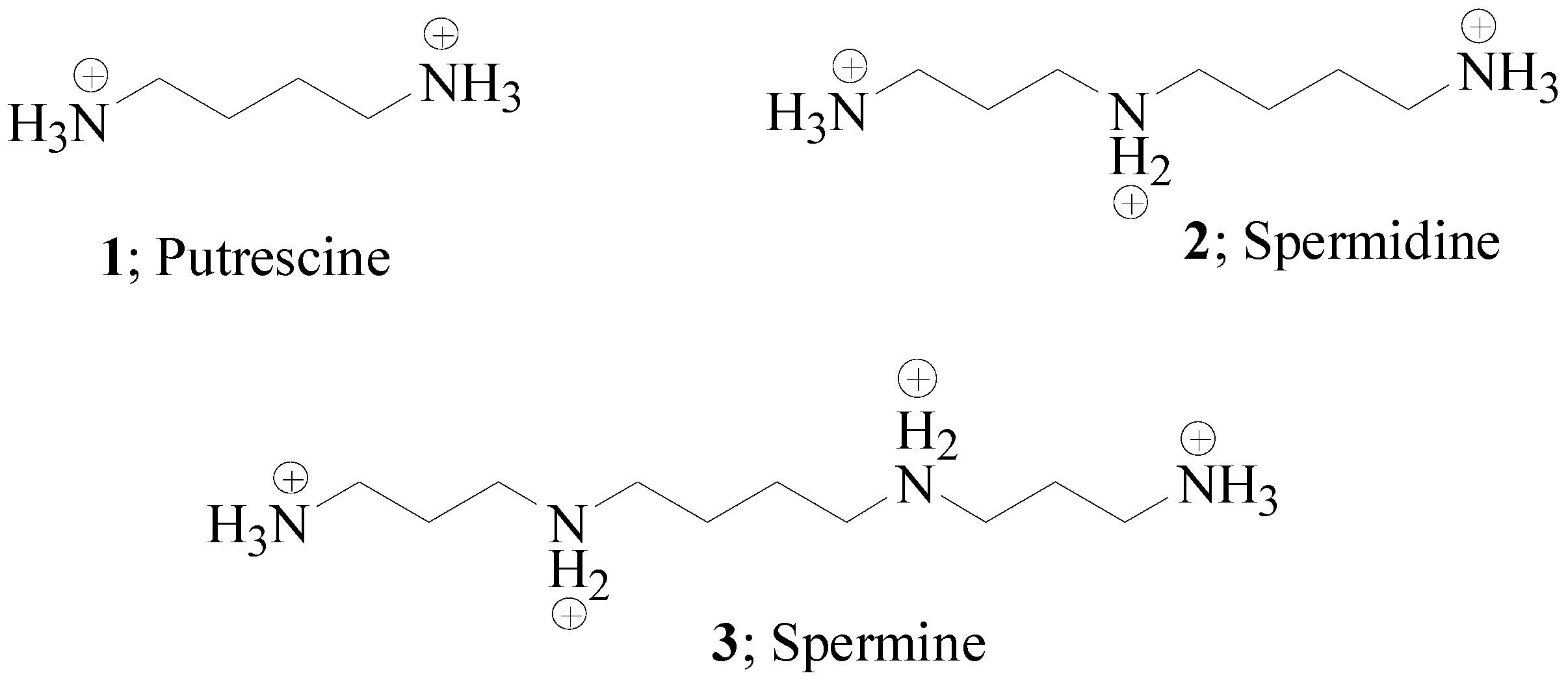
:1. Polyamines in Biology
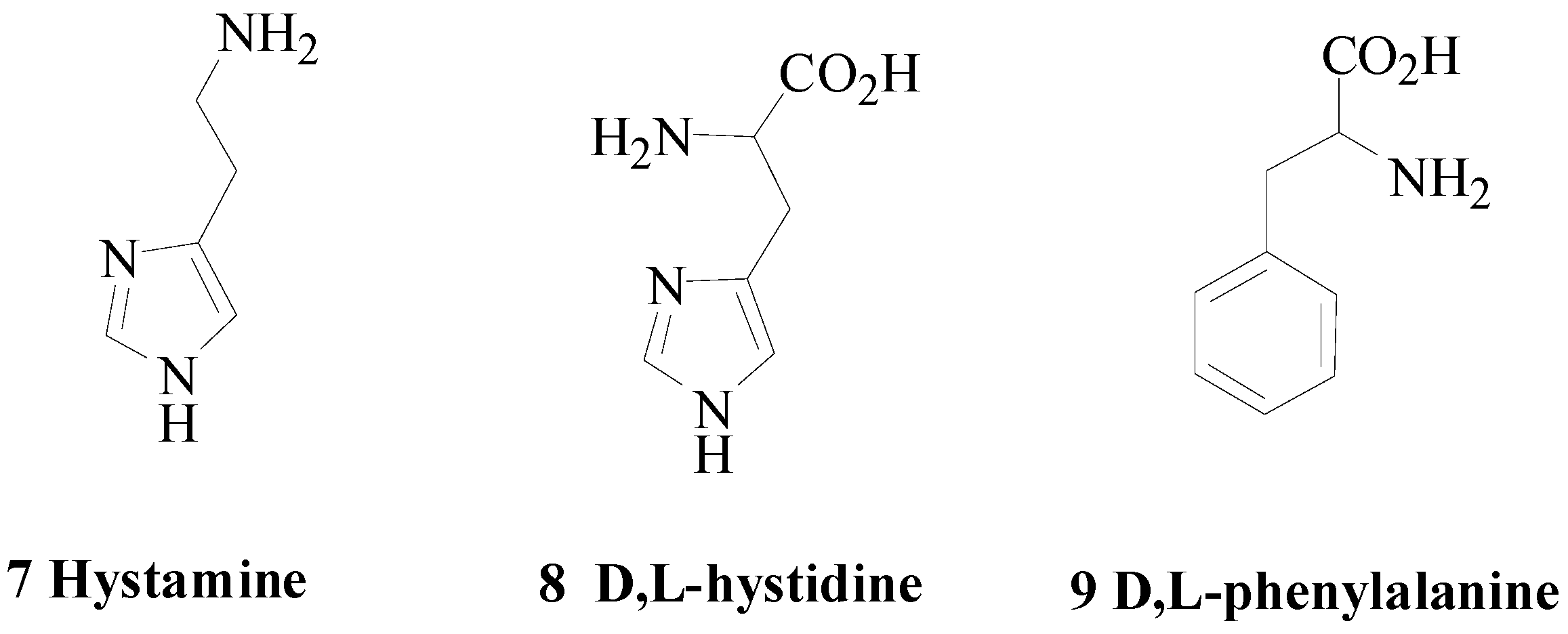
2. Polyamines and CAs
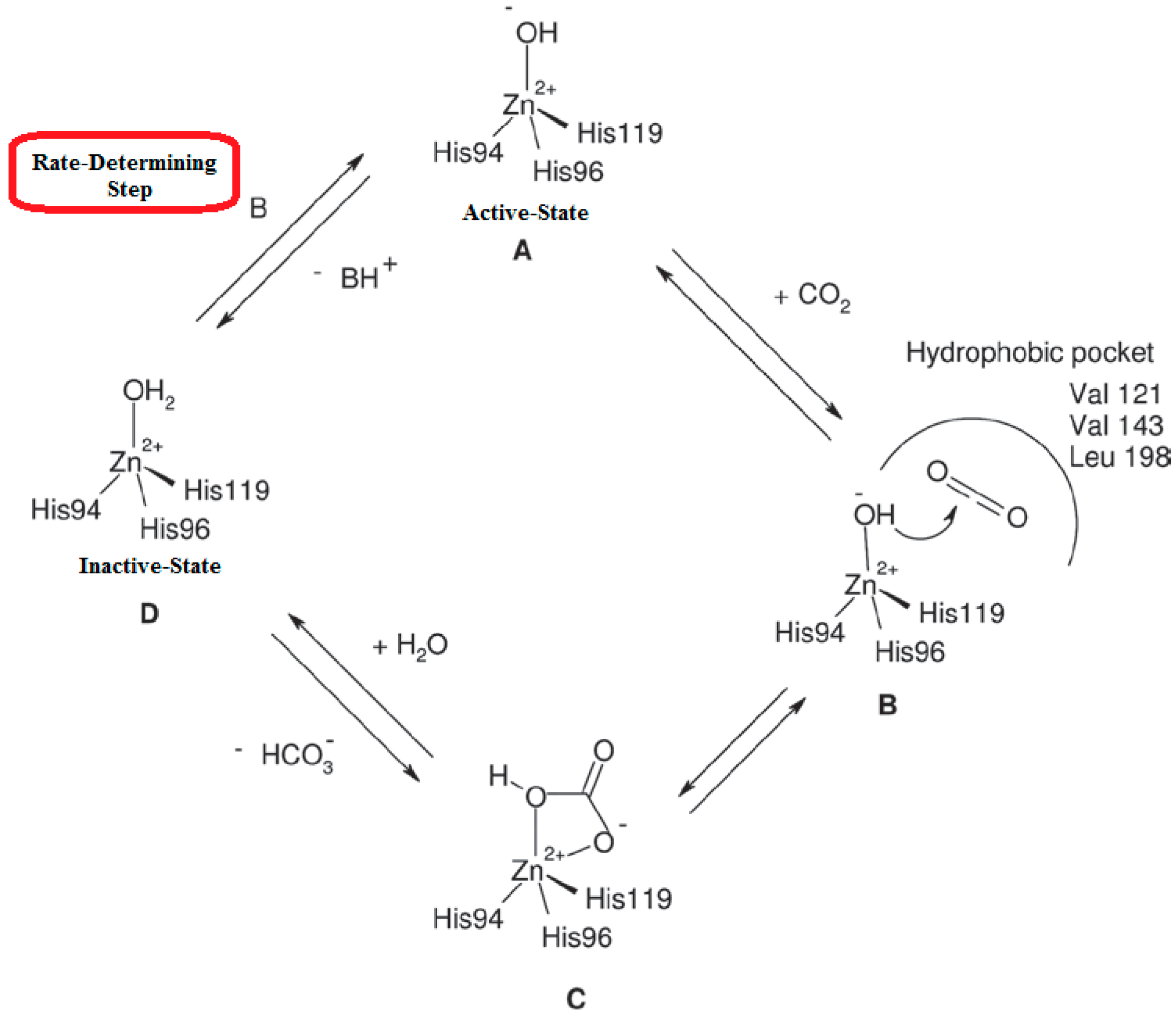
3. Crystallographic Investigation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takahashi, T.; Kakehi, J.-I. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Cell. Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Carbonell, J.; Blázquez, M.A. Regulatory Mechanisms of Polyamine Biosynthesis in Plants. Genes Genom. 2009, 31, 107–118. [Google Scholar] [CrossRef]
- Agostinelli, E.; Marques, M.P.; Calheiros, R.; Gil, F.P.; Tempera, G.; Viceconte, N.; Battaglia, V.; Grancara, S.; Toninello, A. Polyamines: Fundamental characters in chemistry and biology. Amino Acids 2010, 38, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Minguet, E.G.; Vera-Sirera, F.; Marina, A.; Carbonell, J.; Blázquez, M.A. Evolutionary diversification in polyamine biosynthesis. Mol. Biol. Evol. 2008, 25, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging players in bacteria-host interactions. Int. J. Med. Microbiol. 2013, 13, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.J.; Pegg, A.E. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 55–91. [Google Scholar] [CrossRef] [PubMed]
- Ouameur, A.A.; Tajmir-Riahi, H.A. Structural analysis of DNA interactions with biogenic polyamines and cobalt(III)hexamine studied by Fourier transform infrared and capillary electrophoresis. J. Biol. Chem. 2004, 279, 42041–42054. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Temperini, C.; Innocenti, A.; Scozzafava, A.; Kaila, K.; Supuran, C.T. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J. Med. Chem. 2010, 53, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Vullo, D.; Supuran, C.T.; Poulsen, S.A. Natural product polyamines that inhibit human carbonic anhydrases. BioMed Res. Int. 2014, 2014, 374079. [Google Scholar] [CrossRef] [PubMed]
- Heby, O.; Persson, L. Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem. Sci. 1990, 15, 153–158. [Google Scholar] [CrossRef]
- Agostinelli, E.; Condello, M.; Molinari, A.; Tempera, G.; Viceconte, N.; Arancia, G. Cytotoxicity of spermine oxidation products to multidrug resistant melanoma cells (M14 ADR2): Sensitization by MDL 72527, a lysosomotropic compound. Int. J. Oncol. 2009, 35, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Feith, D.J. Polyamines and neoplastic growth. Biochem. Soc. Trans. 2007, 35, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Toninello, A.; Salvi, M.; Mondovì, B. Interaction of biologically active amines with mitochondria and their role in the mitochondrial-mediated pathway of apoptosis. Curr. Med. Chem. 2004, 11, 2349–2374. [Google Scholar] [CrossRef] [PubMed]
- Temperini, C.; Scozzafava, A.; Supuran, C.T. Drug design studies of carbonic anhydrase activators. In Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications; Supuran, C.T., Winum, J.Y., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 473–486. [Google Scholar]
- Supuran, C.T.; Scozzafava, A. Activation of carbonic anhydrase isozymes. In The Carbonic Anhydrases: New Horizons; Chegwidden, W.R., Carter, N., Edwards, Y., Eds.; Birkhauser Verlag: Basel, Switzerland, 2000; pp. 197–219. [Google Scholar]
- Temperini, C.; Scozzafava, A.; Vullo, D.; Supuran, C.T. Carbonic anhydrase activators. Activation of isoforms I, II, IV, VA, VII, and XIV with l- and d-phenylalanine and crystallographic analysis of their adducts with isozyme II: Stereospecific recognition within the active site of an enzyme and its consequences for the drug design. J. Med. Chem. 2006, 49, 3019–3027. [Google Scholar] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Draghici, B.; Vullo, D.; Akocak, S.; Walker, E.A.; Supuran, C.T.; Ilies, M.A. Ethylene bis-imidazoles are highly potent and selective activators for isozymes VA and VII of carbonic anhydrase, with a potential nootropic effect. Chem. Commun. 2014, 50, 5980–5983. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym. Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Kondeti, B.; Tu, C.; Maupin, C.M.; Silverman, D.N.; McKenna, R. Structural insight into activity enhancement and inhibition of H64A carbonic anhydrase II by imidazoles. IUCrJ 2014, 1, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.S.; Kim, C.U.; Sippel, K.H.; Gruner, S.M.; Agbandje-McKenna, M.; Silverman, D.N.; McKenna, R. A short, strong hydrogen bond in the active site of human carbonic anhydrase II. Biochemistry 2010, 49, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines AC, antibacterial bromotyrosine-derived metabolites from the marine sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 5, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and bilains A–C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007, 11, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ki, D.; Kim, S.; Yeom, J.; Kim, Y.; Yun, B. Pistillarin salt, a dicatecholspermidine family member from Gomphus floccosus, inhibits DNA single strand breakage by the fenton reaction. J. Korean Soc. Appl. Biol. Chem. 2011, 2, 312–315. [Google Scholar] [CrossRef]
- Steglich, W.; Steffan, B.; Stroech, K.; Wolf, M. Pistillarin, a characteristic metabolite of Clavariadelphus pistillaris and several Ramaria species (Basidiomycetes). Z. Naturforsch. C 1984, 39, 10–12. [Google Scholar]
- Choomuenwai, V.; Schwartz, B.D.; Beattie, K.D.; Andrews, K.T.; Khokhar, S.; Davis, R.A. The discovery, synthesis and antimalarial evaluation of natural product-based polyamine alkaloids. Tetrahedron Lett. 2013, 54, 5188–5191. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Fechner, G.A.; Boyle, A.; Simpson, M.M.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Su, N.; Chen, H.; et al. Spermatinamine, the first natural product inhibitor of isoprenylcysteine carboxyl methyltransferase, a new cancer target. Bioorg. Med. Chem. Lett. 2007, 24, 6860–6863. [Google Scholar] [CrossRef] [PubMed]
- Alterio, V.; di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; de Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Ludwig, P.A.; Christianson, D.W. Phenol as a carbonic anhydrase inhibitor. J. Am. Chem. Soc. 1994, 116, 3659–3660. [Google Scholar] [CrossRef]

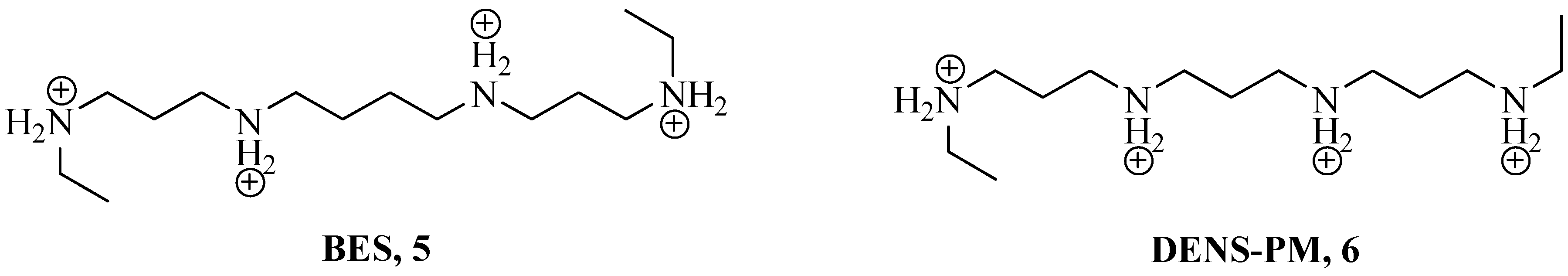
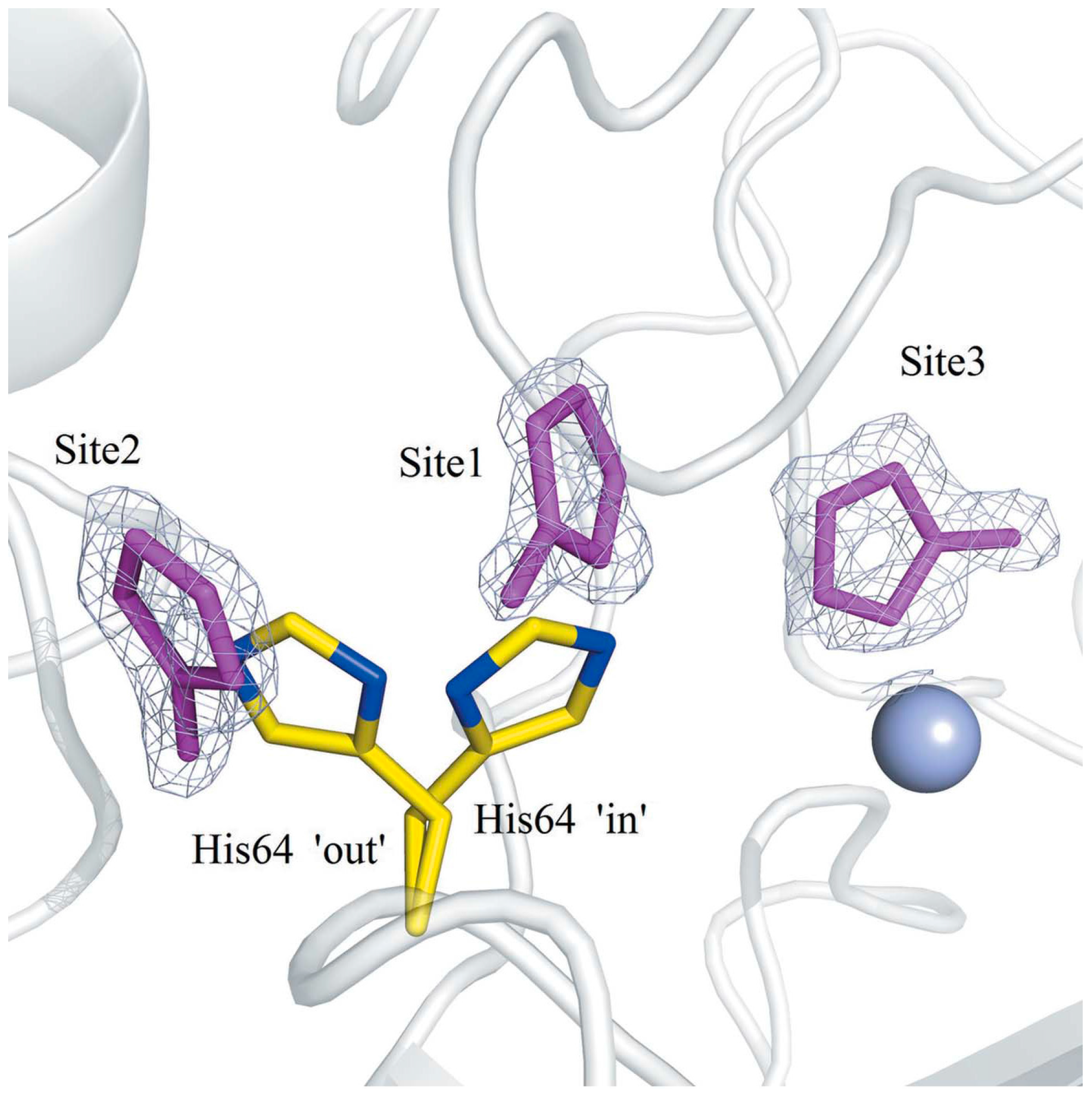
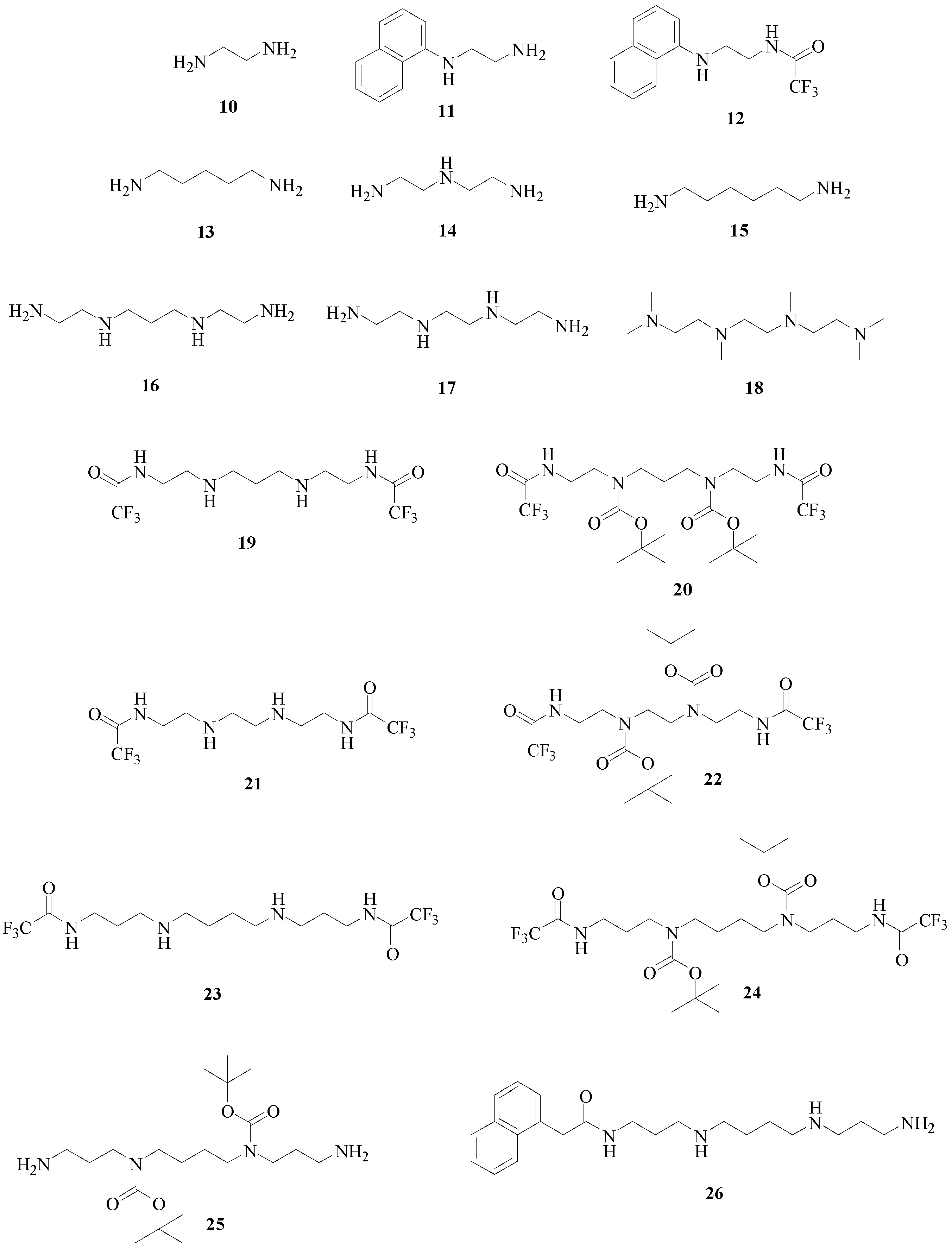
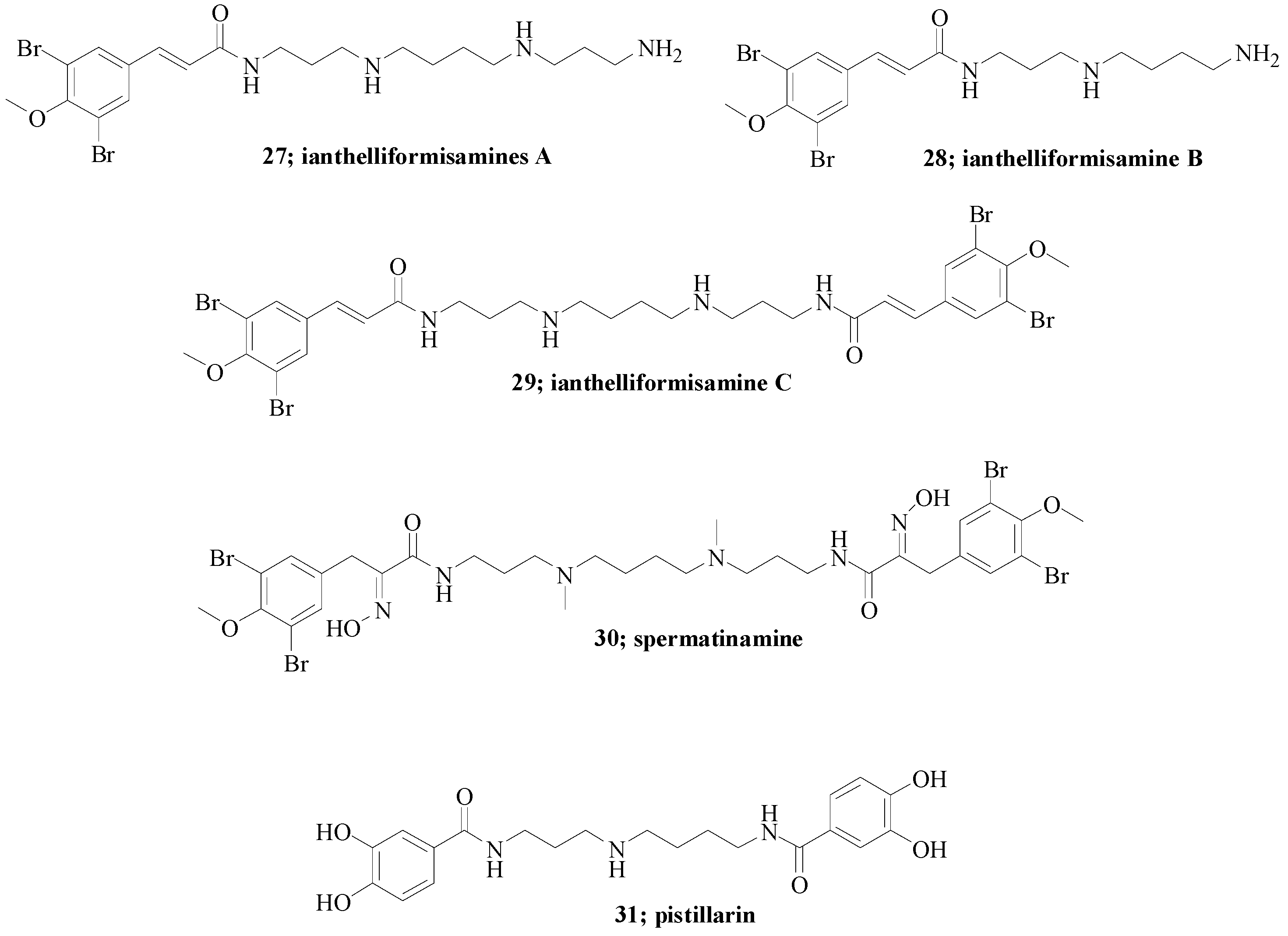

| Entry | KI (μM) * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hCA I | II | III | IV | VA | VB | VI | VII | IX | XII | XIII | XIV | XV | |
| 2 | 1.40 | 1.11 | 11.5 | 0.11 | 1.22 | 1.44 | 1.41 | 1.23 | 1.37 | 44.1 | 11.6 | 1.0 | 10.0 |
| 3 | 231 | 84 | 167 | 0.01 | 0.84 | 0.83 | 0.99 | 0.71 | 13.3 | 27.6 | 22.6 | 0.86 | 74 |
| 11 | >500 | 103 | 0.42 | 0.058 | 0.048 | 0.061 | 0.64 | 0.36 | 0.51 | 0.38 | 0.62 | 0.59 | 0.57 |
| 12 | >500 | 121 | 128 | 12.3 | 106 | 107 | 109 | 1.24 | 12.2 | 21.5 | 127 | 34 | 110 |
| 13 | 13.3 | 11.0 | 0.50 | 0.052 | 0.044 | 0.54 | 0.74 | 0.42 | 0.38 | 0.45 | 0.63 | 0.50 | 0.65 |
| 14 | 415 | 118 | 117 | 116 | 110 | 11.0 | 11.5 | 12.1 | 10.6 | 11.4 | 11.5 | 10.1 | 105 |
| 15 | 12.6 | 34.4 | 0.60 | 0.45 | 0.61 | 0.58 | 0.72 | 0.44 | 0.41 | 0.37 | 0.69 | 0.64 | 0.66 |
| 16 | 115 | 75 | 63 | 44 | 50 | 59 | 53 | 58 | 48 | 68 | 66 | 36 | 66 |
| 17 | 100 | 64 | 48 | 35 | 38 | 49 | 43 | 45 | 39 | 57 | 52 | 12.1 | 59 |
| 18 | >500 | 11.2 | 0.52 | 0.053 | 0.047 | 0.71 | 0.78 | 0.73 | 0.31 | 0.52 | 0.58 | 0.74 | 0.76 |
| 19 | 122 | 112 | 96 | 108 | 62 | 54 | 156 | 108 | 117 | 112 | 131 | 125 | 104 |
| 20 | >500 | >500 | >500 | 309 | 416 | 401 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 21 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 22 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 23 | 136 | 11.3 | 11.5 | 0.116 | 1.26 | 1.05 | 1.10 | 1.09 | 11.4 | 10.2 | 12.6 | 6.7 | 110 |
| 24 | >500 | 107 | 112 | 104 | 125 | 103 | 104 | 107 | 124 | 175 | 179 | 85 | >500 |
| 25 | 137 | 110 | 132 | 103 | 131 | 107 | 114 | 108 | 144 | 165 | 136 | 115 | 236 |
| 26 | 12.3 | 1.13 | 11.6 | 0.018 | 1.03 | 1.05 | 0.11 | 0.10 | 0.12 | 0.19 | 10.2 | 1.03 | 0.78 |
| AAZ | 0.25 | 0.012 | 200 | 0.074 | 0.063 | 0.054 | 0.011 | 0.0025 | 0.025 | 0.0057 | 0.017 | 0.041 | 0.072 |
| Phenol | 10.2 | 5.5 | 2.7 | 9.5 | 218 | >500 | 208 | >500 | 8.8 | 9.2 | >500 | 11.5 | 10.5 |
| Entry | KI (μM) * | |||||
|---|---|---|---|---|---|---|
| hCA I | II | IV | IX | XII | XIV | |
| 2 | 1.40 | 1.11 | 0.11 | 1.37 | 44.1 | 1.0 |
| 3 | 231 | 84 | 0.01 | 13.3 | 27.6 | 0.86 |
| 27 | 1.76 | 0.41 | 6.72 | 0.20 | 2.81 | 2.12 |
| 28 | 0.77 | 0.37 | 9.10 | 0.35 | 3.48 | 2.28 |
| 29 | 0.86 | 0.35 | 9.08 | 0.27 | 3.50 | 6.96 |
| 30 | 0.85 | 0.48 | >20 | 0.34 | >20 | 2.72 |
| 31 | 0.79 | 0.34 | 7.03 | 0.36 | 4.21 | 1.52 |
| AAZ | 0.25 | 0.012 | 0.074 | 0.025 | 0.006 | 0.041 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scozzafava, A.; Supuran, C.T.; Carta, F. Polyamines and α-Carbonic Anhydrases. Molecules 2016, 21, 1726. https://doi.org/10.3390/molecules21121726
Scozzafava A, Supuran CT, Carta F. Polyamines and α-Carbonic Anhydrases. Molecules. 2016; 21(12):1726. https://doi.org/10.3390/molecules21121726
Chicago/Turabian StyleScozzafava, Andrea, Claudiu T. Supuran, and Fabrizio Carta. 2016. "Polyamines and α-Carbonic Anhydrases" Molecules 21, no. 12: 1726. https://doi.org/10.3390/molecules21121726






